Introduction
Over 27,000 cases of rectal cancer and anal cancer are diagnosed annually among men in the US (ACS 2020a). Advances in treatment over the past 20–30 years have resulted in improved survival and decreased local recurrence for these cancers (ACS 2020a). The current 5-year survival rate for early stage rectal cancer is 90% and for early stage anal cancer is 83% (ACS 2020a, 2020b). As a result of these advances in treatments and improved survival rates, survivorship issues, such as sexual functioning, have become increasingly important.
The treatment for rectal cancer is based mainly on stage and usually requires chemotherapy, radiation, and surgery (ACS 2020c). Radiation is generally used preoperatively in combination with sensitizing chemotherapy. After chemoradiation, surgery (low anterior resection, proctectomy with coloanal anastomosis, or abdominoperineal resection, depending on where the cancer is in the rectum) is performed (ACS 2020c). Following surgery, patients will typically receive an additional 4–6 months of chemotherapy, and the complete treatment for rectal cancer generally lasts for 10–12 months following diagnosis. Treatment for anal cancer today generally requires a combination of radiation and chemotherapy (chemoradiation) (ACS 2020d). Radiation therapy typically lasts 5–6 weeks; chemotherapy is typically administered during the first and fifth week (ACS 2020d). In the past, surgery was the only way to cure anal cancer, but today surgery is often not needed (ACS 2020d).
For men, the primary sexual dysfunctions studied following rectal and anal cancer treatment are erectile dysfunction (ED), loss of the ability to ejaculate, and loss of sexual interest (Mannaerts et al. Reference Mannaerts, Schijven and Hendrikx2001; Hendren et al. Reference Hendren, O’Connor and Liu2005; Moriya Reference Moriya2006; Sun et al. Reference Sun, Grant and Wendel2016; Yerramilli et al. Reference Yerramilli, Drapek and Nipp2019). Anal and rectal cancer treatments also have major permanent effects on receptive anal sex, though these data are not often commonly reported in the literature. Studies in this area have reported that as many as 69% of men will have problems with erections and 60% will have difficulty with ejaculation after treatment (Mannaerts et al. Reference Mannaerts, Schijven and Hendrikx2001; Hendren et al. Reference Hendren, O’Connor and Liu2005; Moriya Reference Moriya2006; Milbury et al. Reference Milbury, Cohen and Jenkins2013). Not surprisingly, the number of men who are sexually active is found to significantly decrease after treatment for rectal cancer; 45% report their sex life to be worse following treatment as compared to pretreatment, and 47% report a loss of libido following treatment (Havenga and Welvaart Reference Havenga and Welvaart1991; Hendren et al. Reference Hendren, O’Connor and Liu2005; Moriya Reference Moriya2006; Milbury et al. Reference Milbury, Cohen and Jenkins2013; Breukink and Donovan Reference Breukink and Donovan2013). Despite the significant impact rectal cancer and anal cancer treatment have on the sexual functioning of male survivors, there are limited psychoeducational interventions designed to address this problem (Incrocci and Jensen Reference Incrocci and Jensen2013; Milbury et al. Reference Milbury, Cohen and Jenkins2013). Barsky Reese et al. (Reference Barsky Reese, Porter and Regan2014) and Reese et al. (Reference Reese, Porter and Somers2012) have tested an intimacy enhancing intervention for male and female colorectal patients and their partners in a randomized controlled trial (RCT). This telephone-based, 4-session intervention is focused on teaching skills for coping with sexual concerns and enhancing intimacy (Barsky Reese et al. Reference Barsky Reese, Porter and Regan2014). While this focus is important, there is also a need for interventions to focus specifically on improving the sexual functioning for male rectal/anal cancer survivors.
The current study reports on the development and pilot testing of the Sexual Health Intervention for Men (intervention). This 4-session, psychoeducation-focused intervention was conducted primarily via telephone and developed based on the input of rectal cancer survivors (Ball et al. Reference Ball, Nelson and Shuk2013), clinical expertise, and prior research (Canada et al. Reference Canada, Neese and Sui2005; Schover et al. Reference Schover, Canada and Yuan2012). The intervention combines education about medical treatments with psychosocial discussions related to distress about sexual dysfunction, negative emotions/barriers to treatment, and techniques to improve communication and intimacy. The current study presents results from a pilot RCT. We hypothesized that intervention would improve sexual functioning and secondary psychosexual variables.
Methods
Study design
This study was a pilot RCT testing the impact of the intervention compared to a referral and information control. The aims were to investigate the impact of the intervention on (1) the primary outcome of sexual functioning and (2) secondary outcomes of sexual bother, sexual self-esteem, and cancer-specific distress. The trial received Institutional Review Board (IRB) approval, and informed consent was obtained prior to study entry. Approached patients were told that this study aimed to learn how a new type of sexual health educational program affects erectile function and emotional and social well-being compared to the standard care that patients receive after treatment for rectal or anal cancer.
Consented subjects completed self-report questionnaires with staff reading the questions over the phone (86.8%) or by mail (13.2%). After the baseline assessment, subjects were randomized to either intervention or control. The intervention took, on average, 5 months to complete. All intervention sessions were provided by 1 psychologist (C.N.). Follow-up assessments occurred, on average, 3 months post-completion of intervention (follow-up 1) and 8 months post-completion of intervention (follow-up 2), respectively. Assessments for the control arm were completed on the same schedule. To ensure equal time periods between groups, the control individual assessments were yoked to individual assessments in the intervention arm. Men were paid US$10 for each assessment and were reimbursed for parking if they attended sessions in person.
