The use of algae for human consumption has been documented for hundreds of years in some cultures(Reference Nisizawa, Noda, Kikuchi and Watanabe1). More recently, and due to globalisation, patterns of consumption have changed around the world. For example, the adaptation of macrobiotic diets adopted from Japan by European countries produced the inclusion of marine vegetables in their diets. In 2003, it was estimated that about 1 million tons of algae were harvested in thirty-five countries as food, fuel and cosmetic sources(Reference Marsham, Graham and Tobin2).
In contrast, there are other tropical countries with a vast variety of algae species, with high growing rates, sometimes constituting a problem for ecological and tourism development, where algae consumption is not a regular practice. Paradoxically, in these geographical zones there are important nutritional and economic deficiencies, that the use of their own produced algae could help to ameliorate. The extraordinary nutritional composition of algae in terms of fibre, protein, minerals and vitamins, as well as their low-fat and non-digestible carbohydrate content, make algae a nutritive, low-energy food which represents an important food alternative(Reference Kuda, Tsunekawa, Goto and Araki3–Reference Rúperez7).
Algae are also being studied as a source of antioxidants(Reference Kuda, Tsunekawa, Goto and Araki3, Reference Nagai and Yukimoto8). Epidemiological data obtained in rodents showed the protective effect of red and green algae against intestinal, skin and breast cancer(Reference Funahashi, Imai and Tanaka9). One mechanism proposed to explain inhibition in carcinogenesis was mediated by an algae component that increased antioxidant enzyme activity and reduced lipid oxidation in the rat liver(Reference Yamamoto and Maruyama10).
In spite of the intense investigative effort and the recent knowledge generated in Fe metabolism, anaemia and Fe deficiency constitute important nutritional deficiencies worldwide(11). The most common approaches to combat these deficiencies are Fe supplementation and food fortification which require a highly available source of Fe. Nutritional education and diet changes to include foods with a high content of bioavailable Fe are also important approaches(12, 13).
The search for alternative, bioavailable and inexpensive sources of food Fe is desirable, especially in underdeveloped countries. Algae could be interesting candidates to explore as Fe sources, especially in countries where algae production is feasible. Since Fe bioavailability from algae has not been extensively studied, and due to the relationship between Fe bioavailability and polyphenol content in foods, as well as the role of these two nutrients in the generation and metabolism of antioxidants, the objective of the present work was to study Fe bioavailability, polyphenol content and antioxidant capacity from three species of marine algae (Ulva sp., Sargassum sp. and Porphyra sp.) distributed worldwide.
Materials and methods
Selection of algae for human consumption
Algae were collected by swimming and diving from four beaches in Margarita Island, Venezuela by trained personnel of Biotecmar CA. Material collected was classified and washed with sea water eliminating impurities such as sand, rocks, epiphytes and epifauna. Algae were sun-dried for 6 h and for further 12 h in the shade before they were packed, labelled and refrigerated at 4°C until transported to the laboratory.
After an initial screening for Fe content, three algae were selected for Fe bioavailability studies with maize and wheat breads. These species (Ulva sp., Sargassum sp. and Porphyra sp.) were washed by immersion in tap water for five times, followed by soaking for 30 min with a water change at 15 min. To eliminate excess of water, samples were placed on paper towels for 1 h and freeze dried to constant weight. Dried samples were used for total polyphenol content, reducing power and Fe bioavailability studies.
Total polyphenol content
Total phenolic compounds were determined by the Folin–Ciocalteu method(Reference Singleton and Rossi14). A sample of 200 μl was added to 1 ml 1:10 diluted Folin–Ciocalteu reagent. After incubation for 4 min, 800 μl of sodium carbonate (75 g/l) were added. Samples were incubated for 2 h at room temperature and absorbance measured at 750 nm. A standard curve was prepared with gallic acid (0–5 μg/ml) and results were reported as gallic acid equivalents/g sample. Algae samples were prepared by extracting 1 g samples with 5 ml 0·1 m-HCl for 1 h.
