Introduction
The grainbelt of Western Australia (WA) has a Mediterranean climate, with annual crops produced in the winter/spring growing season and a fallow over the summer/autumn (Department of Agriculture and Food Western Australia 2016). The summer rainfall events are not frequent or reliable enough to support summer cropping in most of this region, but they stimulate germination of weeds and volunteer crop plants (Michael et al. Reference Michael, Borger, MacLeod and Payne2010). These weeds use stored soil moisture and nutrients, reducing yield potential of the following (rainfed) winter grain crop (Anderson et al. Reference Anderson, Sharma, Shackley and D’Antuono2004; Department of Agriculture and Food Western Australia 2016; Hunt and Kirkegaard Reference Hunt and Kirkegaard2011; Turner and Asseng Reference Turner and Asseng2005). Borger et al. (Reference Borger, Riethmuller, Hashem and Zydenbos2010) noted a 25% yield reduction in wheat (Triticum aestivum L.) following growth of uncontrolled windmillgrass (Chloris truncata R. Br.) over the summer fallow, presumably due to removal of moisture and nutrients. Further, summer weeds carry disease and insect pests and impede crop sowing (Cameron and Storrie Reference Cameron and Storrie2014). For example, beet western yellows virus (Polerovirus sp.) is a common disease of crop and pasture species in southwestern Australia and is hosted by summer annual weeds such as volunteer canola (Brassica napus L.), watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai], and fleabane (Erigeron sp.) (Coutts et al. Reference Coutts, Hawkes and Jones2006). Barley yellow dwarf virus (Luteovirus sp.) and cereal yellow dwarf virus (Polerovirus sp.) are hosted by a range of native and introduced annual grass species (Hawkes and Jones Reference Hawkes and Jones2005).
Prior surveys of weeds in WA have described species incidence and density in cropping fields in a single season—the winter annual growing season or the summer fallow (Borger et al. Reference Borger, Michael, Mandel, Hashem and Renton2012; Michael et al. Reference Michael, Borger, MacLeod and Payne2010). These surveys highlight those species that growers have failed to control, providing guidance on what species need further research or extension to improve management tactics. This approach fails to assess total species incidence, as some species have been killed through successful management. Further, surveys in a single year cannot assess variability in species incidence or spread of individual species. Optimal weed control requires an understanding of species invasiveness (Bullock et al. Reference Bullock, Kenward and Hails2002). Roadsides, by comparison, are a habitat that supports weed growth, and they are often not subject to weed control. The current project surveyed roadside weeds in the WA grainbelt over three consecutive summers (from 2015 to 2017). The project aimed to determine the prevalence of summer weeds and highlight the variation in species incidence and density between years.
Materials and Methods
A survey was conducted to assess weed species incidence and density on the roadside of major roads within the WA grainbelt, over three consecutive summers (from 2015 to 2017; Figure 1). The survey was conducted each year during February to April, at least 6 to 8 wk after a summer rainfall event of more than 20 mm (where summer in Australia is December to February), to ensure summer weeds had germinated and reached maturity before assessment. Surveying mature summer weeds (in late summer or early autumn) made identification easier and allowed surveyors to target sites that showed no evidence of weed control practices or disturbance. The usual cause for disturbance of a roadside weed population was that the unsealed gravel road had been graded, a common process in rural WA that repairs the road but disturbs or removes roadside vegetation. Chemical control of weeds on a roadside is unusual, as legislation protects the native roadside vegetation. In 2015, a total of 244 sites were selected approximately every 10 km where mature weeds were visible on the roadside. In 2016 and 2017, sites were revisited, but any site that showed signs of disturbance, weed control, or low rainfall in any year (i.e., sites with no weeds or sparse, immature weeds) was removed from the data set. The final data set had 133 sites common across the 3 yr. At each site, weed species were identified along a 20-m transect. Each species was recorded at low (0 to 10 plants for broadleaf weeds or seed heads for grass weeds m-2), medium (11 to 50 plants or seed heads m-2), or high (>50 plants or seed heads m-2) density. Photos were taken to allow identification of ambiguous species. Native vegetation was not included in the survey, except those native species that are common agronomic weeds: C. truncata, Russian-thistle (Salsola tragus L.) or mulla mulla [Ptilotus polystachyus (Gaudich.) F. Muell.]. Some species were grouped within a genus, due to difficulty with accurate identification (i.e., species in the Erigeron genus), according to the method in Michael et al. (Reference Michael, Borger, MacLeod and Payne2010). Rainfall data over each summer were obtained from the Rainfall Archive at the Bureau of Meteorology (2019).
