The consumption of whole grains has consistently been found to be inversely associated with type 2 diabetes risk in prospective studies(Reference Schulze, Schulz, Heidemann, Schienkiewitz, Hoffmann and Boeing1, Reference de Munter, Hu, Spiegelman, Franz and van Dam2). Whole grains may lead to a prolongation and reduction of intestinal glucose absorption and thus to a lower postprandial insulin secretory demand. However, it is reasonable to assume that the effect of whole grains to reduce type 2 diabetes risk may depend on variation in the genes involved in the process of insulin secretion in the pancreatic β-cells. The transcription factor-7-like 2 (TCF7L2) gene is a major susceptibility locus for type 2 diabetes(Reference Cauchi, El Achhab and Choquet3, Reference Florez4) and polymorphisms in this gene are suggested to lead to diabetes by means of a reduced insulin secretion(Reference Weedon5). Thus, we hypothesised that variation in the TCF7L2 gene might influence the association between the intake of whole grains and type 2 diabetes risk, and evaluated this potential association in the large, population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam cohort study.
Experimental methods
Study population
The EPIC-Potsdam study comprises 27 548 subjects from the general population of the Potsdam area in Northern Germany, mainly aged 35–65 years at recruitment (1994–8)(Reference Boeing, Korfmann and Bergmann6, Reference Bergmann, Bussas and Boeing7). We designed a prospective case–cohort study of 849 incident type 2 diabetes cases identified during a mean follow-up time of 7·1 years and 2500 randomly selected individuals (subcohort) from all participants of the EPIC-Potsdam study. Identification and verification of incident cases have been described in detail before(Reference Schulze, Schulz, Heidemann, Schienkiewitz, Hoffmann and Boeing1). From the subcohort, 2322 subjects remained for analysis after exclusion of participants with a history of diabetes at baseline, with self-reported diabetes during follow-up but without physicians' confirmation, and those without relevant baseline information, blood samples, and fully obtained follow-up data. Furthermore, individuals reporting implausible energy intake (cutpoints of less than 3350 kJ/d or more than 25 100 kJ/d) were excluded from analysis, resulting in a final subcohort population of 2318 subjects. Of the 849 incident cases, 798 remained for analysis after similar exclusion criteria. Because the subcohort is representative of the full cohort at baseline, seventy-four incident cases belonged to the subcohort and 724 cases were so called ‘external cases’ identified in the remainder of the full cohort. Informed consent was obtained from all study participants, and approval was given by the Ethical Committee of the State of Brandenburg, Germany.
Dietary assessment
Frequency of intake and the portion size of 148 foods consumed during the 12 months before examination were measured with a validated FFQ. Information on frequency of intake and portion size was used to calculate the amount of food items in grams consumed on average per d. We calculated total whole-grain intake as the sum of whole-grain bread, whole-grain bread rolls, and whole-grain cereals and considered 50 g as one portion, equalling about one slice of bread or one roll in this population. The validity and reproducibility of the FFQ have been described previously(Reference Bohlscheid-Thomas, Hoting, Boeing and Wahrendorf8, Reference Kroke, Klipstein-Grobusch, Voss, Moseneder, Thielecke, Noack and Boeing9).
Genotyping
Genotyping was performed with the TaqMan System (Applied Biosystems, Foster City, CA, USA) using 5 ng of whole-genome amplified DNA per single nucleotide polymorphism as described before(Reference Hampe, Wollstein, Lu, Frevel, Will, Manaster and Schreiber10). An average genotyping success rate of 98·1 % was achieved. Replicate quality-control samples (6 %) were included and genotyped with ≥ 99 % concordance.
Statistical analysis
Hazard ratios were computed using a weighted Cox proportional hazards model, modified for the case–cohort design according to the Barlow method(Reference Barlow, Ichikawa, Rosner and Izumi11). To determine the interaction effect between TCF7L2 rs7903146, whole-grain intake and diabetes risk, we modelled whole-grain intake as a continuous variable (per 50 g/d portion), calculated genotype × whole-grain intake product terms in a multivariate adjusted Cox model, and performed linear hypothesis tests about the regression coefficients. In the model, genotype CT+TT, ‘CC × whole grains’ and ‘CT+TT × whole grains’ product terms, and additional covariates were included. The product terms are not the classic interaction terms, but allow estimation of the effect of nutrient intake in the genotype groups separately. A parameter of a gene–nutrient product term that is significantly different from zero can be interpreted as a significant nutrient effect in the carriers of the corresponding genotype. The power to detect a genotype-specific environmental effect is somewhat higher than that of a subgroup analysis with separate models for each genotype. We used information on covariates obtained from the baseline examination, including age, sex, waist circumference, BMI, sports activity (0, 0·1–4·0, >4·0 h/week), occupational activity (low, medium, high), educational attainment (less than high school, high school, more than high school), cigarette smoking (never, past, current < twenty cigarettes/d, current ≥ twenty cigarettes/d), alcohol intake (0, 0 ≥ 5, 5 ≥ 10, 10 ≥ 20, 20 ≥ 40, and >40 g/d), red meat (per 150 g/d), processed meat (per 50 g/d), low-fat dairy (per 150 g/d), butter (per 20 g/d), margarine and vegetable oil (per 20 g/d) consumption, coffee intake (per 150 ml/d) and total energy intake (kJ/d). The statistical analyses were performed with SAS release 9.1 (SAS Institute, Cary, NC, USA).
