Global dietary patterns have been going through significant changes, especially in economically emerging countries, such as Brazil. The demographic and economic transformations in the past few years led to a reconfiguration in the patterns of Brazilians’ dietary consumption. Fresh or minimally processed foods have been substituted by a diet based on ultraprocessed foods, with high levels of sugar, fat and salt(1,Reference Louzada, Martins and Canella2) , and low in fibre and micronutrients(Reference Louzada, Martins and Canella2). This consumption pattern is a risk factor for the development of non-communicable chronic diseases(Reference Louzada, Martins and Canella2).
Studies indicate that unhealthy dietary patterns, like Western diets, composed of large amounts of saturated and trans-fat, refined carbohydrates and red meat, are related to high levels of inflammation(Reference Ahluwalia, Andreeva and Kesse-Guyot3). On the other hand, the consumption of healthier diets, such as the Mediterranean diet, which is rich in fruits, vegetable oils, vegetables and legumes, is associated with low levels of inflammation(Reference Viscogliosi, Cipriani and Liguori4).
Faced with the implications of this nutrition transition, the Dietary Inflammatory Index (DII®) was developed with the objective of assessing the inflammatory potential of individuals’ diets. The DII provides a way to evaluate dietary intake based on the pro-inflammatory and anti-inflammatory effect of up to forty-five dietary parameters, such as whole foods such as onion and garlic, spices, macronutrients, vitamins, minerals, flavonoids and other specific nutrients(Reference Shivappa, Steck and Hurley5). More recently, the Energy-adjusted Dietary Inflammatory Index (E-DII™) was created, which is calculated very similarly to the DII, except that it considers the impact of total energy intake(Reference Peres, Bandera and Qin6).
Studies have revealed relationships between high values of DII/E-DII in relation to increased risk of increased levels of inflammatory markers, and development of chronic diseases such as obesity, cancer, kidney disease, diabetes and CVD(Reference Peres, Bandera and Qin6,Reference Mazidi, Shivappa and Wirth7) . These studies have been performed mainly in high-income nations, such as the USA(Reference Mazidi, Shivappa and Wirth7,Reference Shivappa, Blair and Prizment8) and European countries(Reference Phillips, Shivappa and Hébert9), though countries in the Middle East, Asia and Latin America are represented among the fifty-four countries in which the DII/E-DII have been used(Reference Vahid, Shivappa and Hatami10).
It is known that certain factors, such as economic and demographic aspects, can influence food choices. Especially in poor countries, studies have shown that individuals with higher levels of education and income consume more inflammatory diets, with higher values of DII(Reference Shivappa, Blair and Prizment8,Reference Carvalho, Silva and Assunção11,Reference Wirth, Shivappa and Khan12) due to greater and easy access to foods rich in trans and saturated fat and added sugars. However, in affluent countries, the opposite pattern is observed, that is, more affluent people select diets with higher concentrations of phytochemicals, which tend to be strongly anti-inflammatory. In addition, individuals with BMI classified as overweight and obese have a more pro-inflammatory diet, with higher DII values in comparison with normal BMI(Reference Mazidi, Shivappa and Wirth7,Reference Phillips, Shivappa and Hébert9) , showing a relationship between inflammatory dietary patterns and nutritional status.
Studies about the inflammatory potential of diets have been performed mainly in high-income nations, and it is relevant to understand that potential in populations with different characteristics. Also, very few DII-focused studies have been conducted in Latin America(Reference Carvalho, Silva and Assunção11,Reference Silva, Sampaio and Shivappa13) . Brazil is a medium-income country, with continental dimensions, great socio-economic differences among its regions and is going through a rapid and dramatic nutritional transition process. In this context, the objective of the present study is to investigate the inflammatory potential of the Brazilian population’s diet and the associated demographic, socio-economic and anthropometric factors.
Methods
This is a cross-sectional study that used microdata from the Consumer Expenditure Survey (POF) 2008–2009, performed by the Brazilian Institute of Geography and Statistics (IBGE) between 19 May 2008 and 18 May 2009. POF is a residence-based study, representative of the Brazilian population, and considered the most complete research database of its type in the country. The survey investigated household budgets, life conditions, anthropometry, food availability and dietary consumption(14–16). Brazil is a medium-income country, and in the POF data collection period, it presented a human development Index of 0·715(17).