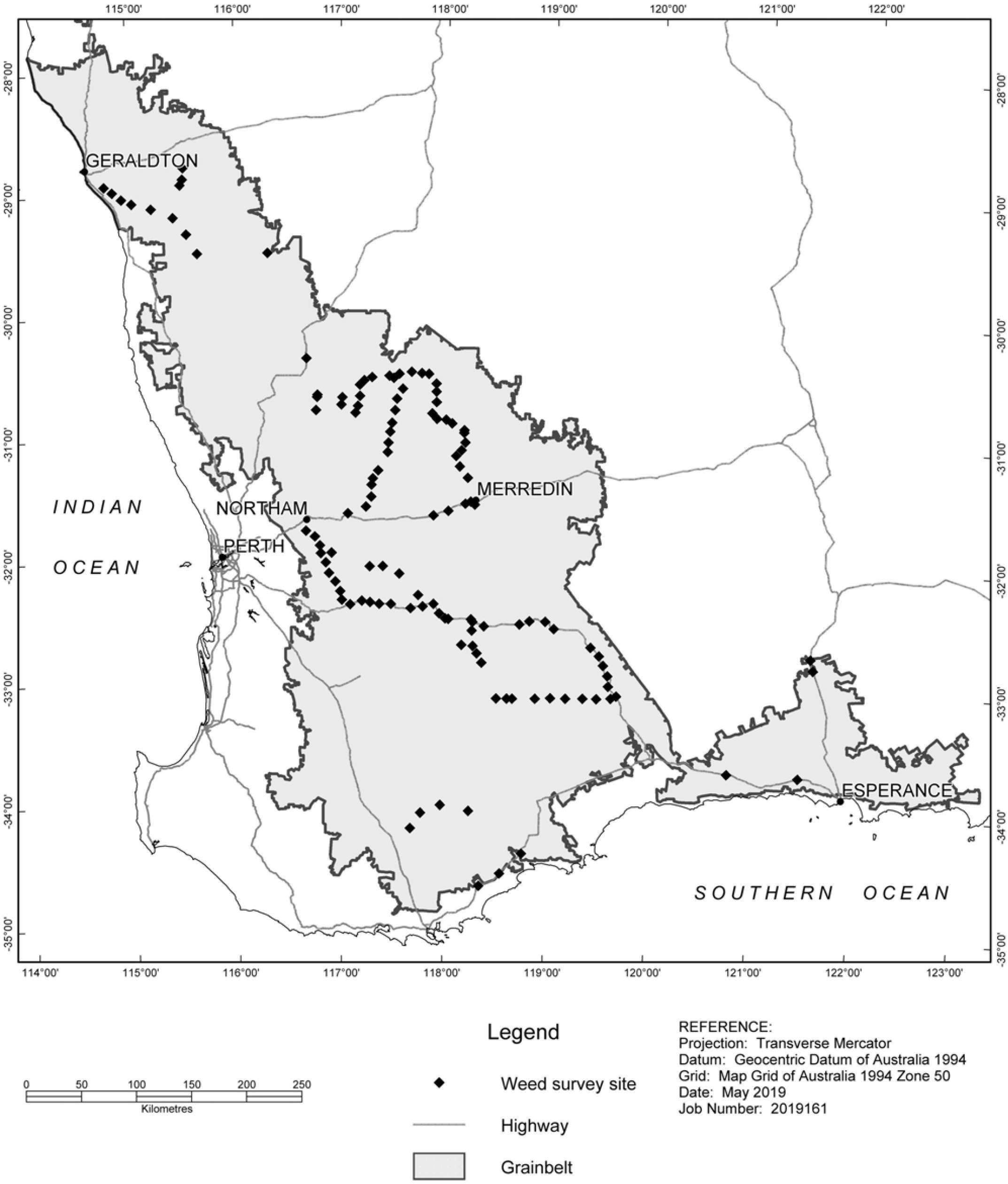
Figure 1. The 133 sites surveyed for summer weed incidence and density within the Western Australian grainbelt over 2015, 2016, and 2017. Map courtesy of the Department of Primary Industries and Regional Development.