Important study design changes
There were 3 important design changes made during the study: (1) There were a number of men who initially agreed to participate, were randomized to the intervention group, and then stated their ED was either not severe enough or they were not bothered enough by their ED to commit the time and effort required for the intervention. To limit this, we increased the severity of ED required to be eligible for the study and added eligibility criteria to include a degree of bother related to ED. (2) Approximately midway through the trial, we modified the randomization from 1:1 to 3:1 (intervention:control) to increase the number of subjects in the intervention arm. (3) To help increase study recruitment, we added anal cancer to the study eligibility criteria as the treatment and effect on sexuality for anal cancer are very similar to those of rectal cancer.
Additionally, while not formally changed in the protocol, the original study timelines did not match the realities of the length of time participants needed for the intervention. The original design was for the intervention to be completed in 7 weeks (i.e., 4 study intervention sessions, 2 weeks apart with the booster sessions occurring during the weeks between intervention sessions). Due to the reality of the tasks to be completed between sessions (doctor visits and trials of ED medications), patients’ schedules and, for many patients, coping with significant bowel side effects of treatment, the average length of time to complete the intervention was approximately 5 months (20.85 weeks), with the 4 intervention sessions occurring generally 4–6 weeks apart and booster sessions 2–3 weeks following each session. However, we note that this timeline is analogous to our clinical experiences of time needed between sessions. The original schedule for follow-up assessments was 2 and 4 months, respectively, following completion of the intervention. However, these occurred on average 3 and 8 months, respectively, following completion of the intervention.
Participants
Patients were recruited from a major northeastern cancer center between March 2010 and June 2013. Those eligible for this pilot RCT were as follows: (1) male; (2) ≥21 years of age; (3) diagnosed with regional (i.e., stages I–III) rectal adenocarcinoma, rectosigmoid with an anastomosis ≤15 cm, or anal cancer; (4) ≥6 months postsurgical or radiation treatment for rectal cancer or ≥6 months postradiation or chemotherapy treatment for anal cancer; (5) with no evidence of recurrent disease; (6) English-speaking; (7) endorsed moderate or low confidence in ability to achieve and maintain erections (≤3 (moderate confidence) on a 5-point Likert scale ranging from 1 (very low confidence) to 5 (very high confidence)); and (8) endorsed bother related to their difficulty with erections (≥2 (a little) on a 5-point Likert scale ranging from 1 (not at all bothered) to 5 (extremely bothered)). Patients only were recruited for this study (no partners).
Interventions
Referral and information control (Control)
Men randomized to this arm received a letter with a referral to the Sexual Medicine Clinic at our institution. These men also received written information about how to address sexual functioning in the form of the American Cancer Society’s booklet on Sexuality after Cancer (ACS 2020e).
Sexual Health Intervention for Men (Intervention)
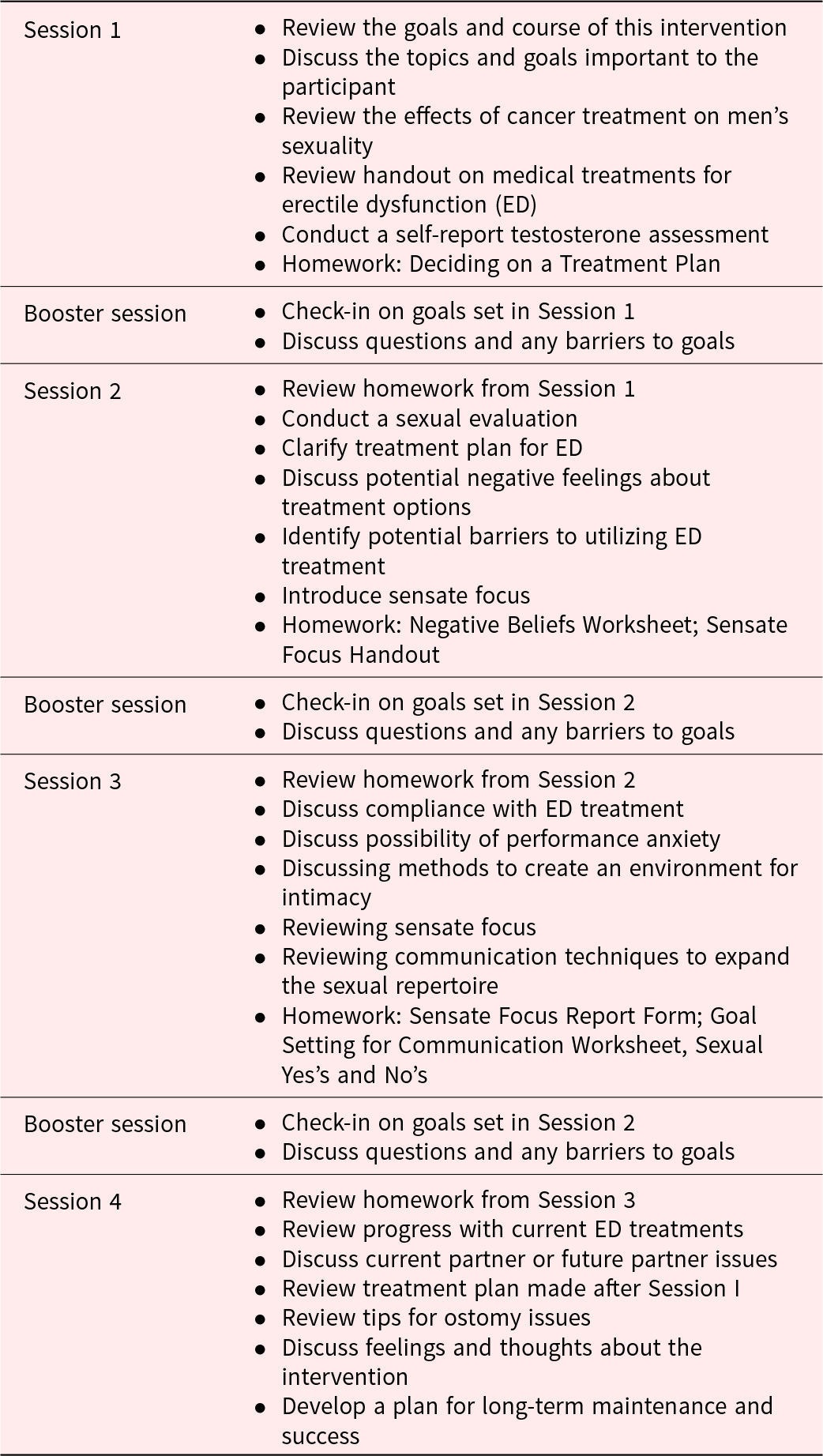
This intervention was based on a psychoeducational intervention developed by Dr. Leslie Schover (a collaborating investigator on this project) to help men with prostate cancer improve their sexual functioning (Canada et al. Reference Canada, Neese and Sui2005; Schover et al. Reference Schover, Canada and Yuan2012). We modified the intervention to shift the focus from prostate cancer to rectal cancer. We then held focus groups with rectal cancer survivors to obtain their feedback to further modify the intervention and to explore their experience with sexual function following rectal cancer treatment (Ball et al. Reference Ball, Nelson and Shuk2013). The modified intervention consisted of four 1-hour individual sessions held every 4–6 weeks, with 3 additional brief (5- to 10-minute) booster sessions provided between each session. The four 1-hour sessions were scheduled to take place over 4–5 months and were conducted over the phone (77%) or in person (23%). All booster sessions were conducted over the phone. Table 1 presents the details of the session content. Twenty-three men completed ≥1 session. Of these, we assessed 6 cases for fidelity of session content. The average fidelity rating of the therapist to the intervention manual was 92% (range, 58%–100%). Of the 23 participants who completed ≥1 session, only 9 self-reported adherence to written homework.