Antioxidant capacity in algae
Antioxidant capacity in algae was measured by the ‘ferric-reducing activity in plasma’ (FRAP) method, reported by Benzie & Strain in 1996(Reference Benzie and Strain15). Tripyridyltriazine reacts with ferric Fe to form a complex, which at low pH is reduced to ferrous Fe generating an intense blue colour absorbing at 593 nm. Briefly, 750 μl FRAP reagent containing tripyridyltriazine and ferric Fe in acetate buffer reacted with 25 μl of sample. The absorbance was registered immediately at 593 nm, and also after 4 min incubation. A standard curve was prepared with ferrous sulfate (100–200 μm) and results were reported as μmol Fe/100 g dry algae. Algae samples were prepared by extracting 2 g samples with 5 ml 0·1 m-HCl for 24 h.
Meal preparation
Six absorption studies were performed using three species of algae, administered with maize or wheat breads. In each absorption study each subject received four meals. In meal 1, bread was prepared without algae addition and meals 2–4 contained increasing amounts of algae. The first three studies were performed with Ulva sp., Sargassum sp. and Porphyra sp. administered with maize bread and the other three studies included the same algae administered with wheat bread.
All studies included a 110 g maize bread (prepared from 50 g non-enriched maize flour donated by Alimentos Polar Comercial, PROMESA, Venezuela) or a 100 g wheat bread (prepared from 50 g non-enriched wheat flour donated by Cargill de Venezuela), with 25 g cheese, 5 g margarine and a glass of water. Radioactive tagging (59Fe or 55Fe) was added to the water used to prepare the dough. In all studies, three different concentrations of each alga (Ulva sp., Sargassum sp. and Porphyra sp.) were administered to the same subject. In one study, the same subject received the basal meal without algae and 2·5, 5·0 or 7·5 g of each alga/bread in the other three meals. In studies 1 and 4, the alga studied was Ulva sp., in studies 2 and 5 Sargassum sp. and in studies 3 and 6, Porphyra sp. The amounts of algae included ranged 2·5–7·5 g algae/maize or wheat bread, since in preliminary experiments it was determined that the acceptance of the breads diminished when higher amounts of algae were included.
For maize experiments (studies 1–3):
Meal 1. Radioactive maize breakfast alone.
Meal 2. Radioactive maize breakfast with 2·5 g Ulva sp., Sargassum sp. or Porphyra sp.
Meal 3. Radioactive maize breakfast with 5·0 g Ulva sp., Sargassum sp. or Porphyra sp.
Meal 4. Radioactive maize breakfast with 7·5 g Ulva sp., Sargassum sp. or Porphyra sp.
Studies 4–6 were similar to studies 1–3 except that the basal breakfast consisted of wheat bread instead of maize bread.
Iron bioavailability studies
Fe absorption studies were performed in eighty-three adult volunteers (twenty-one men and sixty-two women) apparently in good health, although some of them presented mild to moderate Fe deficiency and/or anaemia. The subjects studied included males over 15 years of age, menopausal and child-bearing-age women. To the last group, a pregnancy test was performed. Each subject received four meals in each study and was allowed to participate in only one study. On day 1 of the experiment, pregnancy tests were performed and selected individuals were informed about the objectives and procedures of the study. A written consent form was signed by each volunteer. The study was approved by the Ethical Committee of the Venezuelan Institute for Scientific Research.
Meal 1, tagged with 59Fe (0·0333 MBq (0·9 μCi) per individual), was administered to the subjects after an overnight fast. Meal 2, also extrinsically labelled, but with 55Fe (0·0481 MBq (1·3 μCi) per individual), was fed 4 h later. No food or drink (except for water) was allowed between meals 1 and 2, and 4 h after administration of meal 2. The protocol for the administration of radioactive food in the morning after an overnight fast and the afternoon of the same day was based on experiments previously published(Reference Taylor, Martínez-Torres and Méndez-Castellano16).
