Introduction
Vultures are medium to large-sized scavenging birds of prey (Campbell Reference Campbell2015; del Hoyo et al. Reference del Hoyo, Elliott and Sargatal1994). They are often described as important ecosystem service providers by consuming carrion and other organic waste (Carucci et al. Reference Carucci, Whitehouse-Tedd, Yarnell, Collins, Fitzpatrick and Botha2022; DeVault et al. Reference DeVault, Beasley, Olson, Moleón, Carrete, Margalida, Sekercioglu, Wenny and Whelan2016; Moleόn et al. Reference Moleόn, Sánchez-Zapata, Margalida, Carrete, Owen-Smith and Donázar2014).
Globally, 23 species of vultures occur (Ives et al. Reference Ives, Brenn-White, Buckley, Kendall, Wilton and Deem2022), of which Africa is the vulture-richest continent with 11 species (Anderson Reference Anderson2007; Campbell Reference Campbell2015). Out of these, Ghana is home to eight species (Deikumah Reference Deikumah2020). Six of them, namely, the Hooded Vulture Necrosyrtes monachus, White-headed Vulture Trigonoceps occipitalis, African White-backed Vulture Gyps africanus, Rüppell’s Griffon Gyps rueppellii, Palm-nut Vulture Gypohierax angolensis, and Lappet-faced Vulture Torgos tracheliotos are resident non-migratory species in Ghana, but two, the Egyptian Vulture Neophron percnopterus and Eurasian Griffon Gyps fulvus, are migrants (Di Vittorio and Petrozzi Reference Di Vittorio and Petrozzi2018). According to the International Union for Conservation of Nature (IUCN) (2024), four of Ghana’s eight vulture species are “Critically Endangered”, two are listed as “Least Concern”, and two are “Endangered”.
The population of vultures has declined drastically in the last three decades (Di Vittorio et al. Reference Di Vittorio, Hema, Dendi, Akani, Cortone and López López2018; Safford et al. Reference Safford, Andevski, Botha, Bowden, Crockford and Garbett2019), particularly in West Africa (Henriques et al. Reference Henriques, Granadeiro, Monteiro, Nuno, Lecoq and Cardoso2018). Major threats to vultures as reported globally in several studies are anthropogenic (Ives et al. Reference Ives, Brenn-White, Buckley, Kendall, Wilton and Deem2022; Ogada et al. Reference Ogada, Shaw, Beyers, Buij, Murn and Thiollay2016). Poisoning is the predominant threat among all vultures of the world, particularly in Africa (Margalida et al. Reference Margalida, Ogada and Botha2019). Toxins, especially from lead and pesticides, were the most commonly reported cause of mortality, followed by traumatic injury, which includes collision with infrastructure and gunshot (Ives et al. Reference Ives, Brenn-White, Buckley, Kendall, Wilton and Deem2022). Other threats reported in several studies include the high demand for vulture body parts in traditional medicine, unprovoked persecution, habitat loss, and reduced food availability (Buechley et al. Reference Buechley, Girardello, Santangeli, Ruffo, Ayalew and Abebe2022; Buij et al. Reference Buij, Nikolaus, Whytock, Ingram and Ogada2016; Di Vittorio et al. Reference Di Vittorio, Hema, Dendi, Akani, Cortone and López López2018; Ogada et al. Reference Ogada, Shaw, Beyers, Buij, Murn and Thiollay2016).
Habitat use in African vultures has been assessed extensively by several authors (Bamford et al. Reference Bamford, Monadjem and Hardy2009c; Buechley et al. Reference Buechley, Girardello, Santangeli, Ruffo, Ayalew and Abebe2022; Kane et al. Reference Kane, Monadjem, Aschenborn, Bildstein, Botha and Bracebridge2022; Kendall et al. Reference Kendall, Virani, Hopcraft, Bildstein and Rubenstein2014). It has been revealed that vultures use protected areas extensively (Martens et al. Reference Martens, Pfeiffer, Downs and Venter2018; Pfeiffer et al. Reference Pfeiffer, Downs and Venter2015). However, some vultures can also spend a considerable amount of time outside protected areas (Buechley et al. Reference Buechley, Girardello, Santangeli, Ruffo, Ayalew and Abebe2022; Kane et al. Reference Kane, Monadjem, Aschenborn, Bildstein, Botha and Bracebridge2022; Peters et al. Reference Peters, Beale, Bracebridge, Mgumba and Kendall2022). Gyps vultures, for instance, are known to cover large areas in search of carrion (Delgado-González et al. Reference Delgado-González, Cortés-Avizanda, Serrano, Arrondo, Duriez and Margalida2022; Kane et al. Reference Kane, Monadjem, Aschenborn, Bildstein, Botha and Bracebridge2022; Phipps et al. Reference Phipps, Willis, Wolter and Naidoo2013a). This wide-ranging behaviour, as well as their communal feeding, expose them to a full range of threats such as poisoning, electrocution, and collision with powerlines (Kane et al. Reference Kane, Monadjem, Aschenborn, Bildstein, Botha and Bracebridge2022; Peters et al. Reference Peters, Beale, Bracebridge, Mgumba and Kendall2022; Phipps et al. Reference Phipps, Wolter, Michael, MacTavish and Yarnell2013b).
The distribution and abundance of vultures globally may also be influenced by habitat suitability and seasonal patterns (Holland et al. Reference Holland, Byrne, Hepinstall-Cymerman, Bryan, DeVault and Rhodes2019; Margalida et al. Reference Margalida, García and Cortés-Avizanda2007; Stolen Reference Stolen2000). Habitat characteristics such as tree height, distance to rivers, and food availability are known to influence African vulture distribution (Bamford et al. Reference Bamford, Monadjem and Hardy2009b; Johnson and Murn Reference Johnson and Murn2023). Riparian habitats have been reported to host higher species richness and diversity because this habitat is usually distinct from the surrounding landscape (Seymour and Simmons Reference Seymour and Simmons2008). Seasonal trends in the abundance of vultures may result from changes in the availability of carcasses between seasons (Stolen Reference Stolen2000). Changes in the abundance of vultures within a particular setting may also be due to individuals responding to changes in food availability, as in the case of Turkey Vulture Cathartes aura and Black Vulture Coragyps atratus in Central Florida (Stolen Reference Stolen1996, Reference Stolen2000). This suggests that vultures exhibit spatial differences in resource selection, and habitat types are important in determining their abundance in an area.