POF sampling was conducted in two phases. In the first phase, primary units of sampling were selected, which correspond to the census sectors of the Demographic Census 2000(14). A Master Sample was determined from the sampling with probability proportional to the number of domiciles in the sector. The subsample of census sectors selected for POF 2008–2009, according to the Master Sample, was determined by simple random sampling. Through the selection of census sectors, secondary sampling units, which correspond to permanent private domiciles, were selected by simple random sampling without replacement in each sector(14).
The final POF sample was composed of 189 288 individuals living in 55 970 domiciles. With the objective of evaluating the individual dietary consumption, the National Diet and Nutrition Survey was conducted. For this survey, one in every four domiciles from the final POF sample was randomly selected, with a total of 34 003 individuals in 13 569 domiciles(16). The sample for the present study was composed of 34 003 individuals (Fig. 1).

Fig. 1. Flow chart of the sampling process to assess food consumption in the Consumer Expenditure Survey (POF) 2008–2009.
Adapted from Sperandio et al.(Reference Sperandio, Rodrigues and Franceschini18). IBGE, Brazilian Institute of Geography and Statistics.
Procedures for data collection, variables and instruments
The socio-economic and demographic variables were obtained through interviews performed in the domiciles. More details on the sampling, data collection procedures and instruments utilised in POF were previously described by IBGE(14).
Socio-economic and demographic data
For the present study, the following data were used: sex (female and male); self-reported colour/race (black, pardo or mixed, white, yellow, indigenous and unsure); age categorised in age range (10–19, 20–59 and above 60 years old); level of education evaluated in years of study (<9 years and ≥9 years) and income by monthly household income quartile.
Anthropometric data
The anthropometric measurements used in the study were weight and height. Body weight was measured using a portable electronic scale, and height was measured using a portable stadiometer(15). Relative weight, as a proxy for adiposity, was assessed using the BMI (BMI = weight (kg)/height (m)2). For adolescents, the indicator BMI-for-age in Z score was used and classified according to the growth curves recommended by the WHO (2007). For adults between 20 and 59 years old, the WHO’s classification (2000) was used, and for seniors (≥60 years old), the proposal from the Pan American Health Organization (PAHO, 2002).
Dietary consumption data
The dietary data were collected through dietary records, filled out on two non-consecutive days by the informants and complemented during interviews by the research agents. After the interviews, the researchers completed the transcription of the records to the data entry system. When the individuals were not able to fill out the dietary records, the information was filled out with the assistance of another resident of the domicile(16).
All of the food (including ready-to-eat meals) and drinks consumed within 24 h were recorded, along with the following information: time of the meal, amount consumed in household measurements, preparation and source of the food (inside or outside of the domicile)(16).
The research groups were trained for data collection of personal dietary consumption and received instructional material that included photos of utensils and containers that are frequently used to serve foods and drinks. More details can be found in IBGE(16).
Analysis of dietary data by the National Diet and Nutrition Survey
In order to identify possible reporting errors, the IBGE researchers reviewed all of the dietary records. After review, the data were typed in a portable computer, using a specific software for data entry(16). The software, which is specific for dietary records, had a database (registry of foods and drinks) of approximately 1500 items, which were selected from 5686 records in POF’s 2002–2003 database of food and drink acquisition. However, when necessary, the researchers were able to include new foods or drinks and the preparation type for foods that were not present in the database. For that, fifteen new preparation types and 106 new home measurements were made available(16).
If there were any doubts regarding the household units for measuring the food or drink consumed, the researcher would request the informant to show the utensil used during consumption or the packaging, in case of industrialised products. During the data collection stage, there was a partial analysis of the data collected with verification of response frequency, average of items consumed in the first and in the second day, codification of non-registered items, and verification of items that were included inadequately, such as: packed lunch (marmita)(16).
During the verification of data consistency, twenty-nine individuals were excluded due to incomplete records. In addition, quantities that were considered unrealistic were imputed and that information was recorded in the database. For these, the average estimated portion for each food was taken into account according to previously defined statistic criteria, which included the sex of the informant, age range, state, metro area and reported unit of measurement(16).
The interviewed individuals mentioned 1971 food items, which were classified in twenty-one food groups: fruits; vegetables; cereals; leguminous plants; legumes; roots and tubers; oleaginous items (chestnuts, walnuts and peanuts); flour and pasta; breads; cakes; cookies; meats; eggs; dairy products; sweets; drinks; pizzas; savoury snacks and sandwiches; mixed preparations; sauces and condiments; oils and fats. The quantities consumed, reported in home measurements, were converted to g or ml for the calculation of the amount of each item consumed by the informant, based on the Table of Measures for Food Consumed in Brazil from POF 2008–2009(16).
