Introduction
The West Indian manatee Trichechus manatus is categorized as Endangered on the Brazilian Red List (ICMBio, 2018) and as Vulnerable on the IUCN Red List (Deutsch et al., Reference Deutsch, Self-Sullivan and Mignucci-Giannonni2008). Along the Brazilian coast, manatee populations are patchily distributed from Amapá to Alagoas (Luna et al., Reference Luna, de Lima, de Araújo, Zanon and Passavante2008; de Lima et al., Reference de Lima, Paludo, Soavinski, da Silva and de Oliveira2011) and there is evidence of released animals using the coasts of Sergipe and Bahia states (Deutsch et al., Reference Deutsch, Self-Sullivan and Mignucci-Giannonni2008). The distribution of manatees is influenced by physiological (nutrition and metabolism; St Aubin & Lounsbury, Reference St Aubin, Lounsbury, Geraci and St Aubin1990) and environmental factors (water salinity, temperature and depth, and availability of food resources; Lefebvre et al., Reference Lefebvre, Marmontel, Reid, Rathbun, Domning, Woods and Sergile2001), freshwater supply (Favero et al., Reference Favero, Favero, Choi-Lima, Souza-alves and Le2020) and anthropogenic habitat modifications (Deutsch et al., Reference Deutsch, Self-Sullivan and Mignucci-Giannonni2008). Manatees prefer naturally sheltered areas such as coves, bays and estuaries and are sensitive to disturbance caused by the construction and operation of cities, ports, marinas, shipyards, salt works, shrimp farms and other anthropogenic structures and activities in these areas (Aquasis, Reference Aquasis2016). Such habitat modifications are associated with an increase of manatee calf stranding events along the north-eastern Brazilian coast (Parente et al., Reference Parente, Vergara-Parente and Lima2004; Meirelles, Reference Meirelles2008; Medeiros et al., Reference Medeiros, Rebelo, Dos Santos, Menezes, Almeida and Messias2021). Without access to undisturbed estuaries, females give birth in open water, where newborns may become separated from mothers and stranded on the coast (de Lima et al., Reference de Lima, Paludo, Soavinski, da Silva and de Oliveira2011). Stranded calves found alive are transferred to one of three rehabilitation facilities in north-eastern Brazil (Meirelles, Reference Meirelles2008). After an initial health assessment, they are kept in individual pools for a quarantine period of c. 2 months, and later moved to larger pools with other calves. They are fed soya milk formula, algae and seagrass. At 1 year of age they are moved to an oceanarium and offered a diet of seagrass and algae supplemented with vegetables (carrots and lettuce) and vitamins (Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015).
After rehabilitation and following the Brazilian Manatee Reintroduction Protocol (de Lima et al., Reference de Lima, de Alvite and Vergara-Parente2007), the animals are transferred to enclosures in the Mamanguape River estuary or Tatuamunha River, within two marine protected areas (Mamanguape River Environmental Protection Area in Paraíba and Environmental Protection Area Costa dos Corais in Alagoas, respectively). Manatees spend 15 days–12 months in these areas, adapting in captivity (i.e. enclosures within estuaries) to local environmental conditions (Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015).
The Manatee Reintroduction Programme was created in 1994 with the purpose of re-establishing the original geographical distribution of the manatee in Brazil (de Lima et al., Reference de Lima, de Alvite and Vergara-Parente2007). As a result of this initiative, the number of rescued and rehabilitated manatees increased during 1994–2020, with a total of 48 manatees released (de Lima et al., Reference de Lima, de Alvite, Reid and Bombassaro Júnior2012; Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016, Reference Normande, Attademo, Luna, Borges, Lima and Sommer2019). These efforts to rehabilitate and release stranded manatees have facilitated the reconnection of isolated populations and the restocking of areas where manatees historically occurred (Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016).
Upon release, manatees tend to use estuarial and coastal areas (Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016). Based on their movement patterns, the area used by released individuals, expanding over time from their respective release sites, is conceptually defined as their home range, and the extreme geographical positions reached during movements constitute the limits of their territories (de Lima et al., Reference de Lima, de Alvite, Reid and Bombassaro Júnior2012).