Extensive studies on vulture distribution and habitat use have been conducted globally. The Indian White-backed Vulture Gyps bengalensis is distributed in small patches and prefers to nest on pine trees Pinus roxburghii (Thakur and Narang Reference Thakur and Narang2012). African White-backed vultures use areas where food is available and prefer to nest in tall trees within riparian habitats (Bamford et al. Reference Bamford, Monadjem and Hardy2009b; Johnson and Murn Reference Johnson and Murn2023). Previous studies revealed that White-backed Vultures in Tanzania national parks and game reserves preferred to use areas close to permanent rivers during the dry season (Peters et al. Reference Peters, Beale, Bracebridge, Mgumba and Kendall2022). Carcass utilisation by the White-backed Vulture in north-eastern Swaziland (now Eswatini) was negatively affected by higher vegetation density (Bamford et al. Reference Bamford, Monadjem and Hardy2009a).
While previous authors have documented the population status of vultures in some urban areas in Ghana (Campbell Reference Campbell2009; Ogada and Buij Reference Ogada and Buij2011), and the abundance and diversity of some vulture species in Mole National Park (MNP) (Afrifa et al. Reference Afrifa, Monney and Deikumah2022; Agyei-Ohemeng et al. Reference Agyei-Ohemeng, Danquah and Adu Yeboah2017; Di Vittorio and Petrozzi Reference Di Vittorio and Petrozzi2018; Sulemana et al. Reference Sulemana, Monney and Deikumah2022), studies of habitat use in vultures in Ghana are generally limited. Given the overall declining population trend for vultures in Africa and the underlying causes for the massive declines reported, baseline information on the abundance and habitat use of vulture populations in Ghana is needed, especially in the protected areas of the savanna vegetation zone. As a result, this study focused on estimating the population status of vultures and understanding the factors that influence their abundance and habitat use in the MNP. We predicted that: (1) the abundance of vultures will be higher in the MNP because of the concentration of wildlife in the park (Peters et al. Reference Peters, Beale, Bracebridge, Mgumba and Kendall2022); (2) vulture species will utilise the areas of the park with the highest food resource, mainly within riparian areas (Johnson and Murn Reference Johnson and Murn2023; Peters et al. Reference Peters, Beale, Bracebridge, Mgumba and Kendall2022); (3) vulture abundance will be higher in the dry season compared with the wet season (Kendall et al. Reference Kendall, Virani, Hopcraft, Bildstein and Rubenstein2014); (4) adult vultures will be more abundant than juveniles (Kane et al. Reference Kane, Monadjem, Aschenborn, Bildstein, Botha and Bracebridge2022; Margalida et al. Reference Margalida, Jiménez, Martínez, Sesé, García‐Ferré and Llamas2020).
Methods
Study area
The MNP is the largest and oldest Wildlife Protected Area (WPA) in Ghana and covers an area of about 4,600 km2 (9°30′0″N 2°0′0″W) (Di Vittorio and Petrozzi Reference Di Vittorio and Petrozzi2018) and is located in the savanna region of Ghana (Figure 1). The park comprises a diversity of vegetation types including bovals (open areas dominated by grass), riparian forests, floodplain grasslands, open savanna woodland, and swamps.
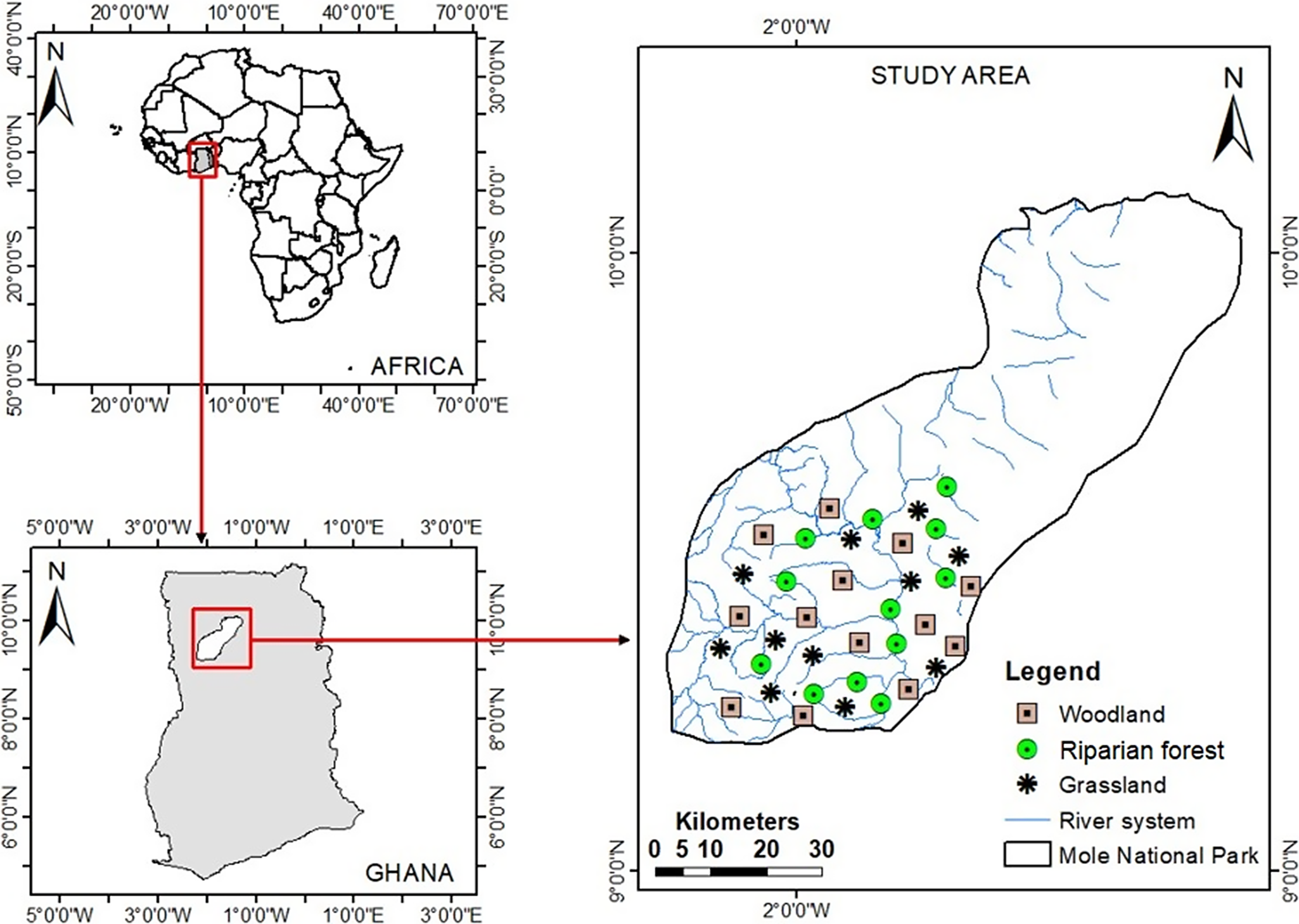
Figure 1. Map of Mole National Park (MNP) showing areas with different habitat types where vultures were surveyed. The top left displays a map of Africa, indicating the location of Ghana, while the lower section shows a map of Ghana and the location of the MNP.