In order to estimate energy content, macronutrients and micronutrients of the food items that were mentioned in the research, the researchers used the Tables of Nutritional Composition of Food Consumed in Brazil, created by IBGE, using data from the Brazilian Food Composition Table and from the United States Department of Agriculture, in addition to the foods’ nutritional labels(16).
In the present study, the dietary parameters that are unavailable in POF’s database were added subsequently. Information about the amount of caffeine and alcohol were obtained exclusively from the United States Department of Agriculture tables. The amounts of flavonoids (flavonols, flavones, isoflavones, flavonones, flavanols and anthocyanidins) were extracted from the Brazilian Food Composition Table, version 6.6 from 2017, the β-carotene was obtained from the United States Department of Agriculture tables, and the missing foods, from the 2017 Brazilian Food Composition Table.
Dietary inflammatory index
The DII was used to assess the dietary inflammatory potential. The DII was created from an extensive literature review, which evaluated the effect of forty-five dietary parameters over inflammatory mediators (IL-1β, IL-4, IL-6, IL-10, TNF-α and C-reactive protein). It is a continuous quantitative measurement that ranges from −8·87 to +7·98. From this literature review, scores were created for each dietary parameter. The DII is calculated from the analysis of food consumption of a specific population. In the calculation, data are standardised with the assistance of a database composed of the combination of information on food consumption in eleven countries (USA, Mexico, Australia, Denmark, Japan, New Zealand, UK, India, South Korea, Taiwan and Bahrain)(Reference Shivappa, Steck and Hurley5). To standardise the DII of the present study, the mean and standard deviation of each food parameter in the combined database of the eleven countries were used. To obtain the DII for each food parameter in the present study, the standardised values were multiplied by the inflammatory effect score obtained by the literature review of the food parameters. Finally, to calculate the individual’s total DII, the DII of each food parameter was added. The development of the DII and the methodology used the calculation in this study were described in detail by Shivappa et al. (Reference Shivappa, Steck and Hurley5).
In order to improve prediction and comparison with the non-adjusted DII scores, the E-DII was created. To calculate the E-DII, the dietary data were expressed in 1000 kcal (4184 kJ) of the food consumed for the dietary parameters analysed in this study. The same computational procedures were used as for the DII; except that the world reference database was also expressed per 1000 kcal (4184 kJ). Higher, that is, more positive scores, DII or E-DII represents more pro-inflammatory diets, while lower or more negative scores indicate more anti-inflammatory diets(Reference Peres, Bandera and Qin6).
The present study utilised data from thirty-four of the forty-five possible dietary parameters in order to obtain the DII and E-DII, such as MUFA, PUFA, alcohol, β-carotene, caffeine, carbohydrates, cholesterol, energy, Fe, fibre, flavonols, flavones, isoflavones, flavonones, flavanols, anthocyanidins, saturated fat, total fat, trans-fat, Mg, n-3, n-6, proteins, riboflavin (vitamin B2), Se, thiamine (vitamin B1), vitamins A, B3, B12, B6, C, D and E and Zn. The dietary parameters oregano, rosemary, onion, ginger, saffron, turmeric, pepper, green/black tea, folic acid, eugenol and garlic were not included in the analysis because there was a lack of information or under-recording of the consumption of such culinary ingredients (spices) and drink.
Statistical analysis
The data from this research project were analysed using STATA® software (version 14.0). The distribution of the numerical variables was analysed through box plot, histogram, kurtosis analysis, asymmetry and Kolmogorov–Smirnov and Shapiro–Wilk tests. From their distribution, the variables were presented as means and standard deviations. The categorical variables were recorded as frequencies and percentages.
The variables sex, age, level of education, income quartile, skin colour/race, regions and nutritional status had the average compared. For such, Student’s t test and ANOVA parametric tests were used.
In order to verify the association of each variable with the E-DII, a bivariate linear regression analysis was performed between demographic, socio-economic and anthropometric variables and the E-DII. The nominal cut-off point for judging whether a relationship was statistically significant was set at α = 0·05.