On day 15, blood (30 ml) was drawn to determine the haematological profile (Hb concentration(Reference Crosby, Munn and Furth17), serum Fe(18), unsaturated binding capacity(19) and serum ferritin concentration(Reference Flowers, Kuizon, Beard, Skikne, Covell and Cook20)) and to measure radioactivity incorporation into erythrocytes. Duplicate (10 ml) and triplicate samples of radioactive food were prepared for radioactive counting using the technique of Dern & Hart(Reference Dern and Hart21, Reference Dern and Hart22). Fe absorption from each meal was calculated from the radioactivity in the subject's blood using an estimate of blood volume based on sex, weight and height(Reference Nadler, Hidalgo and Bloch23).
On the same day (day 15), meals 3 and 4 were administered following the same protocol as for meals 1 and 2. On day 30, a blood sample was taken to measure radioactivity incorporation and serum ferritin concentration.
Statistical analysis
Data analysis was based on comparisons (repeated-measures ANOVA with Bonferroni as a post-test) between absorption values for the four meals in each study. Mean values and standard deviations were calculated for all anthropometric and haematological measurements. Geometric values and standard errors were calculated for all absorption data and ferritin concentrations. The statistical analysis was performed using GraphPad InStat (GraphPad Software Inc., San Diego, CA, USA) and Excel (Microsoft Corporation, Redmond, WA, USA) programs.
Results
The total Fe content of non-enriched flours was 1·7 mg/100 g for maize flour and 2·4 mg/100 g for wheat flour. The mean Fe content of algae used in the bioavailability studies was 57·5 (sd 31·1) mg Fe/100 g dry weight for Ulva sp., 156·9 (sd 32·4) mg Fe/100 g dry weight for Sargassum sp. and 15·5 (sd 2·9) mg Fe/100 g dry weight for Porphyra sp. The algae were included in bread meals at 2·5, 5·0 and 7·5 g algae/bread. Accordingly, the Fe content of the maize meals administered ranged from 1·48 to 2·74 mg Fe for Ulva sp., 2·72 to 6·46 for Sargassum sp. and 0·98 to 1·25 for Porphyra sp. For wheat-containing meals, Fe content fluctuated between 1·83 and 3·09 mg Fe for Ulva sp., 3·07 and 6·81 for Sargassum sp. and 1·33 and 1·59 for Porphyra sp.
Total polyphenol content
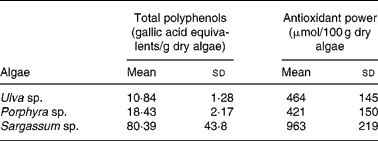
As shown in Table 1, the total polyphenol content was higher in Sargassum sp. compared with the other two species tested. The difference in gallic acid equivalents was up to seven and three times greater for Sargassum sp. than for Ulva sp. or Porphyra sp., respectively.
Table 1 Total polyphenol content and antioxidant power of acidic extracts from Ulva sp., Porphyra sp. and Sargassum sp. selected for human consumption* (Mean values and standard deviations)
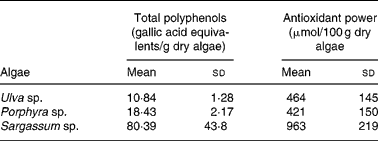
* Selected algae was washed and hydrated by immersion in distilled water for 30 min, as described in Materials and methods. Excess of water was eliminated by extending algae on absorbent paper for 1 h, and freeze-dried to constant weight. Extraction was performed with 0·1 m-HCl for 1 h at room temperature. Extracts were filtered and polyphenols were determined by the method of Singleton & Rossi(Reference Singleton and Rossi14). For the ‘ferric-reducing activity in plasma’ (FRAP) assay, algae were extracted in acid for 24 h (n 4–12).
Antioxidant capacity in algae
Antioxidant capacity expressed as μmol Fe/100 g dry algae showed that Sargassum sp. presented the highest reducing power, which was approximately double compared with Ulva sp. or Porphyra sp., with similar antioxidant capabilities (Table 1).
Meal preparation
The appearance of maize and wheat breads prepared with 2·5, 5·0 and 7·5 g of Sargassum sp., Ulva sp. and Porphyra sp. is shown in Figs. 1 and 2. In spite of the change in appearance as the algae content was increased, there was no problem for the subjects participating in the studies to accept and consume these products, coming back on two other occasions to finish the study.