The park is dominated by open savanna woodland interspersed with grasslands and riparian forests (Burton et al. Reference Burton, Buedi, Balangtaa, Kpelle, Sam and Brashares2011; Dankwa‐Wiredu and Euler Reference Dankwa‐Wiredu and Euler2002). The woodland habitat is mainly dominated by trees that are widely spaced with patches of grasses and shrubs (Sulemana et al. Reference Sulemana, Monney and Deikumah2022). The grassland consists of grasses and sedges with small trees and shrubs scattered all around (IUCN/PACO 2010). The riparian forest is dominated by streams and rivers that provide food, cover, and serve as stopovers for both aquatic and terrestrial species in the park (Naiman et al. Reference Naiman, Fetherston, McKay, Chen, Naiman and Bilby1998).
The Mole, Kulpawn, and Polzen rivers that drain the park flow permanently, while the others (Samole, Lovi, and Zuo) usually dry up or are reduced to stagnant pools in the dry season (Kuuder Reference Kuuder2012). The park consists of some wetlands that provide havens for species, including Lana Pool, Dam 1 and 2, Asibey Pool, and Haraba Pool. In terms of biodiversity, the park harbours over 93 mammal species and at least 344 bird species (IUCN/PACO 2010). Known vulture species in the MNP include White-headed, Hooded, Palm-nut, White-backed, Eurasian Griffon, and Rüppell’s Vultures (Di Vittorio and Petrozzi Reference Di Vittorio and Petrozzi2018; Nsor et al. Reference Nsor, Acquah, Mensah, Kusi-Kyei and Boadi2018; Sulemana et al. Reference Sulemana, Monney and Deikumah2022).
The northern part of Ghana, of which the MNP is part, experiences uni-modal rainfall distribution (single wet season) from April to October (Abizari et al. Reference Abizari, Azupogo, Nagasu, Creemers and Brouwer2017), with a peak in September. The dry season occurs between December and March with an annual mean temperature of 28°C (Sulemana et al. Reference Sulemana, Monney and Deikumah2022). Due to the numerous streams and rivers which drain into the White Volta River, the availability of water in the park is widespread during the wet season (Dankwa‐Wiredu and Euler Reference Dankwa‐Wiredu and Euler2002; Di Vittorio and Petrozzi Reference Di Vittorio and Petrozzi2018) but leaves behind only drinking spots in the dry season.
Vulture populations in Ghana may be threatened by poisoning, trade in vulture parts for use in traditional medicine, and habitat loss (Boakye et al. Reference Boakye, Wiafe and Ziekah2019; Deikumah Reference Deikumah2020). The decline in the silk cotton tree Ceiba pentandra through deforestation and selective logging for plywood has been reported (Deikumah Reference Deikumah2020). Illegal logging of the African rosewood Pterocarpus erinaceus and charcoal production has been severe in the northern part of Ghana (Amadu et al. Reference Amadu, Ayamga and Mabe2021; Dumenu and Bandoh Reference Dumenu and Bandoh2016; Sulemana et al. Reference Sulemana, Monney and Deikumah2022). In the MNP, poaching of elephants and illegal extraction of natural resources has been reported, especially around saltlicks and waterhole areas (Obour et al. Reference Obour, Asare, Ankomah and Larson2016).
Survey design
The three dominant habitat types, namely woodland, grassland, and riparian forest, were selected for the inventory of vulture species because they represent the dominant vegetation composition of the MNP (Figure 1). Riparian forests selected included nearby wetlands.
A line transect sampling method was employed for the observation of vulture species. This method is suitable for bird species that occur in low-density populations, have patchy distributions, inhabit open habitats, and are highly mobile and conspicuous (Bibby et al. Reference Bibby, Burgess, Hill and Mustoe2000). Vulture surveys were conducted in 13 different areas consisting of three habitat types. We conducted three transects per location each day, with each transect 1 km in length. Transects were visited twice daily for both seasons, making four visits per transect and habitat type over the entire study period.
Total survey days and distances for the entire study period were 26 days (13 for each season) and 208 km respectively. Two observers walked along existing trails or created one where possible with the aid of a Garmin GPS device (Garmin eTrex 10), and one person recorded the observations throughout the survey. In a day, 6 hours were spent recording all vultures summing up to about 156 hours for the entire study period. Vultures were counted from either side of the transect within a range of 0–500 m.
Vulture survey
Vulture surveys were carried out between August and November 2019 marking the wet season and between January and March 2020 representing the dry season in Ghana.
Vulture surveys were conducted during 06h30–09h30 and 14h30–17h30 in both wet and dry seasons. An average speed of 2 km/hour was maintained throughout the survey. Identification of vultures was aided by a pair of binoculars (Olivon 8 × 42) and the Field Guide to the Birds of Ghana (Borrow and Demey Reference Borrow and Demey2022). We recorded the activities (perched and/or feeding, and flying) for each individual, but the latter was not used in the analysis on habitat use, although these records were important for the total vulture inventory.
Combining features of the body and plumage (such as bill colour, shape and colour of the neck and throat, wing coloration and patterns, etc.), vultures were then aged into either adults or juveniles (Borrow and Demey Reference Borrow and Demey2022). About 98% of vultures encountered were aged confidently, and the number of individuals of each vulture species was recorded. Where there was uncertainty (about 2%), photographs were taken of specimens using a digital SLR camera (Nikon Camera D5600, focal length of 70–300 mm) and identification and confirmation were sought from expert ornithologists.
The survey was conducted on rain-free days when visibility was clear. All observations were conducted in the southern part of the park (about 1,700 km2 of the total land area) due to accessibility and logistical limitations. The coordinates of the locations where vultures were sighted across various habitats were recorded using a Garmin GPS device.
Data analysis
Observations from various vulture species were pooled to assess their abundance in the MNP, examining the influence of habitat, season, and age.
An initial Shapiro–Wilk test was conducted to check the normality of the response variable, abundance, and to decide the suitability of parametric vs non-parametric tests. Given the non-normal distribution of the data (Shapiro–Wilk normality test W = 0.59, P <0.05), we opted for generalised linear models (GLMs) with a Poisson distribution to perform the quantitative analysis.