Data on habitat use and movement patterns, gathered as part of release and reintroduction programmes, provide insights into movements, ecological aspects and impacts of human activities, which can facilitate the conservation of species and habitats (Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016). Considering the ecological importance of manatees (Aquasis, Reference Aquasis2016), their vulnerability and the threats to which they are exposed (de Lima et al., Reference de Lima, Paludo, Soavinski, da Silva and de Oliveira2011), further studies on their distribution and home ranges are warranted. Mapping habitat use patterns, the most used resources, home ranges and the routes and corridors used between these areas is important for manatee conservation. This information can help identify priority areas for habitat protection and assist in mitigation of anthropogenic impacts. It may also help improve manatee rehabilitation strategies based on the influence of captive management on post-release behaviour. The aim of our study was to identify the home ranges of released manatees in Brazil and evaluate their adaptation to life in the wild.
Study area
We tracked the movements of rescued, rehabilitated and released manatees in the north-eastern Brazilian states of Paraíba, Sergipe and Bahia (Fig. 1). In Paraíba, manatees mainly use the estuary of the Mamanguape River in the municipality of Rio Tinto and the estuary of the Paraíba do Norte River, between the municipalities of Cabedelo and Lucena, where the Cabedelo Port is located, which has considerable motorboat traffic. In Sergipe and Bahia, the study areas were in the estuary of the Vaza Barris River and the Piauí-Fundo-Real estuarine complex. The Piauí River is 132 km long and flows into the Atlantic ocean between the municipalities of Estância (Sergipe) and Jandaíra (Bahia). The climate in the area is characterized by a dry (September–February; mean monthly rainfall 62 mm) and rainy (March–August; mean monthly rainfall 262 mm) season.