To perform the multivariate analysis, a hierarchical theoretical model was developed in order to assess the association between sociodemographic variables and the E-DII. At the first level, the variables included were (age, sex, region and race/skin colour) and a significant P value of 0·1 was considered. At the second level, the variables included were (household income and education) and a P value of 0·1 was admitted. In the final model, the significance value of 0·05 was considered.
Results
Data from a total of 34 003 individuals aged 10 years and older, who participated in the survey, represented the analytical database for this study. From those, 53·8 % were women and they had a mean age of 36·1 (sd 18·3) years. The majority of the individuals had <9 years of education (64·3 %), mixed skin colour (50·5 %), 37·1 % were from the Northeast region of the country and 39·5 % were overweight/obese (Table 1).
Table 1. Demographic, socio-economic and anthropometric characteristics of the participants in the Consumer Expenditure Survey, Brazil, 2008–2009
(Numbers and percentages; medians and interquartile ranges)

* Median and interquartile range values are presented for this variable instead of number of individuals and percentage.
The E-DII ranged from −4·77 to 5·98, with a mean of 1·04 (sd 1·44). The mean of the E-DII for men and women was 1·04 and 1·05, respectively (P = 0·328). The average E-DII of pregnant women and nursing mothers was 1·09 (sd 1·40) and did not differ from the average of the general population (P = 0·229). Seniors had lower inflammatory E-DII mean, and adolescents had a greater number (0·61 v. 1·42; P < 0·001) (Table 2).
Table 2. Description of participants’ diet by Energy-adjusted Dietary Inflammatory Index, Brazil, 2008–2009
(Mean values and standard deviations; 95 % confidence intervals; medians and interquartile ranges)
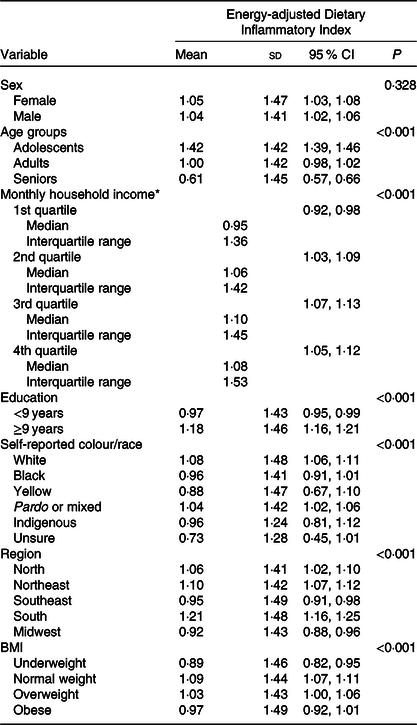
* Median and interquartile range values are presented for this variable instead of mean and standard deviation.
Individuals with higher income had a higher E-DII compared with those with lower income (1·10 v. 0·95; P < 0·001). The E-DII was also higher among individuals with greater levels of education (1·18; P < 0·001). Individuals of race yellow had lower diet inflammatory potential, and white participants had higher inflammatory potential (0·88 v. 1·08; P < 0·001) (Table 2).
Participants from the Midwest and Southeastern regions had a diet with lower inflammatory potential (mean of E-DII 0·92 and 0·95, respectively), while the South and Northeast regions presented more inflammatory values (1·21 and 1·10, respectively) (P < 0·001). Individuals with low weight had lower E-DII values (0·89), whereas participants with normal BMI presented more pro-inflammatory diets (1·09) (P < 0·001) (Table 2).
In Table 3, after bivariate regression analysis, it is noted that obese and overweight male adolescents (β = 0·52; 95 % CI 0·31, 0·73 and β = 0·15; 95 % CI 0·02, 0·28, respectively) from the South (β = 0·37; 95 % CI 0·19, 0·56) had a more pro-inflammatory E-DII. It was also observed that the higher the income, the more pro-inflammatory the E-DII was for adolescents of both sexes. Female adolescents from the South (β = 0·51; 95 % CI 0·32, 0·69) and Northeast (β = 0·23; 95 % CI 0·08, 0·37) had a more pro-inflammatory E-DII.