Four models were developed to explore the factors affecting the abundance of four vulture species, with a consistent set of predictors, i.e. habitat type, season, and age. The habitats – woodland, riparian forest, and grassland – along with two seasonal categories, wet and dry, and age groups, adults and juveniles, were evaluated. Model 1 targeted African White-backed Vulture, Model 2 Hooded Vulture, Model 3 Palm-nut Vulture, and Model 4 White-headed Vulture.
To optimise the models for each vulture species, a stepwise deletion method was applied using the “stepAIC” function from the MASS package in R (Venables and Ripley Reference Venables and Ripley2002). This method systematically evaluated and removed non-significant predictors based on the Akaike information criterion (AIC) (Burnham and Anderson Reference Burnham and Anderson2004), continuing until only statistically significant predictors were retained. This ensured that the final models were both efficient and representative of the data.
We conducted a Wald test to assess whether there was a significant difference in the abundance of African White-backed Vultures between woodland and riparian forest. The test directly compared the coefficients from the GLM, as the initial model used grassland as the reference intercept. We also conducted Likelihood Ratio Tests (LRTs) to determine if season, age, and habitat significantly influenced the abundance of Palm-nut Vultures and White-backed Vultures by comparing models with and without these predictors.
Predictions were generated using the Poisson regression model, incorporating standard error calculations to estimate confidence intervals. The bar chart was constructed using the ggplot2 package in R (Wickham et al. Reference Wickham, Chang and Wickham2016).
Coordinates of vulture sightings recorded using the GPS were extracted using Basecamp and DNR Garmin software. We used QGIS version 3.4.4 (QGIS Development Team 2018) and ArcGIS version 10.6 (ESRI 2018) to map the distribution of vultures across the surveyed areas.
Data were analysed using R statistical software (version 4.2.2) (R Development Core Team 2020).
Results
Overview of vulture surveys
Overall, 466 individuals of four vulture species were counted in the MNP. These included 351 (75%) adults and 115 (25%) juveniles. There were more vultures in the dry season, 311 compared with 155 in the wet season (χ2 = 52.22, df = 1, P <0.05). Hooded Vulture had the highest number of individuals (n = 234), followed by White-backed Vulture (n = 189), Palm-nut Vulture (n = 22), and White-headed Vulture (n = 21). Abundance varied significantly among the four vulture species (Kruskal–Wallis, H = 21.80, df = 3, P <0.001).
Variations in the abundance of African White-backed Vultures relative to habitat type, season, and age
The final model retained all three predictor variables following the stepwise deletion process. Comparing dry season to wet season, the abundance increased by an estimate of 0.266 ± 0.153. This effect is approaching significance (z = 1.738, P = 0.082), indicating a potential increase in vulture numbers during dry seasons, although this result is not statistically significant. Adult vultures were significantly more abundant than juveniles across the studied habitats and seasons, with an estimated increase of 0.554 (± 0.173; z = 3.199, P = 0.001). Compared with grassland, the abundance in riparian forests is significantly higher by an estimate of 0.741 (± 0.322; z = 2.302, P = 0.021). Similarly, woodlands show a significantly higher abundance of vultures than grasslands, with an estimate of 0.805 (± 0.318; z = 2.528, P = 0.011) (Table 1). Additionally, woodlands have a slightly higher vulture abundance compared with riparian forests, with an estimated difference of 0.064, although this difference is not statistically significant (Wald χ2(1) = 0.176, P = 0.675).
Table 1. Parameter estimates from the Poisson regression model examining factors affecting the abundance of African White-backed Vultures Gyps africanus
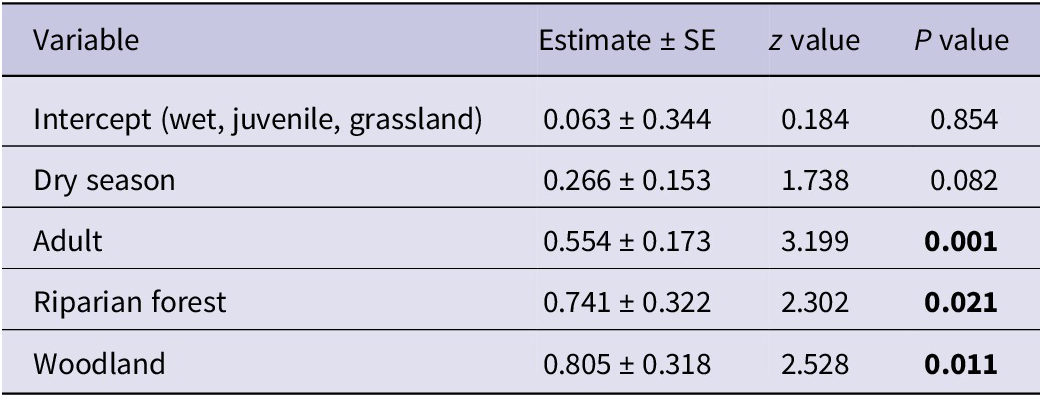
AIC = 286.13.
Variations in the abundance of Hooded Vultures relative to habitat type, season, and age
The final model included only age and habitat as variables, with juvenile age and grassland habitat serving as reference categories. As depicted, riparian forests and woodlands support higher numbers of Hooded Vultures compared with grasslands for both juveniles and adults. Notably, adult vultures were more common than juveniles in all habitats, although with a high variability (Figure 2). See Table 2 for full model details.