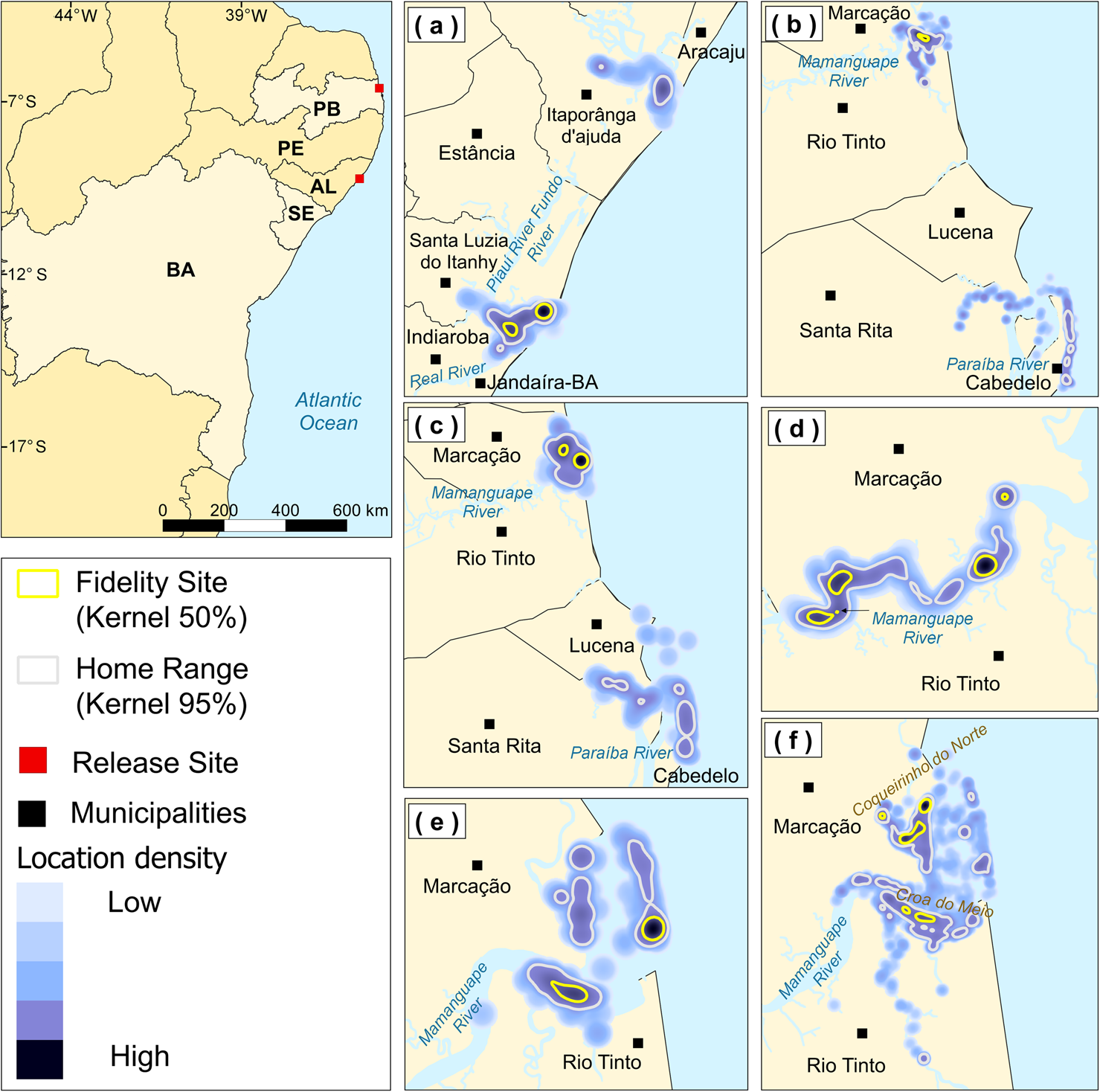
Fig. 1 The study area in north-eastern Brazil (AL, Alagoas; BA, Bahia; PB, Paraíba; PE, Pernambuco; SE, Sergipe), where six released West Indian manatees Trichechus manatus were monitored during 2016–2019. (a) Astro's home range in the Piauí/Fundo/Real rivers estuarine complex and at the mouth of the Vaza Barris River, (b) Mel's and (c) Puã's home ranges at the mouths of the Mamanguape and Paraíba Rivers, (d) Tita's home range in the Mamanguape River, (e) Yara's and (f) Zelinha's home ranges at the mouth of the Mamanguape River.
Methods
Manatee tracking
Six manatees (named Astro, Mel, Puã, Tita, Yara and Zelinha) that had been released during 1994–2012 (de Lima et al., Reference de Lima, de Alvite, Reid and Bombassaro Júnior2012; Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016) were monitored by the Aquatic Mammal Foundation during 2016–2019 (Table 1). The tracking apparatus consisted of a belt attached to each animal's caudal peduncle, a tether (flexible nylon rod attaching the belt to the transmitter housing; de Lima et al., Reference de Lima, de Alvite and Vergara-Parente2007) and a radio transmitter (Nortronic/Aquatic Mammals Foundation, Natal, Brazil). The transmitting assemblage consisted of a GPS device to record geographical coordinates, a satellite transmitter programmed to send signals every 3 hours through the Globalstar network (Globalstar, Covington, USA), and a conventional VHF transmitter operating continuously to transmit locations in real-time (Borges et al., Reference Borges, dos Santos, Rebelo, Mantovani, Círiaco and Marmontel2020).
Table 1 Individual information and results of telemetry monitoring of six West Indian manatees Trichechus manatus on the north-eastern coast of Brazil, 2016–2019.