Table 1. Session outline
Study outcomes
Medical and sociodemographic information
Patients’ medical charts including pathology and laboratory results, physician assessments and reports, and other health information. We did not assess sexual orientation.
Sexual function
International Index of Erectile Function (Rosen et al. Reference Rosen, Riley and Wagner1997).
The International Index of Erectile Function (IIEF) was used to assess sexual functioning on 5 domains: erectile function (EFD), orgasmic function (OFD), sexual desire (SDD), intercourse satisfaction, and overall satisfaction. The IIEF is routinely used in sexual function pharmaceutical clinical trials (Heiman et al. Reference Heiman, Talley and Bailen2007).
Sexual self-esteem and sexual function-related distress
The Self-Esteem and Relationship questionnaire (Althof et al. Reference Althof, Cappelleri and Shpilsky2003).
The Self-Esteem and Relationship (SEAR) questionnaire is an ED-specific measure which assesses self-reported level of self-esteem/confidence in sexual functioning and sexual relationship satisfaction (Heiman et al. Reference Heiman, Talley and Bailen2007). Scores are transformed to a 0–100 scale.
Sexual bother
Sexual bother was assessed with the Sexual Bother subscale of the Prostate Health-Related Quality-of-Life Questionnaire (Befort et al. Reference Befort, Zelefsky and Scardino2005).
Cancer-specific distress
Impact of Events Scale-Revised (Weiss and Marmar Reference Weiss and Marmar1997).
The Impact of Events Scale-Revised (IES-R) assessed severity of cancer distress across domains of hyperarousal, intrusive thoughts, avoidance, and total score (Creamer et al. Reference Creamer, Bell and Balilla2002).
Sample size and power analysis
The sample size projection for this pilot RCT study was 80 subjects. These projections were based on the sample sizes of previous pilot studies in this area (Canada et al. Reference Canada, Neese and Sui2005; Titta et al. Reference Titta, Tavolini and Moro2006; Schover et al. Reference Schover, Canada and Yuan2012), as well as the practicality of running the pilot study during the funding period. We anticipated a 40% dropout rate and those 24 subjects would complete each treatment condition. This dropout rate is comparable to similar studies in men with prostate cancer (Canada et al. Reference Canada, Neese and Sui2005). Assuming a 2-sided significance level of 0.05, this sample size would provide >80% power to detect a between-group effect size of 0.88 of the EFD. Although this between-group effect size of 0.88 was considered large, increasing the chance of a type II error, the primary goal of this pilot study was to determine the promise of this intervention and to establish reliable effect sizes that could be used to power future studies.
Randomization
Participants were originally randomized in a 1:1 ratio which was modified to 3:1 (intervention:control). All consented patients were registered and randomized using the institution’s computerized Protocol Participant Registration system and Clinical Research Database. Participants were stratified on (1) stoma and (2) chemotherapy. Although participants were randomized after registration, they were not informed about arm assignment until after baseline completion.
Blinding
The research assistant who interacted with the subjects related to study logistics and completion of the assessments was blinded to group assignment. The study coordinator, who did not interact with the subjects, tracked group assignment.
Analytic strategy
Means were calculated for all outcome measures by assessment time (baseline, follow-up 1, and follow-up 2) and by treatment arm. Change scores were calculated. Differences in change scores for each assessment time were evaluated by between-subject t-tests. The threshold for statistical significance for all statistical tests was p < 0.05, while p < 0.10 was used to indicate marginal significance.
Effect size estimates (i.e., standardized mean difference or Cohen’s d) were calculated for differences between the study arms in change from baseline to follow-up 1 and baseline to follow-up 2. We used the convention of small (0.2), medium (0.5), and large (0.8) Cohen’s d effect sizes (Cohen Reference Cohen1992).