Analysis
The analysis was performed on the 133 sites that contained mature weeds with no evidence of disturbance in every year. For an individual species, each site was classified according to a binary system of presence (1) or absence (0). Over 3 yr, an individual species at a single site could be defined as one of eight possible “events”: 111, 001, 011, 010, 100, 110, 101, and 000. The 000 event data (an individual weed species was never present at a site) were removed from the data set. Each event was divided by the total remaining number of sites to determine the empirical probability of each event (i.e., a binomial model). The standard error was calculated for a proportion of events, based on the number of sites at which each event occurred. For presentation of results, the events were divided into three categories. “Constant” indicated a weed was present at a site for 3 yr (111). “Introduced” indicated those sites where the species was not initially present in year 1 or year 1 and 2, but was then evident in subsequent years (001, 011). “Nonconstant” indicated those sites in the survey where the weed was present in a year and absent in a subsequent year (100, 110, 101, 010).
Results and Discussion
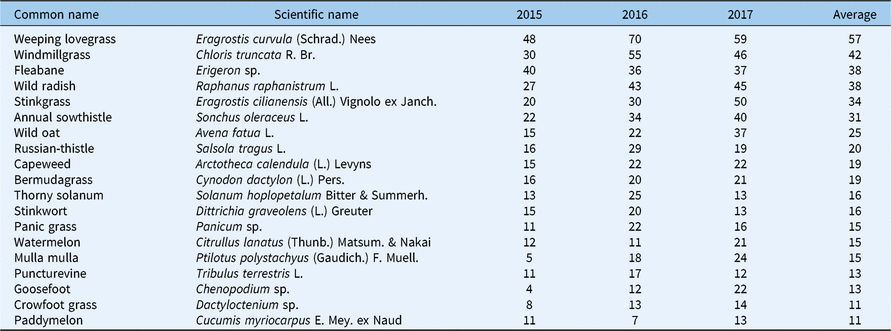
The survey identified a total of 144 separate species (or genera where species were grouped at the genus level). There were 76, 72, and 129 species identified in 2015, 2016, and 2017, respectively. However, only 58 weeds were found at greater than 1% of sites and only 19 weeds were found at greater than 10% of sites (Table 1). Species incidence varied by year. Weeping lovegrass [Eragrostis curvula (Schrad.) Nees] was the most common roadside species in every year. Chloris truncata was always in the top three most common species. The incidence of several species increased from 2015 to 2017, including wild radish (Raphanus raphanistrum L.), stinkgrass [Eragrostis cilianensis (All.) Vignolo ex Janch.], annual sowthistle (Sonchus oleraceus L.), and wild oat (Avena fatua) (Table 1).
Table 1. Summer weed species found at greater than 10% of sites surveyed in the Western Australia grainbelt from 2015 to 2017, the percent of sites each species was found in each year, and the percent of sites each species was found averaged over all years.