Table 3. Bivariate linear regression analysis of the factors associated with the Energy-adjusted Dietary Inflammatory Index in Brazilian adolescents, adults and elderly stratified by sex, Brazil, 2008–2009
(β Values and 95 % confidence intervals)
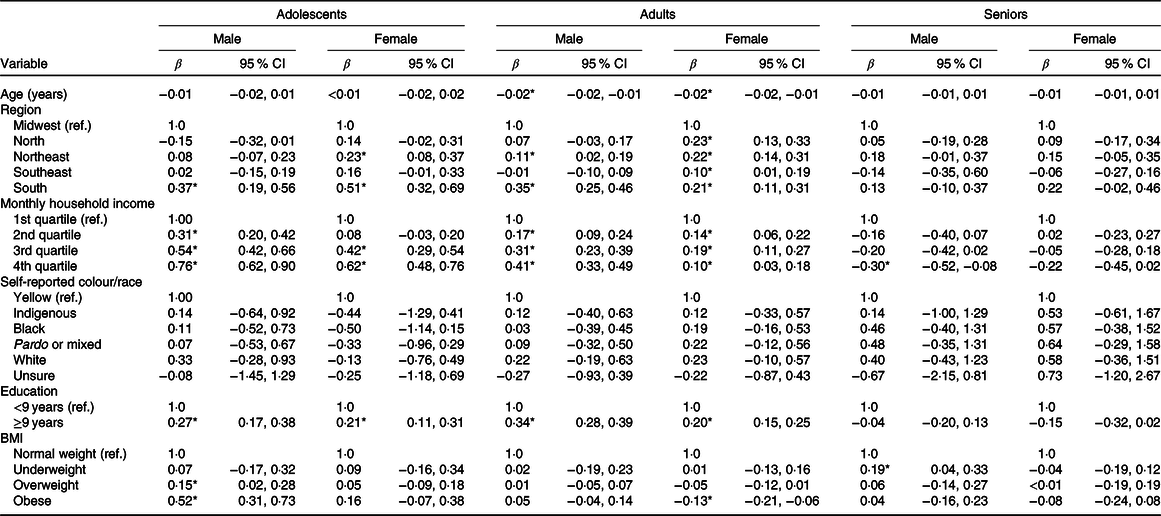
Ref, reference category.
* Statistical significance (P < 0·05).
Individuals with lower levels of education (<9 years) showed a reduction in the E-DII in both sexes and in adolescence, adult life and pregnant women and nursing mothers. On the other hand, the higher the income, the higher was the E-DII for adolescents and adults of both sexes. However, in women, the increase in the E-DII was higher in the third income quartile and in woman with obesity had a reduction in E-DII (β = −0·30; 95 % CI −0·52, −0·08). When compared with the Midwest, women from the all regions showed an increase in the E-DII score, the North (β = 0·23; 95 % CI 0·13, 0·33), Northeast (β = 0·22; 95 % CI 0·14, 0·31), South (β = 0·21; 95 % CI 0·11, 0·31) and Southeast (β = 0·10; 95 % CI 0·01, 0·19). Among men, a higher increase in the E-DII was revealed among residents of the South (β = 0·35; 95 % CI 0·25, 0·46) and Northeast (β = 0·11; 95 % CI 0·02, 0·19). Among seniors, only men with low weight had an increase in the E-DII score (β = 0·19; 95 % CI 0·04, 0·33) and those in the higher income quartile presented lower E-DII score (β = −0·30; 95 % CI −0·52, −0·08).
After bivariate linear regression analysis, only the education variable showed a significant difference (Table 4). Pregnant women and nursing mothers with higher education had lower E-DII scores (β = −0·20; 95 % CI −0·36, −0·04).
Table 4. Bivariate linear regression analysis of the factors associated with the Energy-adjusted Dietary Inflammatory Index in Brazilian pregnant women and nursing mothers, Brazil, 2008–2009
(β Values and 95 % confidence intervals)
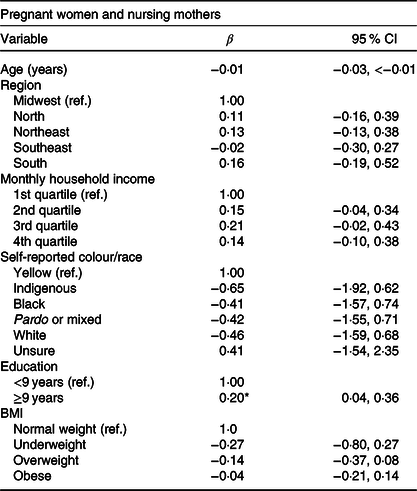
Ref, reference category.
* Statistical significance (P < 0·05).