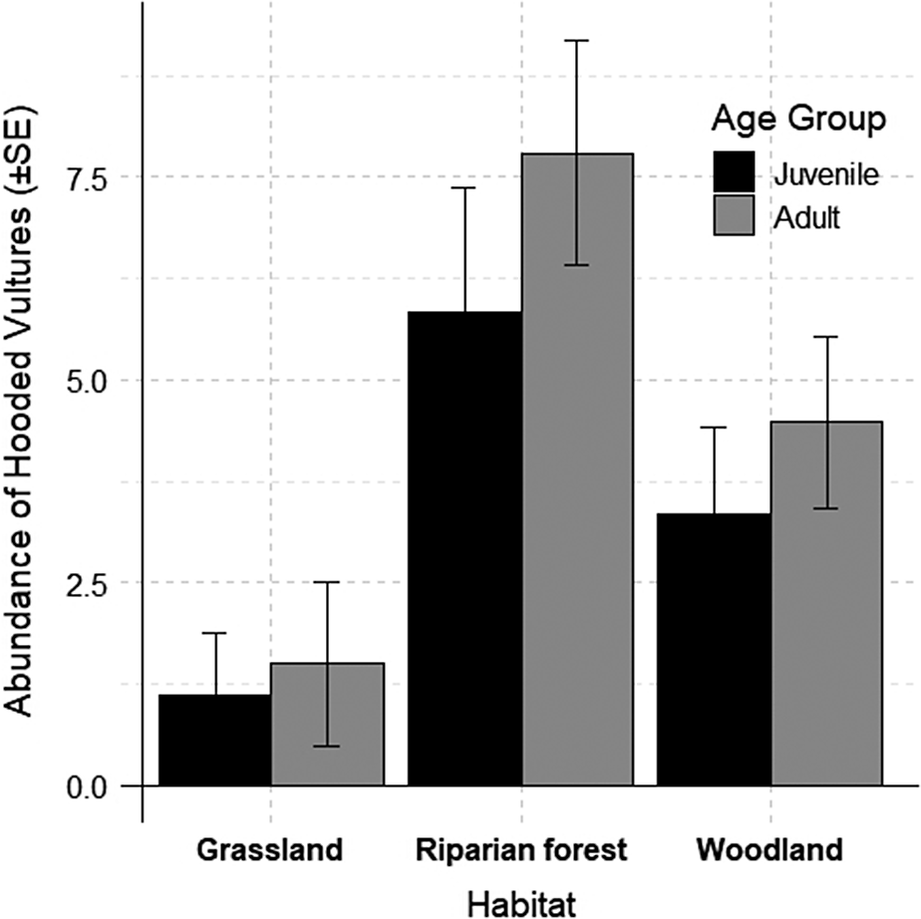
Figure 2. The observed abundance of Hooded Vultures Necrosyrtes monachus across different habitats and age groups. Error bars represent 95% confidence intervals.
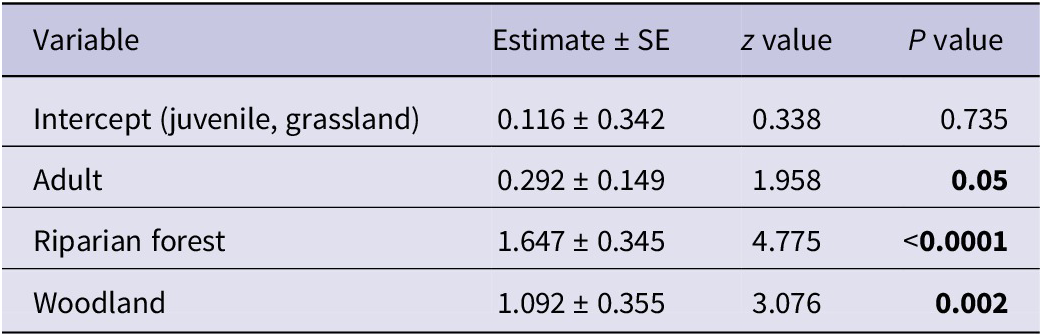
Table 2. Parameter estimates from the Poisson regression model examining factors affecting the abundance of Hooded Vultures Necrosyrtes monachus
AIC = 281.6.
Variations in the abundance of Palm-nut Vultures relative to habitat type, season, and age
Following a stepwise regression process to identify the most statistically significant predictors, the final model retained only the intercept. Significant differences in the abundance of Palm-nut Vultures were not detected for habitat, age, or season (LRT: χ2 (4) = 1.51, P = 0.825). See Table 3 for full model details.
Table 3. Parameter estimates from the Poisson regression model examining factors affecting the abundance of Palm-nut Vultures Gypohierax angolensis
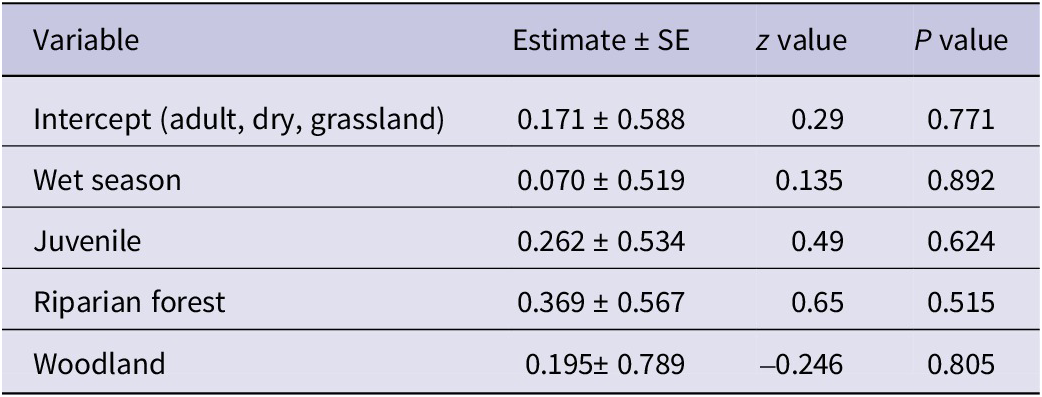
AIC = 45.315.
Variations in the abundance of White-headed Vultures relative to habitat type, season, and age
The analysis for White-headed Vultures showed an intercept estimate of 0.2719 (SE = 0.2182; z = 1.246, P = 0.213). As with Palm-nut Vultures, no significant differences in the abundance of White-headed Vultures were detected for habitat, age, or season (LRT: χ2 (4) = 0.67, P = 0.955). See Table 4 for full model details.
Table 4. Parameter estimates from the Poisson regression model examining factors affecting the abundance of White-headed Vultures Trigonoceps occipitalis
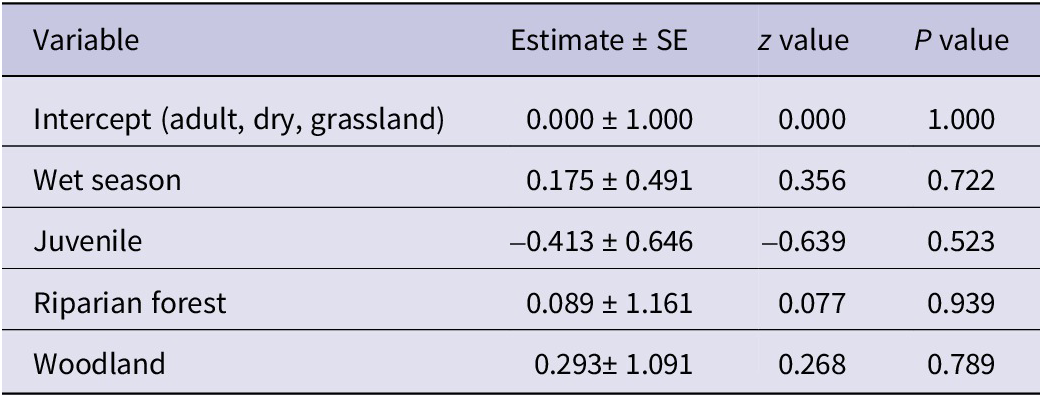
AIC = 46.839.