Species incidence was not consistent (Figure 2). For every species, the probability of occurring at a site in all 3 yr was low, with Chenopodium sp. having zero sites at which it was found over 3 yr. Eragrostis curvula and C. truncata had the greatest probability of being found at individual sites consistently (33% and 31%). With the exception of E. curvula and thorny solanum (Solanum hoplopetalum Bitter & Summerh.), every species had a higher probability of being introduced to a site during the course of the survey, compared with the probability of being present for all three survey years. Ptilotus polystachyus, A. fatua, Chenopodium sp., C. lanatus, and paddymelon (Cucumis myriocarpus E. Mey. ex Naud) all had a greater than 50% chance of being introduced to a site where they were not previously found. Stinkwort [Dittrichia graveolens (L.) Greuter] had an 89% chance of being nonconstant at sites, and puncturevine (Tribulus terrestris L.), Erigeron sp., capeweed [Arctotheca calendula (L.) Levyns], S. australis and bermudagrass [Cynodon dactylon (L.) Pers.] had a greater than 50% chance of being nonconstant. The variation in summer roadside weeds is in contrast to winter annual weeds, for which the weed population can be stable over multiple years, varying in response to species introductions or major changes to agronomic systems (Borger et al. Reference Borger, Riethmuller and D’Antuono2016; Davis et al. Reference Davis, Renner and Gross2005). Because only those sites with mature weeds and no evidence of disturbance were selected for the analysis, the species difference between years is more likely a result of varying climatic conditions (temperature or rainfall) than weed control strategies. Seasonal conditions can vary widely in WA summers, relating both to quantity of rainfall and temperatures during and after rainfall events (Bureau of Meteorology 2019). It is clear that total summer rainfall was above average in December 2016 to February 2017, with the entire grainbelt receiving 50 to 300 mm. By comparison, rainfall in December 2014 to February 2015 ranged from 2 to 100 mm and rainfall in December 2015 to February 2016 ranged from 10 to 200 mm (Bureau of Meteorology 2019). The date of weed emergence is unknown in the current study, making it impossible to relate weed emergence to specific climatic conditions. However, temporal differences in germination resulting from different response to temperature have previously been noted in summer weed species (Baskin and Baskin Reference Baskin and Baskin1977). While seeds are tolerant of high temperatures in dry soil, exposure to high soil temperature in moist conditions can reduce seed viability or induce germination, depending on species (Egley Reference Egley2017). Over the 3 yr, there was increased total incidence (i.e., incidence of every species at every site; Table 2), which may correlate with increasing rainfall over the 3 yr. Internationally, there are some regions where weed emergence is predicted based on meteorological data, but emergence models are not available for WA (Grundy Reference Grundy2003). Because we do not currently have adequate knowledge to predict emergence of summer weed species in WA, it is important to assess the species to be targeted for summer weed control rather than assume consistent species incidence from year to year. Further work is required to determine whether the variation in species incidence on the roadside is also observed in agricultural fields. The variability in seasonal occurrence of individual species means that it is not possible to use these data to assess invasiveness of individual species. While species were introduced to sites where they were not noted in the first year, it is not possible to determine whether they were new to the site or germinating from a seedbank that existed before the survey.
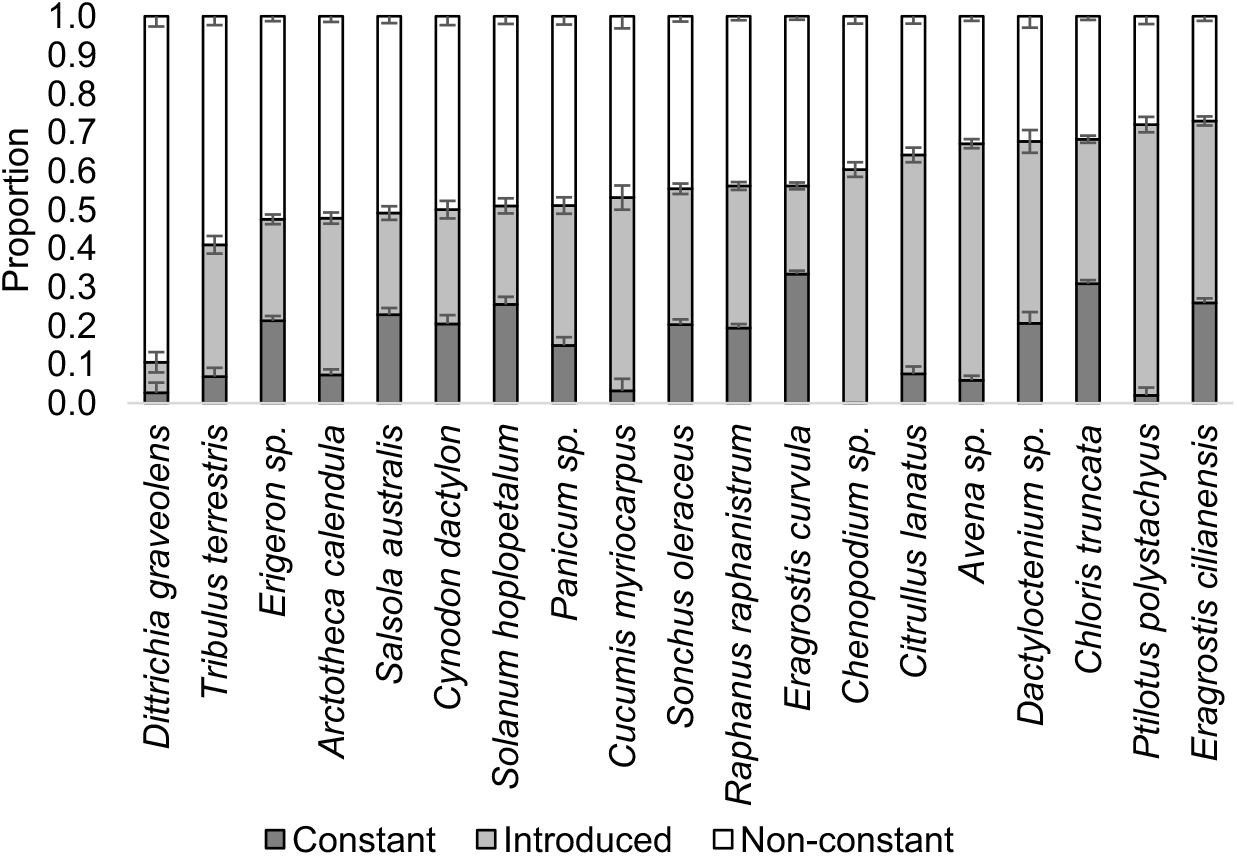
Figure 2. The proportion of sites where each of the 19 most common species were present every year (constant; 111), the sites where the species were introduced in the course of the study (introduced; 001, 011), and the sites where the species were not constant for the duration of the study (nonconstant; 100, 110, 010, 101). Vertical bars indicate the standard error of each probability.