In the final model, the E-DII was associated with the second income quartile (β = 0·17; 95 % CI 0·13, 0·21), the third income quartile (β = 0·28; 95 % CI 0·23, 0·33) and the fourth income quartile (β = 0·24; 95 % CI 0·19, 0·29). Individuals living in the Northeast (β = 0·23; 95 % CI 0·18, 0·28) and South regions (β = 0·25; 95 % CI 0·19, 0·31), white people (β = 0·23; 95 % CI 0·02, 0·44), with ≥9 years of education (β = 0·17; 95 % CI 0·13, 0·20), adults (β = 0·38; 95 % CI 0·34, 0·43) and adolescents (β = 0·87; 95 % CI 0·82, 0·93) had higher E-DII (Table 5).
Table 5. Multivariate linear regression analysis of factors associated with the Energy-adjusted Dietary Inflammatory Index, Brazil, 2008–2009
(β Values and 95 % confidence intervals)
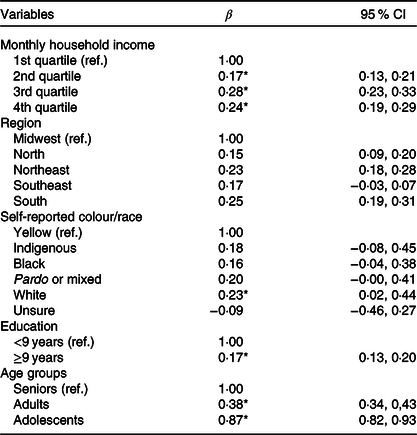
Ref, reference category.
* Statistical significance (P < 0·05).
Discussion
This is the first study to evaluate inflammatory potential of diet in the Brazilian population through the use of E-DII. We observed more pro-inflammatory E-DII values, especially in overweight and obese male adolescents, underweight seniors, and in those with higher income and education levels in bivariate analysis. In the multivariate model, the E-DII was associated with higher income quartiles, was higher in the Northeast and South regions, in white people, individuals with ≥9 years of education and adults and adolescents age group.
The E-DII was developed to improve the comparison of the non-energy-adjusted DII scores. In order to obtain the E-DII, the values of DII are calculated for 1000 kcal (4184 kJ) of the consumed foods, which differentiate it from the DII. In our study, we used both indexes; however, because the E-DII had better output than the DII, which suggests more plausible results, we chose to use only the E-DII in the linear regression analysis.
In Brazil, most studies that have used the DII to assess diet inflammatory profile found similar or higher values than those of the present study. In addition, no study has used a representative sample of the Brazilian population or has investigated its relationship with socio-economic and demographic factors. Carvalho et al. (Reference Carvalho, Silva and Assunção11), in a cross-sectional study with 2017 young adults between 23 and 25 years old, found a DII average of +1·10 and variation of −4·69 to + 5·28. Silva et al. (Reference Silva, Sampaio and Shivappa13), in a cross-sectional study conducted with 137 individuals with multiple sclerosis, from 19 to 64 years old, verified a DII average of 3·30 and variation of −1·34 to +7·13.
International studies that have used the E-DII showed lower average scores compared with what we found in this research(Reference Peres, Bandera and Qin6,Reference Harmon, Wirth and Boushey19) . In the USA, in a case–control study, Peres et al. (Reference Peres, Bandera and Qin6) investigated the E-DII and the risk of ovarian cancer in women of African descent between 20 and 79 years old. The authors observed that the E-DII ranged from −4·15 to 3·19, with an average of −0·51 (sd 1·87). In a prospective cohort, in order to evaluate the E-DII and the risk for colorectal cancer in 190 963 individuals of both sexes and between 45 and 75 years old, Harmon et al. (Reference Harmon, Wirth and Boushey19) found an E-DII of −6·64 to 4·95. In another prospective cohort, Zheng et al. (Reference Zheng, Wirth and Merchant20) verified the survival rate of post-colorectal cancer women between 50 and 79 years old and the relationship with the E-DII. There was a variation of −6·8 to 3·25.
Although the aforementioned studies have shown lower values of E-DII than those of the present study, it is important to highlight that their samples are mainly composed of adults and seniors and such population does have the tendency of modifying and improving the quality of their diets as age advances due to treatment and/or prevention of pathologies(Reference Assumpção, Domene and Fisberg21), which could contribute to the reduction of the E-DII. Since the present study includes adolescents, this age group could have added higher values of E-DII to the sample.