Results
Cox proportional hazard ratios of TCF7L2 single nucleotide polymorphisms located in the 64·6 kb linkage-disequilibrium region of the gene with type 2 diabetes are shown in Table 1. Among those TCF7L2 polymorphisms, rs7903146 C>T showed the strongest association with type 2 diabetes risk (hazard ratio for T-allele carriers = 1·51; 95 % CI 1·21, 1·87) when we adjusted for age, sex, BMI and waist circumference.
Table 1 Association of transcription factor-7-like 2 (TCF7L2) single nucleotide polymorphisms with type 2 diabetes risk*
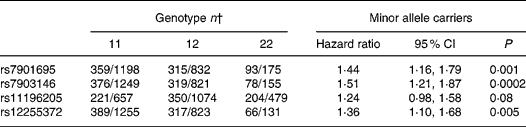
11, Homozygote major allele carriers; 12, heterozygotes; 22, homozygote minor allele carriers.
* Hazard ratios and 95 % CI adjusted for age, sex, BMI and waist circumference.
† Number of incident cases and non-diabetic subjects from the subcohort.
We then evaluated whether TCF7L2 rs7903146 and whole grain interact in the prediction of type 2 diabetes by including the product terms of genotype × whole-grain intake into the model. Whole-grain intake was significantly inversely associated with type 2 diabetes risk in rs7903146 C homozygotes (hazard ratio per 50 g/d portion = 0·86; 95 % CI 0·75, 0·99) after adjustment for age, sex, anthropometry, lifestyle and dietary confounders (Fig. 1). However, no association of whole-grain intake with diabetes risk was seen among T-allele carriers (hazard ratio per 50 g/d portion = 1·08; 95 % CI 0·96, 1·23). The difference between genotype-specific hazard ratios was statistically significant (P = 0·016). These associations were similar in crude models only adjusted for age (data not shown). The exclusion of study subjects (n 178) with baseline glucose concentrations greater then 1260 mg/l in our analyses only produced minor changes in risk ratios and the difference between genotype groups remained significant (data not shown).

Fig. 1 Hazard ratios (HR) of type 2 diabetes for whole grains (50 g/d portion) according to transcription factor-7-like 2 (TCF7L2) rs7903146 genotype. HR for whole grains were adjusted for age, sex, BMI, waist circumference, educational attainment, occupational activity, sports activity, cigarette smoking, consumption of alcohol, red meat, processed meat, low-fat dairy, butter, margarine and vegetable fat, coffee, and total energy intake (kJ/d). Vertical bars represent 95 % CI. n, Number of incident cases and non-diabetic subjects from the subcohort. For the CC genotype, HR = 0·86 (95 % CI 0·75, 0·99); for the CT+TT genotype, HR = 1·08 (95 % CI 0·96, 1·23). * HR for the CT+TT genotype was significantly different from that for the CC genotype (P < 0·016).
Discussion
In the US Diabetes Prevention Program, there was no apparent difference in diabetes-free survival under an intensive lifestyle intervention between TCF7L2 rs7903146 genotypes(Reference Florez, Jablonski, Bayley, Pollin, de Bakker, Shuldiner, Knowler, Nathan and Altshuler12), allowing the assumption that behavioural intervention can mitigate the risk conferred by the genetic background. However, the lifestyle intervention aimed at a reduction of body weight by increasing physical activity and by modifying diet, in particular fat intake, and succeeded primarily by improving insulin sensitivity. However, genetic variation in TCF7L2 is specifically associated with impaired insulin secretion(Reference Weedon5). In the present study, we found that the protective effect of whole-grain intake on diabetes risk exclusively applied to CC genotype carriers of rs7903146, whereas subjects carrying the T-allele seemed to exhibit no benefit from whole-grain consumption. This suggests that the detrimental effect of the risk allele might mitigate the beneficial effect of whole grains on type 2 diabetes risk. The beneficial effect of whole grains may at least partly be related to their high fibre content. Proposed mechanisms, for soluble fibres in particular, involve delayed gastric emptying, inhibition of α-amylase, and a reduction of intestinal transit times, leading to a prolongation and reduction of intestinal glucose absorption and thus to a lower postprandial insulin secretory demand. Fibres in whole grains may also stimulate gastrointestinal hormone secretion, in particular gastric inhibitory polypeptide and glucagon-like peptide-1 (GLP-1)(Reference Hagander, Schersten, Asp, Sartor, Agardh, Schrezenmeir, Kasper, Ahren and Lundquist13, Reference Juntunen, Niskanen, Liukkonen, Poutanen, Holst and Mykkanen14). Because the insulin-secretory defect of the TCF7L2 variant may arise from reduced GLP-1 efficiency rather then GLP-1 secretion(Reference Schäfer, Tschritter and Machicao15, Reference Shu, Sauter, Schulthess, Matveyenko, Oberholzer and Maedler16), the beneficial effects of fibre-rich whole grains might be diminished in the context of a genetically disturbed GLP-1 action.
In conclusion, we found that the inverse association between whole-grain consumption and type 2 diabetes risk might depend on variation in TCF7L2.
Acknowledgements
The present study was supported by grants from the European Union (EUGENE2, LSHM-CT 204 512013), the German Ministry of Science and Technology (NGFN2, 01GS0487) and the Federal Ministry of Education and Research (AZ 0313437D). E. F. was responsible for analysis and manuscript preparation; H. B. was responsible for design, subject evaluation and data collection; A. F. was responsible for revision of the manuscript; F. D. was responsible for data collection and revision of the manuscript; H. G. J. was responsible for revision of the manuscript and M. S. was responsible for design, subject evaluation, data collection and revision of the manuscript. None of the authors had any financial, personal or other conflict of interest.