Distribution of vultures in the MNP
White-backed Vultures and Hooded Vultures were widely distributed across the study area, appearing in 12 out of the 13 survey areas for White-backed Vultures and 10 areas for Hooded Vultures. In contrast, White-headed Vultures were observed in seven areas, while Palm-nut Vultures were recorded in six areas. Figure 3 illustrates the distribution of vulture sightings across the surveyed areas through a heatmap plot.
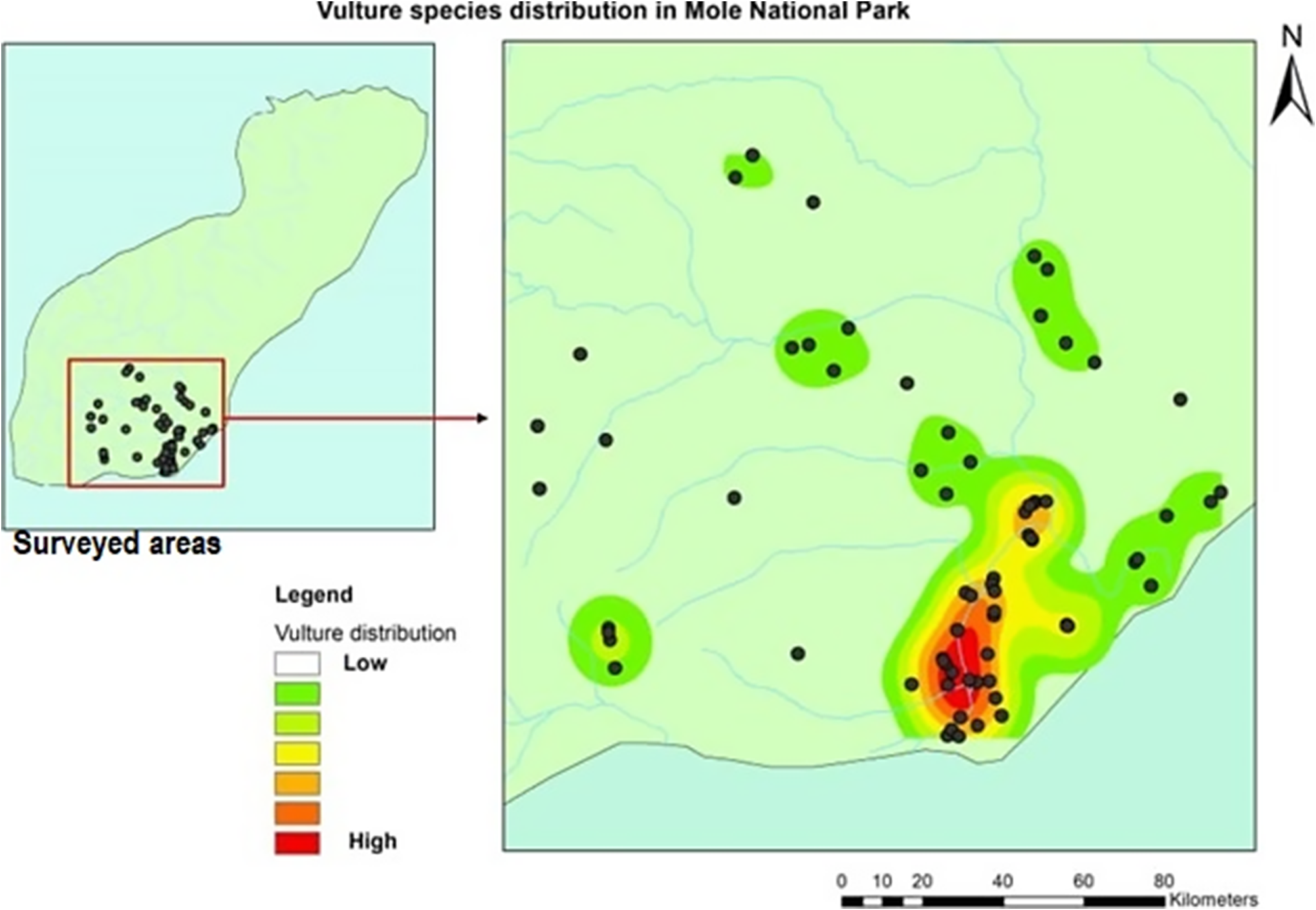
Figure 3. Heatmap patterns of the distribution of vultures with areas of high and low abundance across the survey areas in Mole National Park (MNP).
Discussion
This study sheds light on the population dynamics and species diversity of vultures in the MNP, exploring how their presence is influenced by habitat types, seasons, and age. Notably, the study documented four vulture species, three of which are “Critically Endangered”, i.e. Hooded Vulture, White-backed Vulture, and White-headed Vulture, and one, Palm-nut Vulture, is in the “Least Concern” category of the IUCN Red List of Threatened Species. Previous research in the MNP identified six vulture species, including Hooded, White-headed, Palm-nut, White-backed Vultures, Eurasian Griffon, and Rüppell’s Griffon (Di Vittorio and Petrozzi Reference Di Vittorio and Petrozzi2018), indicating the potential existence of other vulture species in the area. However, our survey did not encounter these additional species.
Population status and distribution of vultures in the MNP
Our survey detected 466 individuals in the MNP across four vulture species, reflecting the rich wildlife diversity within its boundaries. Among these, Hooded Vulture and White-backed Vulture were the most common and widely distributed species, while Palm-nut Vulture and White-headed Vulture were less frequently observed. These findings are consistent with earlier research (Barlow and Fulford Reference Barlow and Fulford2013; Di Vittorio and Petrozzi Reference Di Vittorio and Petrozzi2018; Henriques et al. Reference Henriques, Granadeiro, Monteiro, Nuno, Lecoq and Cardoso2018), which noted the dominance of Hooded Vultures and White-backed Vultures over other species in similar habitats in Gambia, Ghana, and Guinea-Bissau. Similarly, a recent survey conducted between 2020 and 2022 in the MNP corroborated our findings, indicating that Hooded Vultures and African White-backed Vultures were more common and widespread than White-headed Vultures (Goded et al. Reference Goded, Annorbah, Boissier, Rosamond, Yiadom and Kolani2023). In contrast, the João Vieira and Poilão Marine National Park in the Bijagós Archipelago of Guinea-Bissau, covering only 495 km², recorded substantially higher densities of Palm-nut Vultures compared with those observed in the MNP.