Results
Study enrollment, dropout, and completion
A total of 244 subjects met eligibility criteria, and 90 men (37%) agreed to participate and were randomized, 52 to intervention and 38 to control (see Figure 1). Of the 52 subjects randomized to intervention arm, 14 (27%) completed all components of the intervention, 9 (17%) partially completed, while 7 (13%) dropped prior to baseline and 22 (42%) dropped out prior to the first session. Of the 22 subjects randomized to intervention who did not receive the intervention, over half (n = 16) stated that their ED was not severe enough and/or they were not bothered enough by their ED to warrant making the time commitment. As described above, we thus modified study entry criteria to increase ED severity and to include a question to assess ED bother, which helped resolve these types of dropouts. Of the remaining men who did not receive intervention, 1 was technically ineligible and should not have been consented, 2 were lost to follow-up, 1 experienced time constraints, and 2 were diagnosed with a medical condition following consent that precluded their participation in the intervention. A roughly equal number from each arm (9 intervention and 8 control) dropped out between when they “started” the study, which was defined as starting the first session of intervention or completing the first assessment in control and completing the final 8-month follow-up. A total of 44 men completed follow-up 1 (Intervention, n = 14; Control, n = 30), and 38 men completed follow-up 2 (Intervention, n = 14; Control, n = 24). 1 participant in the control arm completed follow-up 1; however, he did not complete the baseline assessment, so he was excluded from analyses. For participants randomized to the intervention arm, the vast majority participated by phone. Seventy-seven percent of the sessions were completed by phone. Only 1 participant completed all 4 sessions in-person.
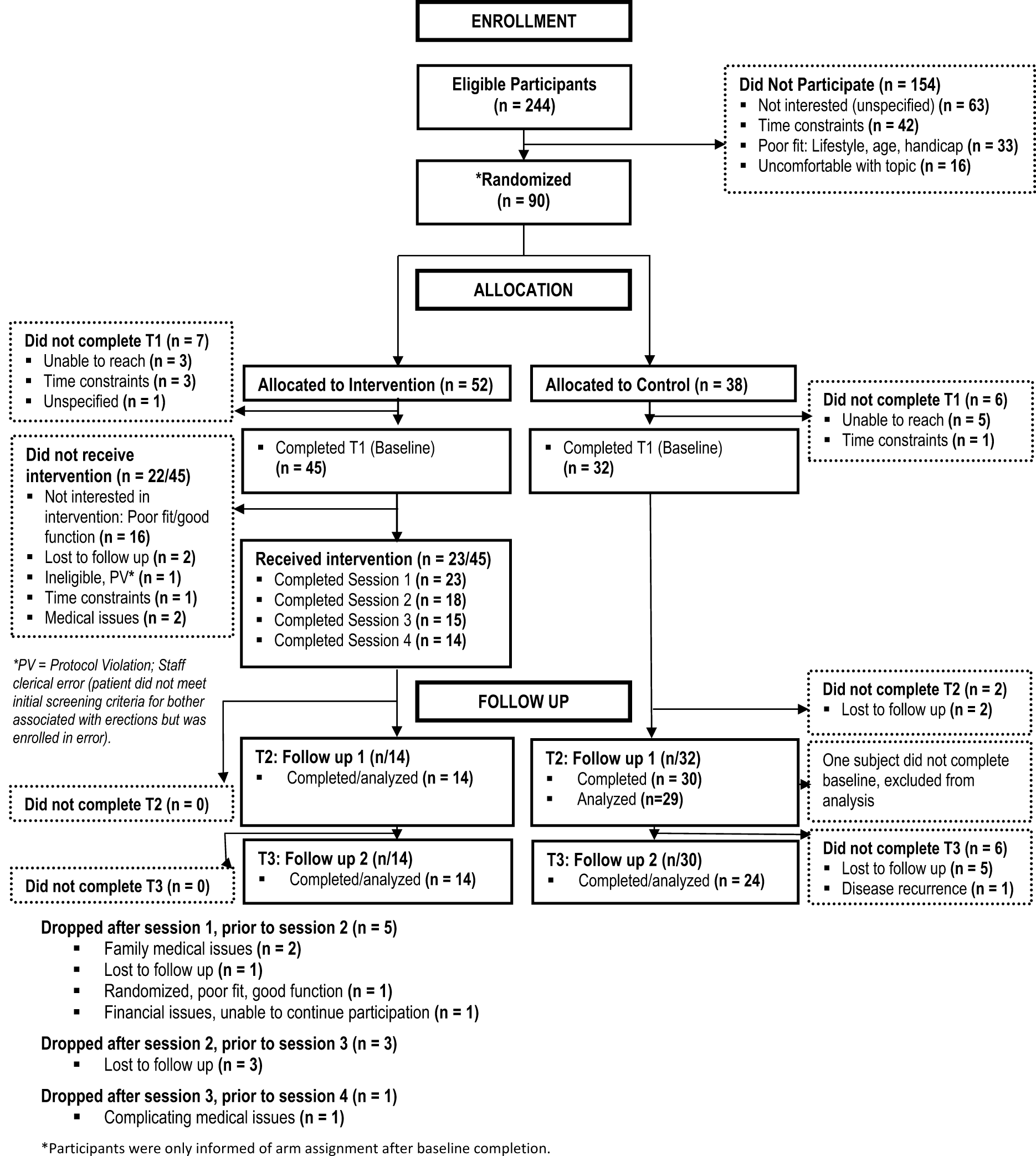
Figure 1. Consort diagram of RCT.
Subject demographics
The average man who completed follow-up 1 (n = 43) was 58 years old (SD = 9.5 years), non-Hispanic/Caucasian (90.5%), and married (85.7%) (vs. partnered but non-married, 14.3%). Cancer diagnoses were 88% rectal and 12% anal. The majority had stage III (64.5%) rectal cancer, and most had received surgical (90.7%), radiation (90.7%), and chemotherapy (90.7%) treatments. At study entry, patients were an average of 4.4 (SD = 3.2) years post-surgery. Sociodemographic, disease, and treatment characteristics for the sample are provided (see Table 2). Despite the disproportionate number of dropouts in the intervention group, there were no differences in demographic characteristics between the 2 groups. Of specific concern with this, dropout was the chance that the groups would report differential baseline sexual function. There were no significant differences at baseline in the percentages who were sexually active (Intervention, 64%; Control, 69%, p = 0.99; Table 2), and there were no significant differences on any of the domains of the IIEF. Specifically, there was no significant difference in the EFD (Intervention, EFD = 10.21; Control, EFD = 12.85, p = 0.33, Table 3).