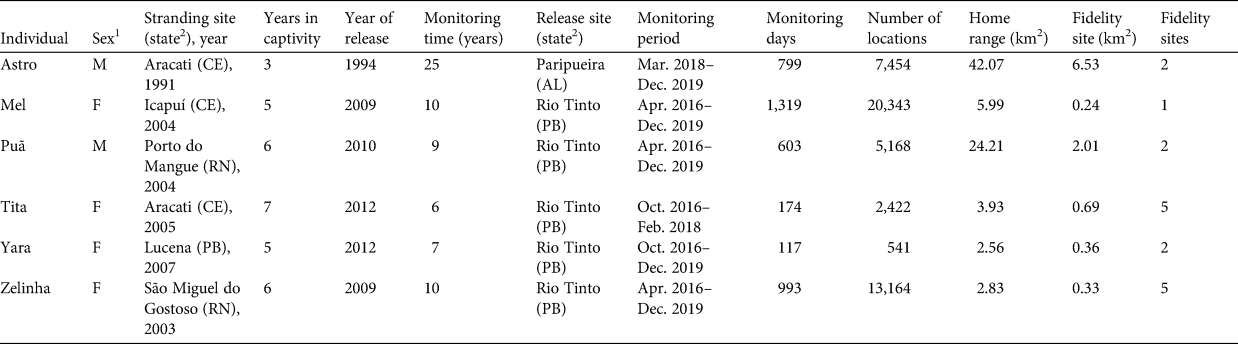
1 F, female; M, male.
2 CE, Ceará; RN, Rio Grande do Norte; Al, Alagoas; PB, Paraíba.
An experienced team periodically captured the released manatees during periods of low tide for clinical management and deployment of the satellite tags. The animals were captured with a custom-made 150-m long net deployed in a circle from a boat. Prior to fitting the tracking equipment, they were evaluated clinically, and biological samples and biometrics were collected (White & Francis-Floyd, Reference White, Francis-Floyd and Dierauf1990). Captures for clinical management occurred every 6 months or according to the needs of individual animals (de Lima et al., Reference de Lima, de Alvite and Vergara-Parente2007). When lost transmitters needed to be replaced or adjustments made to the belts because of changes in the animal's weight, this was done by diving or by recapturing the animal.
Data collection
We received information on the location of individuals (date, time and geographical coordinates) from the satellite system on a daily basis, and downloaded the data through the Globalstar system via e-mail, a smartphone application or the system's digital platform. We used the VHF system to visually locate individuals during fieldwork, to collect data on behaviour, areas used and interactions with people. Field surveys were conducted daily, with observers travelling on foot, by car or in small motorboats. Data collected included geographical location, name of the site, identification of the individual, transmitter number, water depth, distance to high tide line, weather and tidal conditions, visibility, behaviour of the individual, presence of people and type of any interaction, and composition of the research team. Each hour of monitoring included 15 minutes of behaviour observation.
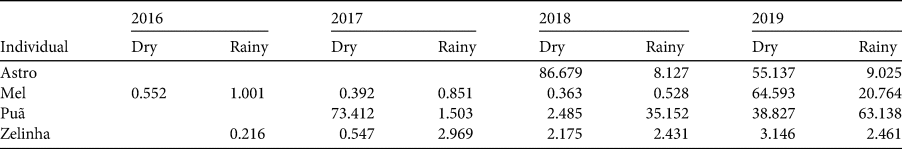
Locations were visualized using QGIS 3.10 (QGIS Development Team, 2019), resulting in the identification of the main areas used by each individual monitored, in the dry and rainy seasons. We used the Home Range tool in QGIS to calculate the areas of use, defining an animal's home range as the area containing 95% of the locations obtained; this excludes sporadic displacements that are not considered as part of the individual's home range (Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016). We had sufficient data to calculate home ranges in the dry and rainy seasons for four individuals: Astro, Mel, Puã and Zelinha.
We used a Kruskal–Wallis test to examine any differences in the home range (response variable) among individuals (Astro, Mel, Puã and Zelinha; categorical variable), and Mann–Whitney tests to examine any differences between seasons (dry and rainy) and sexes (male and female). When there were significant differences, we used Dunn's post hoc test to identify heterogeneous groups. All tests were performed in R 3.5.0 (R Core Team, 2018).
We used the kernel estimator to calculate the home range (95% kernel) of each animal monitored (Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015). Areas with greater concentration of coordinates were classified as fidelity sites (50% kernel; Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Padilla-Saldívar, Hernández-Arana, Slone, Reid and Morales-Vela2012); the number of coordinates that comprised these sites was 50% of all locations recorded for each manatee (Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015).
Assessment of adaptation of released manatees
We evaluated the adaptation of released manatees to the wild considering a number of criteria for success: capacity to find food sources and feed in the short term, remaining in adequate habitat, use of freshwater sources, body measurements appropriate for the animal's age, no evidence of disease or parasites, interaction with native and/or other released manatees, evidence of reproductive behaviour, little affinity with people or gradual loss of any such affinity, and (for females) gestation in the medium and long term (5–10 years; de Lima et al., Reference de Lima, de Alvite and Vergara-Parente2007).
In addition to these criteria, manatees surviving for 1 year after release, using adequate habitats, not requiring repeated rescuing, and exhibiting good physical condition were considered successful releases (Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015; Adimey et al., Reference Adimey, Ross, Hall, Reid, Barlas, Diagne and Bonde2016).