The differences in vulture numbers may be influenced by various factors such as habitat choice, behaviour, and conservation status. Hooded Vultures are adaptable scavengers found in many habitats. Their high numbers may be attributed to their generalist feeding behaviour, allowing them to exploit a wide range of food sources and thrive in diverse environments (Goded et al. Reference Goded, Annorbah, Boissier, Rosamond, Yiadom and Kolani2023; Kendall et al. Reference Kendall, Virani, Kirui, Thomsett and Githiru2012). Additionally, their ability to coexist with human populations (Mullié et al. Reference Mullié, Couzi, Diop, Piot, Peters and Reynaud2017; Mundy et al. Reference Mundy, Butchart, Ledger and Piper1992; Salewski Reference Salewski2021) and take advantage of anthropogenic food sources could also contribute to their abundance (Daboné et al. Reference Daboné, Buij, Oueda, Adjakpa, Guenda and Weesie2019). They have a broad distribution and efficient scavenging skills (Attwell Reference Attwell1963). White-backed Vultures, the next most common, are widespread in Africa (Grimes Reference Grimes1987). Their large roosts and social structure (Attwell Reference Attwell1963; Dowsett-Lemaire and Dowsett Reference Dowsett-Lemaire and Dowsett2014; Goded et al. Reference Goded, Annorbah, Boissier, Rosamond, Yiadom and Kolani2023) make them more visible during surveys (Kemp et al. Reference Kemp, Kirwan, Christie, Sharpe, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). For Palm-nut Vultures and White-headed Vultures, no significant differences in abundance were detected across the three habitats considered. This lack of habitat preference may be due to the low overall sample size, or the characterisation of habitats not being detailed enough to impact the number of vultures seen.
Variations in the abundance of African White-backed Vultures relative to habitat type, season, and age
Our study found a preference of White-backed Vultures for riparian forests and woodlands over grasslands. Riparian forests, with their proximity to water-bodies, attract diverse wildlife, providing reliable food sources for vultures. Additionally, White-backed Vultures are known to nest along riparian areas (Houston Reference Houston1976), which could account for their higher abundance in these habitats. Vultures are known to follow large carnivores to riparian zones where animals gather during the dry season, ensuring a supply of carrion (Attwell Reference Attwell1963; Selva et al. Reference Selva, Moleón, Sebastián-González, DeVault, Quaggiotto, Bailey, Olea, Mateo-Tomás and Sánchez-Zapata2019). Similar studies in the MNP found White-backed Vultures roosting close to water-bodies (Goded et al. Reference Goded, Annorbah, Boissier, Rosamond, Yiadom and Kolani2023), which aligns with our study and previous studies across Africa that emphasise the importance of riparian habitats for White-backed Vultures (Bamford et al. Reference Bamford, Monadjem and Hardy2009b; Johnson and Murn Reference Johnson and Murn2023; Peters et al. Reference Peters, Beale, Bracebridge, Mgumba and Kendall2022).
The dense vegetation of riparian forests may offer shade and protection from predators. While adult vultures have few natural predators due to their size and strength (Mundy et al. Reference Mundy, Butchart, Ledger and Piper1992), their chicks are often vulnerable to predation from birds and mammals (Johnson and Murn Reference Johnson and Murn2019; Marlow Reference Marlow1983; Mundy et al. Reference Mundy, Robertson, Komen and O’Connor1986; Tella and Manosa Reference Tella and Manosa1993).
Unexpectedly, we observed a higher abundance of White-backed Vultures in woodlands compared with grasslands. Grasslands generally have higher wildlife densities (Bhola et al. Reference Bhola, Ogutu, Piepho, Said, Reid and Hobbs2012; Georgiadis et al. Reference Georgiadis, Olwero, Ojwang and Romañach2007) and better carcass accessibility and visibility (Jachmann Reference Jachmann2002). The most likely reason for this observation is the timing of our surveys, which were primarily conducted in the early morning and late afternoon. During these times, vultures are more likely to be roosting than foraging, and woodlands would be the preferred habitats for roosting. Woodlands provide essential cover and ideal perch sites (Holland et al. Reference Holland, Byrne, Hepinstall-Cymerman, Bryan, DeVault and Rhodes2019), which are scarce in open grasslands.
Disparities in numbers between adults and juveniles could arise from low productivity among vultures (Ogada et al. Reference Ogada, Keesing and Virani2012). Vultures do not reproduce every year, they lay a single egg per breeding season, and each member of the pair makes a similar contribution to parental tasks (Bamford et al. Reference Bamford, Monadjem and Hardy2009b; Margalida and Bertran Reference Margalida and Bertran2000), leading to naturally higher proportions of adults within the population. Studies of White-backed Vulture populations in different areas have found similar patterns, where vultures lay only one egg per breeding season and invest heavily in parental activities (Houston Reference Houston1976; Johnson Reference Johnson2018; Yadav Ruby and Kanaujia Reference Yadav Ruby and Kanaujia2018). For instance, a study in Riba-Côa, north-eastern Portugal, found that 67.9% of Griffon Vultures were adults, compared with only 18.2% of juveniles (Van Beest et al. Reference Van Beest, Van Den Bremer, De Boer, Heitkönig and Monteiro2008). Similarly, the estimated adult population of Pyrenean Bearded Vultures Gypaetus barbatus outnumbered juveniles (Margalida et al. Reference Margalida, Jiménez, Martínez, Sesé, García‐Ferré and Llamas2020). In Saudi Arabia, adult and subadult Egyptian Vultures outnumbered immature and juvenile individuals (Shobrak et al. Reference Shobrak, Alasmari, Alqthami, Alqthami, Al-Otaibi and Zoubi2020). Other studies have documented higher mortality rates among juveniles. For example, predation on White-backed Vulture chicks by the ratel Mellivora capensis has been observed in the Kalahari Gemsbok National Park, located in the north-western Cape Province (Marlow Reference Marlow1983). Further studies are needed to fully understand the survival dynamics of these populations.
Variations in vulture numbers between adults and juveniles might also stem from juveniles’ larger home ranges. Younger birds may travel beyond the national park boundaries in search of food or to avoid competition within the park (Kane et al. Reference Kane, Monadjem, Aschenborn, Bildstein, Botha and Bracebridge2022; Mundy et al. Reference Mundy, Butchart, Ledger and Piper1992). Further research within the MNP is necessary to fully understand this variability. Immature vultures primarily focus on locating food, whereas adults have additional ecological needs beyond food (Milanesi et al. Reference Milanesi, Giraudo, Morand, Viterbi and Bogliani2016). Studies elsewhere show that immature White-backed Vultures often spend significant time outside protected areas (Kane et al. Reference Kane, Monadjem, Aschenborn, Bildstein, Botha and Bracebridge2022; Phipps et al. Reference Phipps, Willis, Wolter and Naidoo2013a).