Younger individuals tend to consume more foods and nutrients with higher inflammatory potential. Aslani et al. (Reference Aslani, Qorbani and Hébert22) identified that Iranian adolescents had a more pro-inflammatory diet due to the high consumption of fast food and sugars, such as cookies and crackers. Shivappa et al. (Reference Shivappa, Blair and Prizment23) observed a more pro-inflammatory diet in European adolescents that consumed more bread, chocolate, margarine, butter, animal fat, sodas, isotonics, meats, cakes, pies, cookies, sugars, honey, jam, and less vegetables, fruits, fruit juices and fish. In Brazil, data from POF confirmed higher consumption of these types of food by adolescents, as well as a reduction in this pattern as age advanced(16).
Another aspect that could explain the pursuit of more pro-inflammatory foods is that, during adolescence, diet is more focused on the pleasure of eating rather than the nutritional value of the food(Reference da Silva, Teixeira and Ferreira24), and there is not much concern about the development of non-communicable chronic diseases in the long term(Reference Gambardella, Frutuoso and Franch25). In addition, factors like family environment also determine dietary behaviour. Parents’ food choices and the food acquired for the entire home’s consumption could influence children’s and adolescents’ diets(Reference Bauer, Laska and Fulkerson26).
Regarding education and income, adolescents and adults with higher income and education levels had a more pro-inflammatory E-DII in the present study. However, in high-income nations, the results indicate a reverse relationship between diet, level of education and income. In Europe and in the USA, individuals with higher income and greater education levels had more anti-inflammatory diets when compared with those with lower education and income(Reference Wirth, Shivappa and Davis27). In Brazil, a medium-income country, there still seems to be a direct relationship between diet inflammatory potential, income and education, supporting Carvalho et al. (Reference Carvalho, Silva and Assunção11) findings, which observed that women from the most pro-inflammatory DII tertile had higher levels of education. Moratoya et al. (Reference Moratoya, Carvalhaes and Wander28), in a study on changes in the consumption pattern in Brazil and in the world, suggest that individuals with higher income do not necessarily consume healthier foods, since they have more access to processed food products such as fast food and sweets, which are less accessible to those with lower incomes. Similarly, POF demonstrates that individuals from the highest income quartile, in spite of consuming more fruits and raw salads, also consume more sodas, pizzas, and fried and baked savoury snacks. These results confirm a dietary pattern of higher consumption of ultra-processed products among higher-income individuals. This entails a greater consumption of pro-inflammatory dietary parameters (saturated and trans-fat, cholesterol, carbohydrates, etc.)(16), justifying the E-DII increase in this group. Thereby, the same individuals with higher income, due to their greater purchasing power, have more access and consumption of fruits and vegetables and also of ultra-processed and processed foods(Reference Canuto, Fanton and Lira29,Reference Bielemann, Motta and Minten30) , which may contribute to an increase in the E-DII.
However, in our study, seniors with the highest income quartile had reduced E-DII values. In a study carried out in the USA with seniors, it was noted that those with high income had a higher consumption of grains, vegetables, fruits and milk compared with those with low income(Reference Guthrie and Lin31). Such consumption characteristic results in a lower E-DII, therefore, more anti-inflammatory. This probably occurs because seniors have some restrictions to eat fast food and ultraprocessed foods due to health limitations, so they use their higher income for buy more health foods than ultraprocessed foods.
The present research showed that the South and Northeast of Brazil have higher values of a more pro-inflammatory E-DII in adolescents and adults. POF data reveal that the South presents greater consumption of Na and foods high in fat, SFA and MUFA, trans-fat, and total sugars, and lower average consumption per capita (g/d) of fresh fish compared with other Brazilian regions. It is worth mentioning that such dietary pattern contributes to an increase in the E-DII. National research reveals that in the Northeast region, there is higher consumption of carbohydrates and lower intake of fibres by women(16), in addition to the consumption of fruits and vegetables being below the national average(Reference Jaime, Santos and Oliveira32). High-fibre foods such as fruits and vegetables play a crucial role for a more anti-inflammatory E-DII. Therefore, lower intakes of such foods in this region result in a more pro-inflammatory diet.