Table 2. Participant demographic and medical characteristics
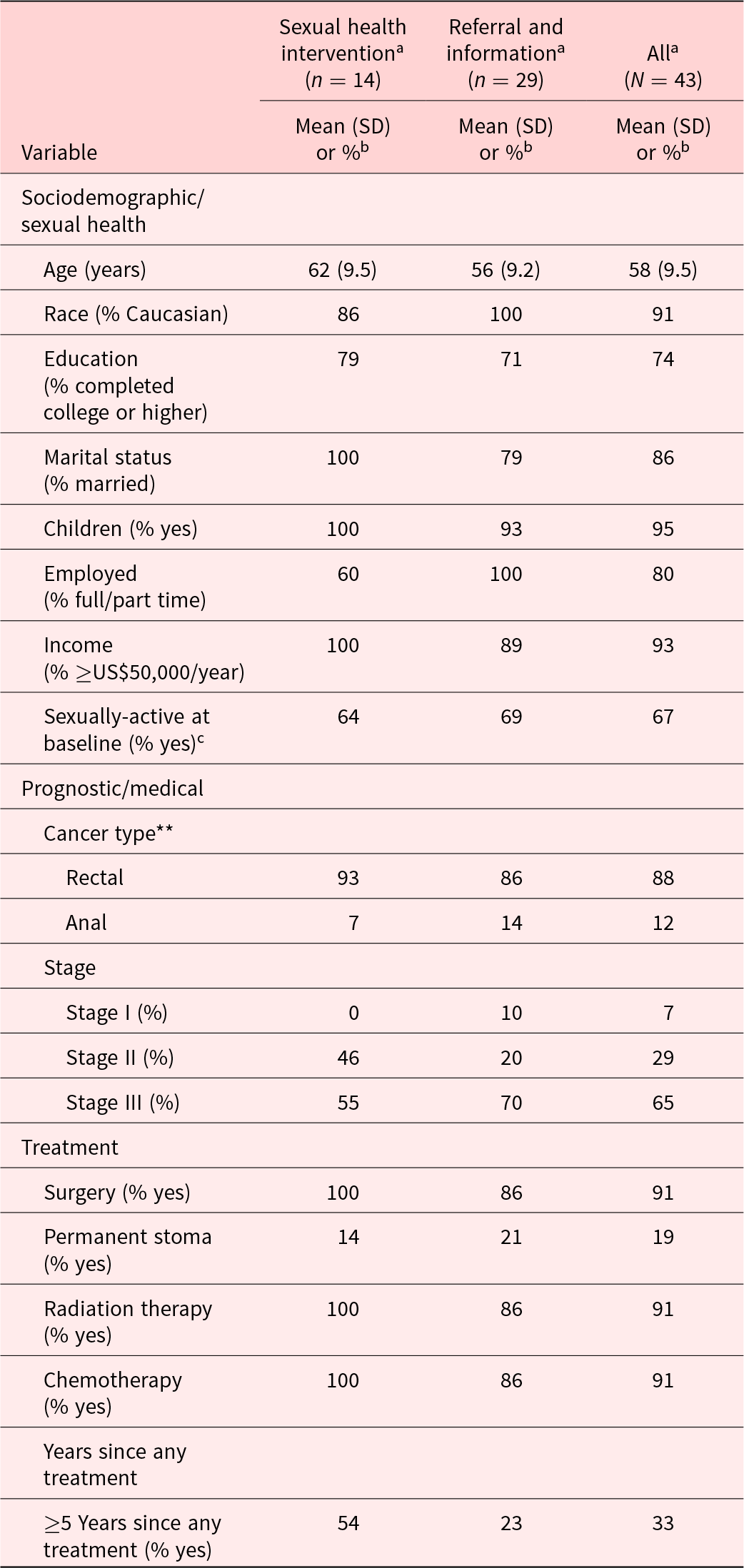
a Patients with at least 1 follow-up.
b Percentages calculated using only valid, non-missing values.
c Defined as having a baseline IIEF ED domain total score as 6 or higher.