Results
The combined total number of monitoring days for all manatees was 4,005. Astro was the only individual to use areas in the states of Sergipe and Bahia, with a home range of 42.07 km2 and two fidelity sites (Fig. 1a). In the Vaza Barris River, he travelled 14.24 km upstream and 0.42 km from the coast. His main area of use was the estuarine complex of the Piauí/Fundo/Real Rivers, where he travelled 12.56 km upstream (but used these areas with less intensity) and ranged 0.93 km from the coast.
Mel and Puã were the only animals monitored in the state of Paraíba that visited areas beyond the limits of the Mamanguape River estuary, with a larger home range than others in the state. Mel had a home range of 5.99 km2, mainly encompassing the estuary of the Mamanguape River, where her sole fidelity site was located. She travelled up to 3.59 km upstream and 0.55 km from the coast. She also travelled c. 28 km south, to the beaches of Cabedelo, making use of coastal areas and the Paraíba River estuary, where she moved 7.71 km upstream and 0.57 km from the coast (Fig. 1b).
Puã's home range was 24.21 km2, encompassing the estuaries of the Mamanguape and Paraíba Rivers. This individual mainly used the Mamanguape River estuary, where two fidelity sites were identified. He travelled 3.08 km upstream and 0.52 km from the coast. In the Paraíba River, he travelled 7.06 km upstream and 0.68 km offshore (Fig. 1c).
Tita, Yara and Zelinha used areas within the estuary of the Mamanguape River, in Paraíba. Tita's home range differed from that of the others, using only upstream areas of the Mamanguape River and reaching 10.17 km upriver. Tita was never recorded near the mouth of the river. Her home range was 3.93 km2, with five fidelity sites (Fig. 1d).
Yara's 2.56 km2 home range encompassed two fidelity sites. She mostly used the area near the reefs, including one fidelity site. She also often used Croa do Meio and the mouth of the Camurupim River, and travelled 3.56 km upriver and up to 0.69 km from the coast (Fig. 1e). Both of her fidelity sites were located in areas regularly used by manatees for feeding. Yara also used two tributaries near the mouth of the Mamanguape River, where salinity is low: the Sinibú and the Caracabú, on the left and right bank of the Mamanguape, respectively (her captive adaptation location was at the Caracabú tributary).
Zelinha's home range, including five fidelity sites, was 2.83 km2. This manatee mainly visited Coqueirinho Beach to the north, the mouth of the Camurupim River, and Croa do Meio, reaching 4.85 km upstream and 0.54 km from the coast (Fig. 1f).
Males Astro and Puã had the largest home ranges, and females Tita and Zelinha had the largest number of fidelity sites. All fidelity sites were within protected areas (in estuaries, bays or upstream on the river).
Integrating data from all manatees monitored, we mapped the main areas used by the animals. In Paraíba, they mainly used two rivers: Mamanguape and Paraíba. In the estuary of the Mamanguape River, the manatees travelled up to c. 10 km upstream from the mouth. Two of the five animals monitored in Paraíba (Mel and Puã) made trips from the Mamanguape River to the beaches of the Cabedelo municipality, c. 28 km away. There, the animals used the estuary of the Paraíba River and one of its tributaries (Guia River), reaching up to 7.71 km upstream, and frequently used the coastal areas. Reefs, which reduce the effects of waves and currents (Barbier et al., Reference Barbier, Hacker, Kennedy, Koch, Stier and Silliman2011), are found at both locations. The animals ventured up to 0.68 km offshore, but did not use the open sea beyond these reef formations. In Sergipe and Bahia, Astro mainly used the estuaries of the Vaza Barris River in Sergipe and the Piauí/Fundo/Real estuarine complex, on the border between the two states, travelling c. 37.23 km between these two areas.
There were no significant differences between the dry and rainy season ranges of four manatees (Astro, Mel, Puã and Zelinha; Mann–Whitney: U = 67; P = 0.57) throughout the monitoring period (Table 2). There was, however, a significant difference in the home range size of individual manatees (Kruskal–Wallis: H (3) = 9.86; P = 0.01), and males had significantly larger home ranges than females (Mann–Whitney: U = 19; P = 0.01).