Although our study observed an increase in White-backed Vulture abundance during the dry season, this trend was not statistically significant, suggesting that seasonal changes may have a less direct impact on their distribution than initially assumed. The lack of seasonal effect may suggest that the population of White-backed Vultures does not migrate in and out of the study area seasonally. Instead, it is likely that vultures move to different locations within the park where food and other resources are more abundant during various times of the year.
Variations in the abundance of Hooded Vultures relative to habitat type, season, and age
The results of the Poisson regression model offered valuable insights into the factors influencing Hooded Vulture population dynamics within the study area. Both habitat type and age significantly impact their abundance.
Riparian forests are particularly favourable for Hooded Vultures, showing a higher abundance compared with grasslands. This highlights the importance of riparian ecosystems in providing suitable foraging and roosting habitat. Notably, the highest numbers of Hooded Vultures were recorded at Dam 1 and 2 wetlands which held water frequently throughout the study period, showing the significance of wetlands to these vultures. Studies in the MNP have observed Hooded Vultures roosting close to water-bodies (Goded et al. Reference Goded, Annorbah, Boissier, Rosamond, Yiadom and Kolani2023). Observations during the study also noted Hooded Vultures feeding on shellfish along rivers and streams. Previous studies have reported similar behaviours, with Hooded Vultures feeding on jellyfish, squid, fly larvae in ghost crabs, and fiddler crabs Afruca tangeri along some beaches in The Gambia, Ghana, and Guinea-Bissau (Barlow Reference Barlow2004; Barlow et al. Reference Barlow, Mendy, Cryer and Dobbs2021). Higher vulture abundance in the southern region of the MNP, particularly near water sources, supports these findings (Goded et al. Reference Goded, Annorbah, Boissier, Rosamond, Yiadom and Kolani2023). Woodland habitats also positively correlate with Hooded Vulture abundance, although slightly less pronounced than that observed in riparian forests.
Age also influences Hooded Vulture abundance, with adults exhibiting higher numbers than juveniles. This observation may reflect differences in ranging behaviour. Studies tracking Hooded Vultures in South Africa, Botswana, Ethiopia, Kenya, The Gambia, and Mozambique revealed that immature vultures have larger home ranges than adults (Thompson et al. Reference Thompson, Barber, Bechard, Botha, Wolter and Neser2020). The lower numbers of juveniles may also be explained by their age distribution, as juvenile birds cover a smaller age range (1–5 or 6 years) compared with adults who can live into their 40s.
Comparing these results with those for African White-backed Vultures, it is evident that both species exhibit similar habitat preferences. Both species show a preference for riparian forests, suggesting shared ecological needs and responses to environmental variables. This similarity highlights how important riparian habitats are for protecting these vulture species.
Variations in the abundance of Palm-nut Vulture and White-headed Vulture relative to habitat type, season, and age
The lack of significant predictors other than the intercept suggests that both Palm-nut Vultures and White-headed Vultures may exhibit consistent ecological preferences within our study area. This implies that both species might possess adaptability to a range of environmental conditions present in the examined area. Such adaptability could indicate the ability of these vultures to thrive in diverse habitats and environmental settings within their respective ranges. Research conducted in the Marine National Park, located in the Bijagós Archipelago of Guinea-Bissau, found that the density of Palm-nut Vultures was influenced by seasonal changes, with higher counts observed in the dry season (Carneiro et al. Reference Carneiro, Henriques, Barbosa, Tchantchalam, Regalla and Patrício2017).
However, it is essential to note that these interpretations could also arise from limitations in our data set, such as small sample sizes or a lack of variability in the predictor variables we considered. These findings emphasise the importance of further research to better understand the factors influencing Palm-nut Vulture and White-headed Vulture abundance. Future studies could benefit from larger data sets or the inclusion of additional ecological variables that might have a more distinct impact on these vulture populations.
One potential limitation of our study is the relatively small size of our data sets, particularly for Palm-nut Vultures and White-headed Vultures. With limited data for these species, our analysis may not have fully captured all the factors influencing their abundance and habitat preferences. In addition, the timing of our surveys, which were conducted in the early mornings and late afternoons, may have biased our understanding of habitat use. Vultures are most active when thermals are available, generally from 09h00 to 15h00. As a result, our study mainly accounts for roosting vultures, reflecting habitat preferences for roosting or nesting rather than foraging. These limitations emphasise the need for larger, more comprehensive data sets and surveys conducted at various times of the day to provide a more accurate assessment of vulture populations and their habitat preferences.
Conclusions
Our study provides key insights into vulture populations in the MNP, highlighting the vital role of habitat conservation for species like the African White-backed Vulture and Hooded Vulture. We observed a higher abundance of vultures within the park, likely attributed to the rich concentration of wildlife. However, it is important to note that our study did not compare vulture abundance inside the park with areas outside, emphasising the need for future research in the study area. Both African White-backed Vulture and Hooded Vulture were concentrated within the riparian areas, likely due to the availability of roosting sites and potential food resources. The greater number of adult vultures compared with juveniles likely reflects their low reproduction rate and the larger home ranges of immature vultures, resulting in a higher proportion of adults in the population.
We recommend expanding this research throughout the MNP and its surrounding areas to gain a clearer understanding of population trends and habitat use, especially considering the declining vulture numbers in West Africa. Investigating home ranges and habitat preferences further is crucial. This approach will provide valuable insights and help identify threats to vultures within and around the park. To improve conservation efforts, park management should prioritise the protection of riparian forests and increase ranger patrols in these critical areas to protect these endangered species effectively.
Acknowledgements
We are grateful to the Leventis Foundation and the A.P. Leventis Ornithological Research Institute for sponsoring this study. We also thank the management of the MNP for their cooperation and for providing staff to ensure our safety during the surveys. Additionally, we appreciate the assistance of Ulf Ottosson, Dr Talatu Tende, and Dr Soladoye B. Iwajomo, lecturers from the A.P. Leventis Ornithological Research Institute, Nigeria, for their help with identifying some of the vulture species encountered during the study and for their other technical support. We are also thankful to Sulemana Abdulai and Joseph K. Afrifa for their technical assistance.
Appendix
Photographs of vulture species taken during the survey: (1–3) adult and juvenile Hooded Vulture Necrosyrtes monachus; (4–6) adult and juvenile White-backed Vulture Gyps africanus; (7 and 8) male and female White-headed Vulture Trigonoceps occipitalis; (9) juvenile White-headed Vulture; (10 and 11) adult Palm-nut Vulture Gypohierax angolensis; (12) immature Palm-nut Vulture.