** 1 Patient in control arm was missing diagnosis in self-report data.
Table 3. Mean scores at each assessment time by treatment arm and significant treatment arm differences in score changes from baseline to follow-up 1 and follow-up 2
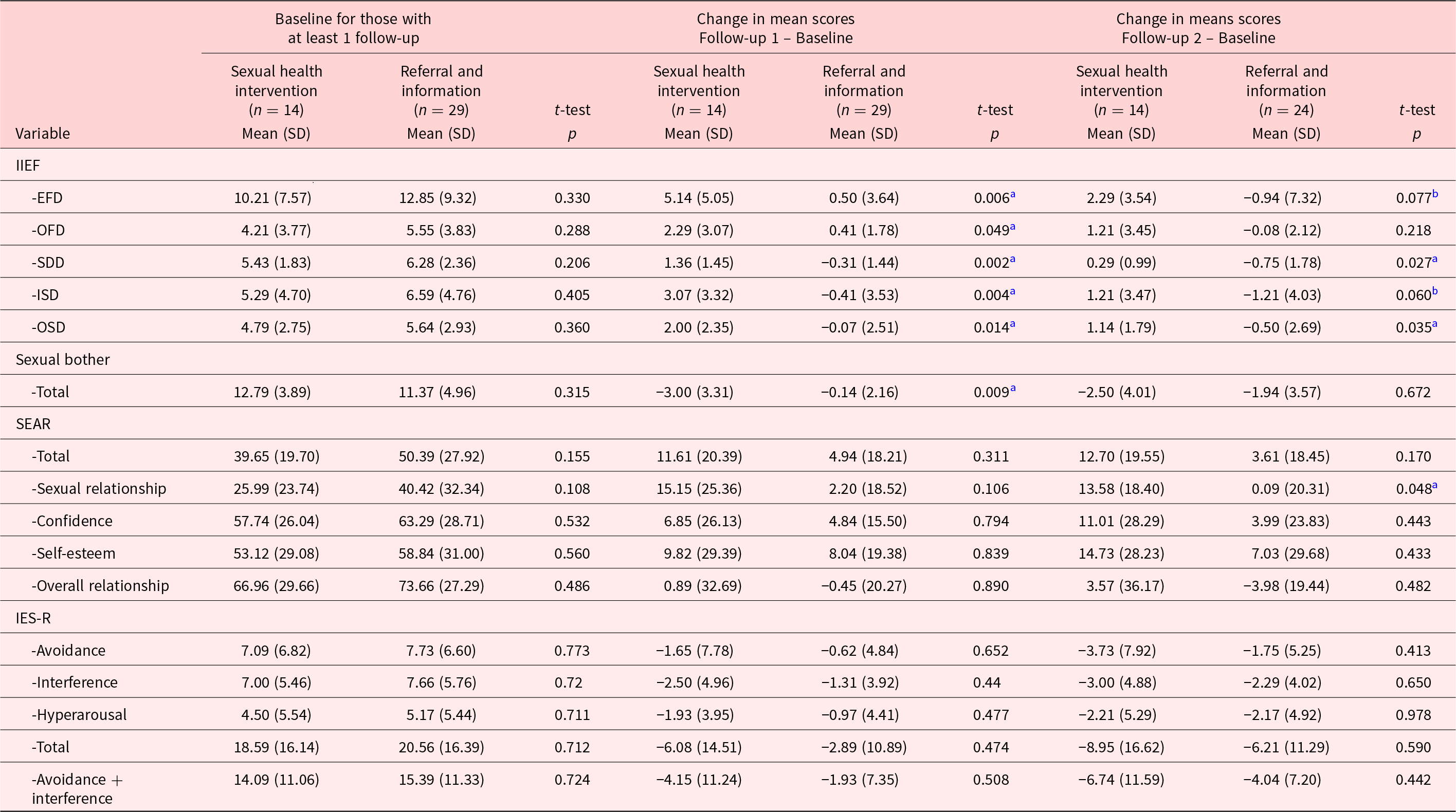
IIEF = the International Index of Erectile Function, erectile function (EFD), orgasmic function (OFD), sexual desire (SDD), intercourse satisfaction (ISD), and overall satisfaction (OSD). IES-R = Impact of Events Scale-Revised; SEAR = Self-Esteem and Relationship.
SEAR scores transformed to a 0–100 scale.
a Two-sample t-test significant, p< 0.05.
b Two-sample t-test approaching significance, p< 0.10.
Completers vs. non-completers
Additionally, since there were a large number of men who dropped out of the study, we also conducted a completer vs. non-completer analysis to determine if these two groups differed on any outcome variables at baseline. Completers were defined as those who completed the follow-up 1 assessment. The completers and non-completers were similar at baseline, and there were no differences in any of the outcome variables, except for the IIEF SDD. The non-completers scored higher at baseline on the SDD (p = 0.03) than the completers.
Study outcomes
Men participating in the intervention arm improved compared to men in the control arm on all IIEF subscales from baseline to follow-up 1 and from baseline to follow-up 2. Magnitudes of effect sizes for differences in changes from baseline between the 2 study arms are shown in Table 4. For all endpoints, mean changes from baseline to follow-up 1 were statistically significantly different for intervention versus control, with effect sizes from d = 0.8 (IIEF OFD) to d = 1.2 (IIEF SD). The effect for the EFD was d = 1.1. Improvements were generally sustained overall from baseline to follow-up 2 assessment for all IIEF values (p = 0.01–p = 0.08), except the orgasm domain, and demonstrated moderate–to-large effects (d = 0.5–d = 0.8).
Table 4. Cohen’s d effect sizes in favor of intervention compared to control
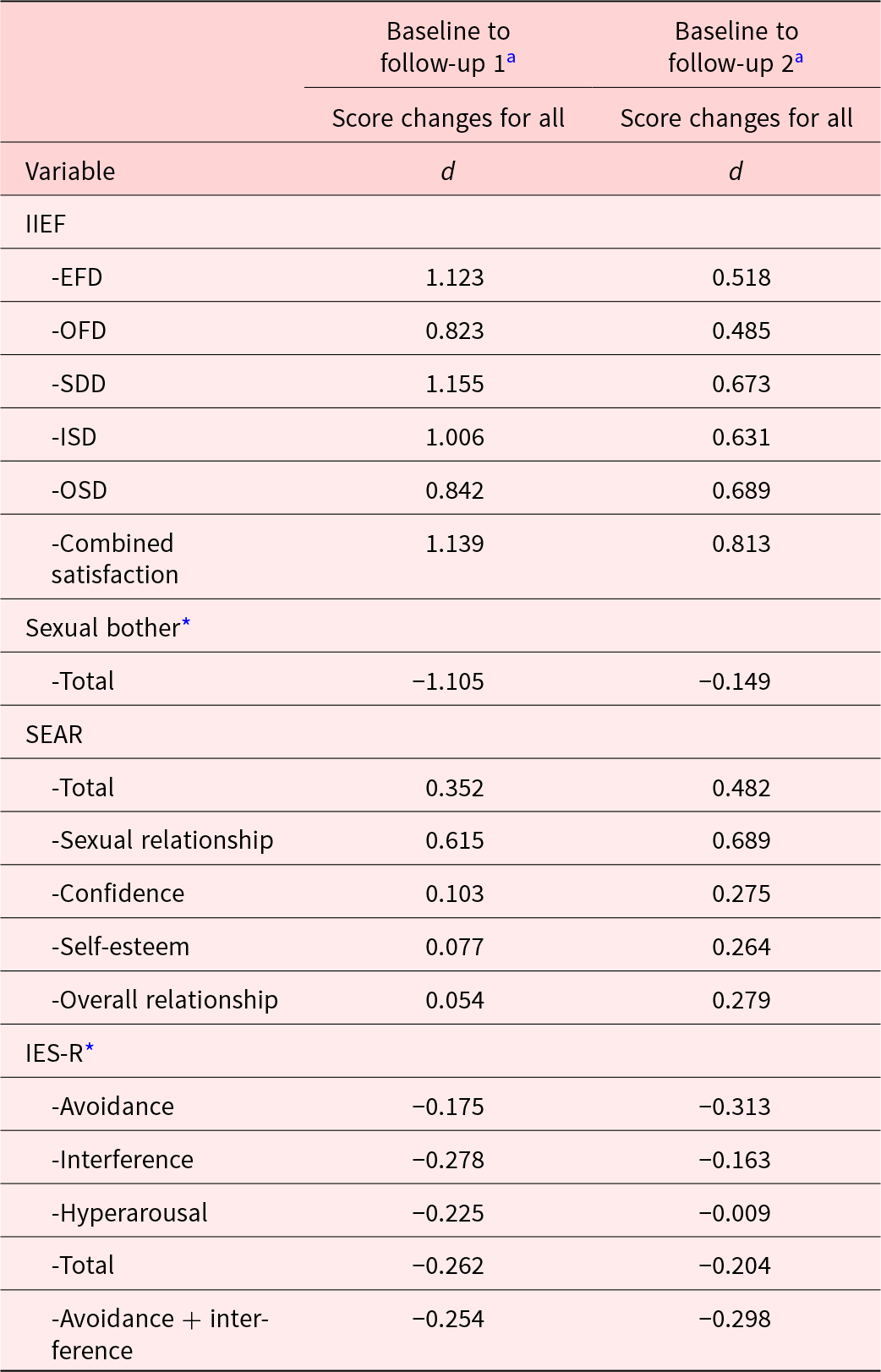
IIEF = The International Index of Erectile Function, erectile function (EFD), orgasmic function (OFD), sexual desire (SDD), intercourse satisfaction (ISD), and overall satisfaction (OSD).
SEAR = Self-Esteem and Relationship.
IES-R = Impact of Events Scale-Revised.
a Small, medium, and large effect sizes correspond with d values of 0.2, 0.5, and 0.8, respectively (Cohen Reference Cohen1992).
* Negative values indicate “improved” psychosocial outcome in favor of intervention comparted to control (i.e., reduced sexual bother and reduced Impact of Event scores).