Table 2 Home range size (95% kernel, km2) of four West Indian manatees monitored with telemetry along the north-eastern coast of Brazil, by season (dry or rainy), during 2016–2019.
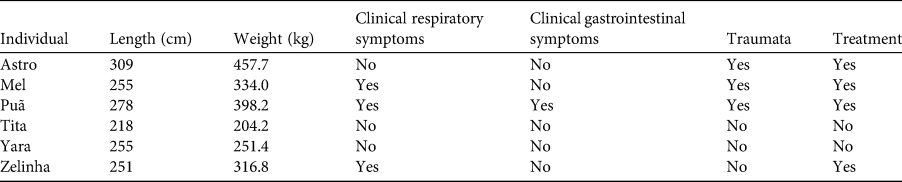
During the study period, some individuals exhibited symptoms of illness or injury (Table 3). Three (Mel, Puã and Zelinha) had a mild nasal secretion that did not require medicinal intervention and resolved before the end of the study period. Puã also exhibited gastrointestinal problems, which regressed spontaneously. Astro, Mel and Puã had injuries caused by trauma (some sustained in collisions with motorized watercraft) and were submitted to an individual evaluation and therapeutic protocol. Tita, who had a similar haematological pattern as the other animals, was found dead at Boa Vista Port in February 2018 in a tributary of the Mamanguape River. The necropsy revealed faecaloma, pulmonary embolism and cardiopulmonary arrest.
Table 3 Total body length, weight (at last evaluation) and clinical data of six released West Indian manatees on the north-eastern coast of Brazil, 2016–2019.
Manatees used freshwater sources 1–11 times per month in the dry season and 1–12 times per month in the rainy season. There were no significant differences among individuals (Kruskal–Wallis: H (2) = 1.201; P = 0.54) or between seasons (Mann–Whitney: U = 718.5; P = 0.06; Table 4).
Table 4 Monthly frequency of use of freshwater sources by three West Indian manatees monitored with telemetry along the north-eastern coast of Brazil, 2016–2019.

Discussion
The manatees monitored in this study used estuarine areas more intensely than the open sea. The same pattern was reported for 20 manatees released along the coast of north-eastern Brazil (Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016), which used the estuarine environment with greatest intensity, followed by mixed habitats and the marine environment, suggesting that this preference may be linked to sources of freshwater in estuaries.
The use of upstream areas of rivers varied by individual, with some travelling up to 14 km upstream. These movements are believed to be motivated by the search for sources of freshwater, as reported in previous studies (Ross, Reference Ross2007; Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Padilla-Saldívar, Hernández-Arana, Slone, Reid and Morales-Vela2012; Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016).
Astro, Mel and Puã travelled the longest distances, and these travels were always between two estuaries. Some of the individuals we monitored had been studied previously. De Lima & de Passavante (Reference de Lima and de Passavante2013) reported three fidelity sites used by Astro during 1994–2004: Maré Mansa beach in Alagoas during the early post-release period, and the estuaries of the Vaza Barris River and Piauí/Real/Fundo complex, both in Sergipe. Sixteen years later (our study), he still used the estuary of the Vaza Barris River, but his fidelity sites were in the Piauí/Real/Fundo estuarine complex.
Normande et al. (Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016) analysed the home range of four individuals monitored in this study (Mel, Puã, Tita and Zelinha) during November 2008–June 2013. Compared to this earlier period, the home ranges of Mel, Tita and Zelinha decreased by 0.42, 2.09 and 3.82 km2, respectively, whereas Puã's home range increased by 2.02 km2. The reductions in home range size may indicate that the animals identified the best feeding sites after 2013 and subsequently did not need to travel as far in search of food. Tita, Yara and Zelinha have remained within the limits of the Mamanguape River estuary since their release. De Lima et al. (Reference de Lima, de Alvite, Reid and Bombassaro Júnior2012) observed similar behaviour, with released manatees staying near their release site.