Regarding BMI, obese and overweight male adolescents had a higher E-DII. Corréa-Rodriguez et al. (Reference Correa-Rodríguez, González-Jiménez and Rueda-Medina33), in a cross-sectional study, verified an association of the DII with cardiovascular risk factors in 428 students between 9 and 17 years old in two cities in Spain. The authors observed a positive relationship between pro-inflammatory diets and a diagnosis of overweight and obesity in these individuals. Therefore, the food consumption profile that contributes to a more pro-inflammatory diet is also one that promotes an increase in the BMI, resulting in obesity. In adult women with obesity, there was a reduction in E-DII values. According to a population-based study by Assumpção et al. (Reference Assumpção, Domene and Fisberg21), women with chronic diseases tend to have a better diet quality, as they develop greater concern for their own health.
However, male seniors with low weight had diets with the highest E-DII values, which could be due to the insufficient quality and quantity of the food consumed. Gomes et al. (Reference Gomes, Soares and Gonçalves34), in an investigation on risk factors for low-quality diets by seniors, concluded that those with low weight had greater intake of food that are poor in energy, fibres, vitamins and minerals. As a consequence, their energy intake was insufficient to meet the nutritional demands in this stage of life.
Another factor that may interfere in the quality of the food consumed by seniors is teeth loss. A study on auto-perception of oral health and food selection by senior users of dental prosthesis revealed a reduction in masticatory function and, possibly, interference in food choices(Reference Lima, Soares and Passos35). In this context, seniors started to seek smooth and easy-to-chew food items(Reference Mahan and Escott-Stump36) and those with easy acquisition and preparation. Therefore, tend to consume soft foods, easily chewable, poor in fibre, vitamins and minerals(Reference Campos, Monteiro and Ornelas37). Consequently, there is a limitation in foods with higher energy density, such as whole grains, fresh fruits and vegetables(Reference Lima, Soares and Passos35), which have anti-inflammatory scores in the E-DII. Therefore, this population seeks foods that are rich in simple carbohydrates, sugars and those that are poor in fibres, characterising a more pro-inflammatory diet. Another explanation would be dependence on others to purchase and prepare food. Male seniors without partners, such as widowers, may develop some sort of isolation, giving less importance to health and, consequently, to diet(Reference Santos and Ribeiro38). Thus, they seek foods that are easier to prepare, and these foods can have less vitamins and minerals.
Conclusion
It is concluded that the Brazilian population has a diet with high inflammatory potential, especially adolescents, white people and those with higher levels of income and education, exposing them as groups with higher risk for the development of diseases associated with inflammation. It was observed that the socio-economic risk profile for a diet with higher inflammatory potential in medium-income nations is different from what is observed in high-income countries.
Public policies must be enhanced in order to promote the population’s awareness towards the consumption of a healthier and less inflammatory diet, especially individuals with higher income and education levels, adolescents and those with obesity. Populations with lower income and education must be the target of actions that promote the intake of foods and nutrients with less inflammatory potential, such as tax reduction on fresh foods and basic staples and increased taxation on unhealthy food products.
Limitations and strengths
The present study had as a limitation the difficulty to quantify the intake of culinary ingredients that are considered anti-inflammatory, such as garlic, onion, saffron, ginger, oregano, rosemary, turmeric and pepper, limiting the number of dietary parameters analysed in the research. Nonetheless, studies that have not evaluated dietary parameters similar to those of the present study suggested that the reduction of this number does not compromise the discriminatory potential of the index(Reference Shivappa, Steck and Hurley5,Reference Tabung, Steck and Zhang39) .
Despite its weaknesses, the study has several strengths. One positive aspect is the fact that this is a population-based study with a large sample size, which evaluated the socio-economic factors associated with diet inflammatory potential in a population of a medium-income country, with pronounced socio-economic differences and that is going through a rapid nutritional transition. This allowed for the identification of individuals with risk characteristics for consumption of a more inflammatory diet.
Acknowledgements
This project was financed by the National Council for Scientific and Technological Development (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Authors’ contributions were as follows: N. O. P. – conceptualisation, methodology, formal analysis, writing the original draft, review and editing; C. A. d. C. – conceptualisation, supervision, investigation, formal analysis, writing the original draft, review and editing; N. S. – conceptualisation, reviewing the manuscript and editing; K. D. S. M. – formal analysis, writing the original draft, review and editing; P. C. d. A. F. V. – formal analysis, reviewing the manuscript and editing; N. S. – methodology, formal analysis, reviewing the manuscript and editing; J. R. H. – methodology, formal analysis, reviewing the manuscript and editing; A. K. T. d. C. F. – conceptualisation, supervision, investigation, formal analysis, writing the original draft, review and editing.
The authors declare that there are no conflicts of interest.