Statistical significance and magnitudes of effect sizes for sexual bother, SEAR, and IES questionnaires are also shown in Tables 3 and 4. For sexual bother, intervention participants improved compared to control at follow-up 1 (p = 0.009, d = 1.1); however, these effects were not sustained at follow-up 2. Although changes on the SEAR and IES-R were not statistically significantly different between the arms, the differences indicated moderate effects at follow-up 1 (d = 0.4 to d = 0.6) for SEAR total score and the SEAR sexual relationship subscale and small effects (d = 0.2 to d = 0.3) for the IES-R subscales in favor of intervention compared to control. Interestingly, the treatment effect sizes were larger at follow-up 2 than at follow-up 1 for all SEAR subscales and the IES-R avoidance subscale suggesting smaller early treatment effects but larger later effects.
Discussion
We presented results for this pilot RCT, which suggests that a psychoeducational intervention helped improve sexual function in men following rectal and anal cancer treatment. The purpose of the intervention was to provide education related to sexual medicine treatments in the context of psychosocial discussions related to distress about sexual dysfunction, negative emotions/barriers to treatment, and techniques to improve communication and intimacy. While the goal of this pilot study was to outline effect sizes and initial effectiveness of the intervention (as opposed to finding statistical significance), we were encouraged that a number of our results did reach statistical significance, both at first follow-up, on average 3 months after completion of the intervention, and at the second follow-up which was given on average 8 months after completing the intervention.
As expected, since we modeled our intervention after an intervention tested in men with prostate cancer (Rosen, Riley et al. Reference Rosen, Riley and Wagner1997, ACS 2020e), our results were similar to those reported in prostate cancer. In men with prostate cancer, the original intervention produced sound results. It is heartening to see these outcomes carried over into a sample of rectal and anal cancer patients as there are important differences in patient experiences with these cancers compared to prostate cancer. The initial diagnosis of rectal and anal cancer tends to be more life threatening, and the treatment is likely to be more complicated encompassing multiple treatment modalities (radiation, surgery, and chemo) as compared to a single modality treatment (radiation or surgery) that most men with prostate cancer will undergo. Patients treated for rectal or anal cancer also have the potential for significant bowel side effects in addition to sexual side effects. Prostate cancer patients also experience side effects from treatment (sexual, urinary, and bowel); however, the bowel side effects related to rectal and anal cancer are considered to be more severe, chronic, and potentially debilitating than side effects of prostate cancer treatment. In our qualitative work in the first phase of this project (Sun et al. Reference Sun, Grant and Wendel2016), it was clear that men were significantly concerned about their bowel side effects and downgraded the importance of sexual function as a result. Considering these differences between prostate cancer and rectal/anal cancer, there was no guarantee the success of this intervention would carry over to rectal and anal cancer.