We did not record any of the individuals studied in areas beyond the reef barrier, but it is believed that manatees use these areas as corridors for movements between estuaries (de Lima et al., Reference de Lima, de Alvite, Reid and Bombassaro Júnior2012; Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016). Paludo & Langguth (Reference Paludo and Langguth2002) reported similar findings from a study of native manatees in the state of Rio Grande do Norte. The fact that there are few GPS locations recorded from these areas may also be linked to water depth and animal movements, as manatees use open waters for fast movements between estuaries, during which the transmitter remains mostly submerged and thus no signal is transmitted (Edwards et al., Reference Edwards, Martin, Deutsch, Muller, Koslovsky, Smith and Barlas2016).
The fidelity sites identified in this study were in sheltered areas with low incidence of waves and currents (estuaries, bays and areas protected by reefs), which demonstrates the importance of such areas to manatees. Similar findings have been reported for north-eastern Brazil (de Lima & de Passavante, Reference de Lima and de Passavante2013; Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016) and Mexico (Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Padilla-Saldívar, Hernández-Arana, Slone, Reid and Morales-Vela2012). In Paraíba, all fidelity sites were located in the estuary of the Mamanguape River, and three individuals were not recorded in any other estuaries. This shows how important this estuary is for the species in Brazil. Animals with small home ranges and few fidelity sites may become vulnerable to the degradation of these environments (de Lima & de Passavante, Reference de Lima and de Passavante2013). It is therefore important to release manatees in protected areas (de Lima et al., Reference de Lima, de Alvite and Vergara-Parente2007), where suitable habitats are protected.
Tita's fidelity sites were located further upstream than those of other individuals, reaching 10 km upriver. No other monitored individuals were observed close to these upstream sites, but fishermen interviewed by da Silva et al. (Reference da Silva, Paludo, de Oliveira, de Lima and Soavinski2011) reported the occurrence of manatees in the area.
The differences in home ranges between seasons were non-significant in this study, but de Lima & de Passavante (Reference de Lima and de Passavante2013) described seasonal patterns of manatee movements in north-eastern Brazil, with larger home ranges and more movement in the dry season. According to de Lima et al. (Reference de Lima, de Alvite, Reid and Bombassaro Júnior2012), these movements, occurring mainly at the end of the rainy season and during the dry season, are exploratory. In contrast, Normande et al. (Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016) found no clear seasonal pattern when analysing long-distance movements of manatees.
In studies of Florida manatees, seasonal movements were often associated with changes in water temperature (Deutsch et al., Reference Deutsch, Reid, Bonde, Easton, Kochman and O'Shea2003; Flamm et al., Reference Flamm, Weigle, Wright, Ross and Aglietti2005). In Brazil, waters are warm, with a mean annual temperature of > 24 °C (da Silva et al., Reference da Silva, Paludo, de Oliveira, de Lima and Soavinski2011; de Lima et al., Reference de Lima, Paludo, Soavinski, da Silva and de Oliveira2011). In regions where temperature is not a motivating factor, seasonality may be motivated by the availability of freshwater and adequate habitats in the dry and rainy seasons (Reynolds et al., Reference Reynolds, Powell, Taylor, Perrin, Würsig and Thewissen2009). Availability of food sources is considered a determinant factor for habitat use by manatees in Brazil (Paludo & Langguth, Reference Paludo and Langguth2002), but the triggers of seasonal movements are still not well understood. De Lima et al. (Reference de Lima, de Alvite, Reid and Bombassaro Júnior2012) suggested that food may not be the main motivating factor because these movements always start at a fidelity site where conditions are favourable for the animals. In a study of native manatees in the state of Piauí, areas of use were more strongly correlated with sources of freshwater than any other resource, suggesting that the distribution of manatees is driven by the proximity of freshwater (Favero et al., Reference Favero, Favero, Choi-Lima, Souza-alves and Le2020).
In our study, male manatees had larger home ranges than females, as in Mexico and Florida (Flamm et al., Reference Flamm, Weigle, Wright, Ross and Aglietti2005; Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Padilla-Saldívar, Hernández-Arana, Slone, Reid and Morales-Vela2012). However, Normande et al. (Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016) found no differences in home range size between males and females in Brazil. Flamm et al. (Reference Flamm, Weigle, Wright, Ross and Aglietti2005) described manatee movement patterns in Florida for solitary males, solitary females and females with calves; they found that males had significantly higher movement rates than both categories of females. The search for receptive females has been suggested as a probable reason for the greater distances travelled by males (Deutsch et al., Reference Deutsch, Reid, Bonde, Easton, Kochman and O'Shea2003), and this may maximize their individual reproductive success (Castelblanco-Martínez et al., Reference Castelblanco-Martínez, Padilla-Saldívar, Hernández-Arana, Slone, Reid and Morales-Vela2012).