Table 2. The total weed incidence in each year (i.e., the total occurrence of all species at all sites) and the percent of incidences in each year for weeds found at a low, medium, or high density.
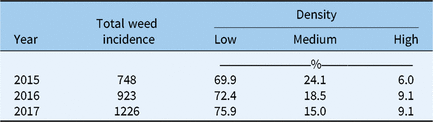
Density remained consistent between years, in spite of increased rainfall, with 70% to 76% of weed species at low density in 2015 to 2017 (Table 2). Only 6% to 9% of weed species incidence occurred at high density in each year. Density in an agricultural field is likely to be a function of total rainfall, and weed control practices if sites showing evidence of disturbance are not removed from the data set (Michael et al. Reference Michael, Borger, MacLeod and Payne2010; Streibig et al. Reference Streibig, Combellack and Amor1989). In the current study, sites were only assessed after major rainfall events, so it is reasonable to assume that rainfall was not a consistently limiting factor to density. Density is also affected by habitat size and competition with native vegetation, which are likely to be consistent between years (because sites showing signs of disturbance to weeds and native vegetation were removed from the data set). Native species were not assessed, and so the impact of competition between native species and agronomic weeds cannot be compared. The size of native vegetation patches is negatively correlated to level of weed infestation in WA (Stenhouse Reference Stenhouse2004). Because roadside native vegetation is in a small area, weed infestation is likely to be high, and this would reduce the condition of the native vegetation (Stenhouse Reference Stenhouse2004). Ideally, weeds would be removed from these environments, but it is difficult to kill weeds using herbicide without also killing native vegetation, and so management is an ongoing challenge for natural resource management groups (Stenhouse Reference Stenhouse2004).
Several key weeds of the winter annual cropping system of WA, including R. raphanistrum and A. fatua, were among the most common species in the current survey of summer weeds (Borger et al. Reference Borger, Michael, Mandel, Hashem and Renton2012). Emergence throughout the year has been observed for other winter annual species (Mennan and Ngouajio Reference Mennan and Ngouajio2006). While winter annual species generally produce seed that remains dormant over the summer fallow, a proportion of the seedbank responds to summer rainfall events (Chapman et al. Reference Chapman, Bolger and Le Coultre1999; Puckridge and French Reference Puckridge and French1983). Raphanus raphanistrum and A. fatua both have widespread resistance to herbicides, and reducing the seedbank throughout the year is the key goal in managing these species (Jones and Medd Reference Jones and Medd2000; Owen and Powles Reference Owen and Powles2016; Walsh and Powles Reference Walsh and Powles2007). These species are the second and third most detrimental weed species in Australian cropping systems after rigid ryegrass (Lolium rigidum Gaudin), partially due to this plasticity in emergence time (Llewellyn et al. Reference Llewellyn, Ronning, Clarke, Mayfield, Walker and Ouzman2016). To optimize control programs, further research is required on the quantity, germinability, and dormancy of seed produced by summer populations of these weed species compared with winter populations. Raphanus raphanistrum seed can remain dormant for more than 5 yr, making it vital that no additional seed enters the seedbank from summer cohorts (Cheam Reference Cheam2006). However, applying nonselective herbicides to control both summer and winter cohorts of R. raphanistrum or A. fatua will exacerbate the development of resistance (Owen et al. Reference Owen, Martinez and Powles2015; Owen and Powles Reference Owen and Powles2009). Future research is required to develop effective and practical management programs to control these species throughout the year.