In addition to the similarity in the overall pattern of results between our intervention and the intervention tested in prostate cancer, there are also differences to highlight. There are signals that the intervention was more effective in men with rectal and anal cancer as compared to prostate cancer, and the effect of the intervention also appears to be more durable in this current study. This may be due to modifications made to the intervention. First, we added brief booster sessions between the study sessions, and the time to complete the intervention was on average 5 months. The brief booster sessions were an important aspect to help keep subjects on track during the intervention. These changes also allowed for more intervention time and a greater duration of the intervention compared to the one tested in prostate cancer. The current study also added a self-assessment to identify low testosterone. As a result, some men in the intervention decided to pursue testosterone replacement therapy, and this could help explain the moderate effects we found at the second follow-up for sexual function domains, such as sexual desire and sexual satisfaction. Another difference was the choice of secondary psychosocial outcomes. When designing this current study, we chose secondary psychosocial variables that focused more specifically on sexual outcomes, such as sexual bother, sexual self-esteem, and sexual relationship, as opposed to variables such as relationship quality and depression. This explicit focus may have helped produce effects at both follow-up time points for this intervention.
A primary concern for this study is the large number of men who dropped out of the intervention arm. One source of this dropout was that the participants who completed the baseline questionnaire were randomized to the intervention group and then elected to drop from the study prior to initiating the intervention. Many of these men stated that they would complete questionnaires but did not want to make the time commitment to the intervention. One reason to conduct a pilot study is to learn about recruiting difficulties related to a specific population. We modified our eligibility criteria related to severity and bother of ED, which helped limit this source of dropout. There was also dropout during the intervention. While some of this dropout was attributed to medical issues, there was a portion who were lost to follow-up. One reason for this type of dropout (and dropout in general) may be attributed to a sense of avoidance to address sexual dysfunction. The literature indicates men tend to avoid pursing treatments for ED despite the desire to address this condition. Additionally, a number of studies have documented the distress men experience related to ED. This can lead to men pairing thoughts of sexual activity with anxiety, fear, or dread, which can lead to avoidance (Nelson et al. Reference Nelson, Lacey and Kenowitz2015). It may also be possible that men had endured ED symptoms and bother for so long that they accepted them as a new normal. In future studies, it may be helpful to initiate an intervention sooner and integrate strategies to reduce avoidance into these types of psychosocial interventions to help increase uptake of the intervention and reduce dropout. Additionally, the intervention in the study did not include partners as part of the intervention. Relationship and communication dynamics are important aspects for men to pursue sexual relations with their partners. This lack of inclusion of the partner could have led to dropout and may be an important aspect to include in the future. Lastly, we have also conducted a companion study in women with rectal cancer, which also had significant dropout. Time commitment and logistical concerns were aspects of dropout in both studies (see brief report (Shaffer et al. Reference Shaffer, Nelson and DuHamel2018) for comparison of dropout between men and women), and streamlining logistics and increasing access to the intervention (i.e., telehealth, online) may be important to reduce dropout.
The strengths of this study include using an intervention which has a sound research foundation, modifying the intervention with patient feedback to appropriately address rectal and anal cancer survivors, testing the intervention in a pilot RCT, and including variables which assess sexual specific psychosocial outcomes. While we believe these are important strengths, the study does have weaknesses. As stated above, the most glaring weakness was the dropout in the intervention arm. Additionally, while we added eligibility for anal cancer patients, this was added after the manual was modified and the study started recruitment. As a result, there was no content added to address anal cancer-specific concerns. In future studies, it may be important to address sexual transmission of a human papillomavirus-related cancer or shame about a malignancy sometimes linked to anal intercourse (Shaffer et al. Reference Shaffer, Nelson and DuHamel2018). We also did not track visits to a sexual medicine urologist or actively record types of sexual dysfunction treatments used (e.g., to address ED or low libido). Lastly, most of this sample were White and highly educated, all were English-speaking, and all were recruited from 1 urban cancer center and as such, caution should be taken when generalizing to more diverse populations.
In the end, there were a number of lessons learned through this pilot study which we hope can inform future research in this population. First, we found that the intervention was effective as a more intensive intervention for men with at least moderate ED and bother. We learned that if men are experiencing mild difficulty with ED and they are not bothered by it, they viewed this intervention as too intense and time consuming. Instead, patients experiencing mild symptoms preferred a referral to sexual medicine resources and declined additional psychoeducational help to address their ED. Future studies may consider implementing different levels of care to address variations in symptoms and bother. Second, questionnaires in this study were administered either by staff reading the questions over the phone (86.8%) or by mail (13.2%). Part of the rationale for administering the measures over the phone with trained staff was to attempt to keep missing data to a minimum and avoid having items get unintentionally missed items. As the use of online self-administered survey platforms is now easily accessible and approved by IRBs, we highly suggest these online platforms in the future as they help reduce potential that responses are affected by social desirability bias because of fear of shame or judgment when surveys are administered by staff over the phone or in person. Third, our eligibility criteria only included patients who were at least 6 months post-treatment, because men following rectal cancer treatment reported bowel side effects were their most significant side effect and they generally were not thinking about erectile function until later in the recovery process (Ball et al. Reference Ball, Nelson and Shuk2013); however, given that patients with ED are often advised to start treatment as early as possible, future studies should enroll patients as soon after the start of treatment as possible. Fourth, participants in this study received a modest US$10 for each assessment. Future studies may consider a more significant compensation to potentially reduce dropout.
In the male rectal and anal cancer population, pilot data support the utility of a brief psychoeducation intervention to improve sexual functioning and adjustment. This study provides initial evidence for combining a psychoeducational intervention with medical interventions to address sexual dysfunction following rectal and anal cancer.
Acknowledgments
We thank the study participants and the research professionals and trainees at Memorial Sloan Kettering Cancer Center and Icahn School of Medicine at Mount Sinai.
Funding
This work was supported by the National Cancer Institute [R21 CA137434; T32CA009461; P30CA008748].
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Approval was obtained from the Institutional Review Board at Memorial Sloan Kettering Cancer Center (IRB # 08-073). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.