Astro and Tita were the only individuals not recorded to interact with other native or released manatees. Astro used the coasts of Sergipe and Bahia, where manatees occurred historically. Considering the presence of other manatees in the estuary of the Mamanguape River (da Silva et al., Reference da Silva, Paludo, de Oliveira, de Lima and Soavinski2011), it is likely that Tita did interact with other manatees, but we did not record this.
All animals evaluated adapted to life in the wild and were considered successful releases, according to the Brazilian Manatee Reintroduction Protocol (de Lima et al., Reference de Lima, de Alvite and Vergara-Parente2007; Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015; Adimey et al., Reference Adimey, Ross, Hall, Reid, Barlas, Diagne and Bonde2016). Success in the rescue and release process requires long-term, continuous monitoring with the support of veterinary interventions whenever necessary, use of monitoring systems with accurate transmission of geographical coordinates, and releasing animals up to 5 years of age (Normande et al., Reference Normande, Luna, Malhado, Borges, Viana Junior, Attademo and Ladle2015). Additionally, it is important to engage local communities, especially fishers, in the participatory monitoring of animals, both in reporting manatee occurrence and recovering lost transmitters.
Tita, Yara and Zelinha remained throughout the entire monitoring period in the estuary of the Mamanguape River, where they re-adaptated in captivity (enclosures inside estuaries), demonstrating the importance of this step to post-release success. According to de Lima & de Passavante (Reference de Lima and de Passavante2013), a site can be considered to have fulfilled its function as a temporary adaptation area when the animals use the surrounding region for a time after their release; when released manatees immediately move away from the site, it means it had no influence on the adaptation process.
In Brazil, one of the goals of the reintroduction programme is to restock areas of historical occurrence (Normande et al., Reference Normande, Malhado, Reid, Viana, Savaget and Correia2016). This was achieved by Astro, making the states of Sergipe and Bahia the current southernmost distribution limit of the species.
The identification of spatio-temporal patterns of habitat use by manatees, the mapping of intensely used areas and the evaluation of the adaptation of released animals to life in the wild provide important data that can assist protected area managers in defining priority areas for conservation. The information will contribute to the Management Plan for the Mamanguape River Environmental Protection Area through regulation of coastal development and activities such as fishing, boat traffic and tourist activities. The data, which were shared with the relevant government institutions, will also contribute to the National Action Plan for the Conservation of Manatees (ICMBio, 2011).
Acknowledgements
We thank the Aquatic Mammal Foundation for logistical and technical support; the Graduate Program in Ecology and Environmental Monitoring and the Cartography and Geoprocessing Lab-UFPB for scientific and academic support; Environmental Protection Areas of Costa do Corais and the Mamanguape River, the National Research and Conservation Center for Marine Biodiversity of the Northeast/ICMBio for support with field work; Nortronic for technical assistance with transmitters; James Reid, Susan Butler, Robert Bonde (U.S. Geological Survey/Sirenia Project) for their support and cooperation; the Long Live the Manatee project of the Aquatic Mammal Foundation, the Petrobras Socioenvironmental Program and the Manatee Conservation Program of the Aquatic Mammal Foundation in partnership with the Boticário Group Foundation for the Conservation of Nature (Fundação Grupo Boticário de Conservação à Natureza) for funding; and the anonymous reviewers for their comments.
Author contributions
Study design: SSdS, VAR, MM, JCGB; contribution to the manufacture of the tagging accessories: SSdS, ICN; development of the radio transmitters and assistance in their use: JPD, JEM, RDC, REGdS, JCGB; fieldwork: SSdS, VAR, AOBC, IdSM, TMGV; data analysis and writing: SSdS, ICN, IdSM, MM, JCGB; revision: all authors.
Conflicts of interest
None.
Ethical standards
This research was carried out under ICMBio/SISBio (Biodiversity Authorization and Information System) permits #25820 and #54205 and otherwise abided by the Oryx guidelines on ethical standards.