A roadside survey allows weeds to be identified in the absence of control programs, but not all agronomic species can survive on a roadside, due to reduced disturbance. Michael et al. (Reference Michael, Borger, MacLeod and Payne2010) found 52 species (or genera, if identification of individual species was not possible) in crop/pasture fields over the summer of 2006. This is a lower species incidence than was found in each of the 3 yr of this roadside survey, indicating that roadsides have greater weed species diversity. The current work cannot be used to determine whether this is due to reduced weed management on roadsides or altered environment (less disturbance). However, of the 19 roadside species found at greater than 10% of sites, 14 were found in fields in 2006 (Michael et al. Reference Michael, Borger, MacLeod and Payne2010). This highlights that roadsides give a reasonable approximation of species incidence in fields. Some of the species not found in the field may be favored in an environment of low disturbance, and so are more likely to be weeds of pasture than crop. However, the higher diversity of species noted on the roadside highlights those species that have potential to invade cropping fields in future. For example, D. graveolens, while not found in fields by Michael et al. (Reference Michael, Borger, MacLeod and Payne2010), is increasingly invasive in California and may be more problematic for Western Australian growers in future (Brownsey et al. Reference Brownsey, Kyser and DiTomaso2013). For the current research, a field survey could not be completed, as there is a legal requirement to contact growers before entering their properties, and this process takes a prohibitively long time. Future research comparing roadside and field weed populations is required to clarify species differences between the two environments. The present research highlighted the fact that roadside weeds have consistent density and variable diversity that are affected by seasonal conditions. However, future research on weed species abundance, coverage, or biomass in fields and roadsides may indicate the potential invasiveness of different roadside species.
Conclusions
Species incidence of summer weeds was not consistent between years, which was surprising, as winter weed species occur consistently over multiple years. Further research to determine germination requirements of individual summer weed species would allow a prediction of summer weed species emergence. In the short term, growers and natural resource management groups that may be required to control summer roadside weeds should assess species incidence, rather than assuming consistent incidence between years. The present research also highlighted that many key weeds of the winter annual cropping system also commonly grow over the summer/autumn fallow. It is important to ensure complete control of these winter annual plants when they emerge in summer, as this will reduce the seedbank available to germinate in winter and directly compete with crops. Because these weed species are killed with nonselective herbicides in summer and again in late autumn at the start of the winter annual cropping system, they are highly susceptible to the development of herbicide resistance. Complete control of these species over summer will avoid resistance development. Management can include herbicide application where there is no native vegetation on the roadside, but is a more complex issue when it is necessary to control these species on roadsides that should not be sprayed because native vegetation is present. However, ranking the most common summer roadside weeds can assist natural resource management groups to devise (or conduct further research on) optimal control methods to remove the most prevalent roadside weeds.
Author ORCIDs
Catherine Borger https://orcid.org/0000-0001-8541-3215
Acknowledgments
This research was undertaken as part of the Emerging Summer Weeds project (UA00149), funded by the Grains Research and Development Corporation. Thanks are due to Mohammad Amjad, Cameron Wild, Alex Douglas, Sally Peltzer, Dave Nicholson, Bec Swift, Adrian Rossi, Jolie Delroy, Edwina Cockburn, Alice Butler, Emma Clarke, Kylie Chambers, Neville Chittleborough, Harmohinder Dhammu, Greg Doncon, Barbara Sage, and John Moore for assisting in the survey. No conflicts of interest have been declared.