Fig. 1 Maize bread (arepa) prepared with 2·5, 5·0 and 7·5 g of (a) Ulva sp., (b) Sargassum sp. and (c) Porphyra sp. selected for human consumption and Fe bioavailability experiments.

Fig. 2 Wheat bread prepared with 2·5, 5·0 and 7·5 g of (a) Ulva sp., (b) Sargassum sp. and (c) Porphyra sp. selected for human consumption and Fe bioavailability experiments.
Iron bioavailability studies
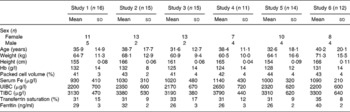
The anthropometrical and haematological characteristics of the subjects participating in all the absorption studies that were performed are presented in Table 2.
Table 2 Anthropometrical and haematological characteristics of subjects participating in iron absorption studies from preparations of algae with maize or wheat (Mean values and standard deviations)
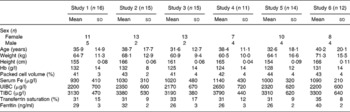
UIBC, unsaturated Fe-binding capacity; TIBC, total Fe-binding capacity.
Anthropometrical parameters were similar for all groups studied, including the higher number of female over male participants. Fe metabolism parameters were also similar among groups. Hb concentrations were comparable between groups and none of them could be classified as anaemic or Fe-deficient groups based on mean Hb and ferritin concentrations, respectively. However, when analysed subject by subject, in groups 1–6 it was found that five, zero, five, four, three and three individuals were anaemic, respectively.
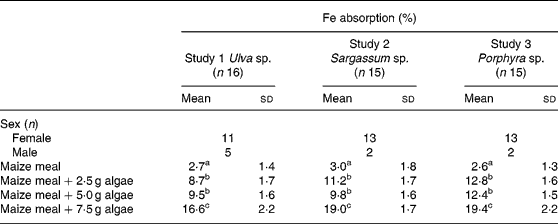
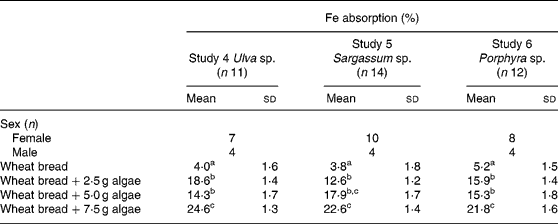
Fe bioavailability from maize bread containing different concentrations of algae (2·5, 5·0 and 7·5 g algae/maize bread) significantly increased Fe absorption compared with the maize bread given alone. In Table 3, it can also be noticed that the addition of any of the three species analysed (Ulva sp., Sargassum sp. or Porphyra sp.) was not statistically different between the 2·5 and 5·0 g doses. However, inclusion of 7·5 g portions of any of the algae tested significantly increased Fe absorption compared with 2·5 or 5·0 g portions.
Table 3 Iron absorption in human subjects from maize-based diets containing three concentrations of different species of marine algae (Ulva sp., Sargassum sp. and Porphyra sp.) (Mean values and standard deviations)
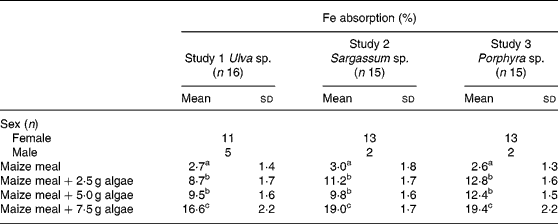
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
When calculated as doses of Fe absorbed from the maize-bread meals, based on the mean Fe content of each meal and their percentage of absorption, the amount of Fe absorbed was 0·13, 0·14 and 0·24 mg from Porphyra sp., 0·13, 0·20 and 0·45 mg from Ulva sp. and 0·30, 0·45 and 1·23 mg from Sargassum sp. for maize breads prepared with 2·5, 5·0 and 7·5 g algae/maize bread, respectively.
Fe absorption from the same algae, but administered with wheat bread, is shown in Table 4. The inclusion of the three algae studied, in any of the concentrations tested, significantly increased Fe absorption compared with the bread without algae addition. As observed with maize bread, the use of 2·5 or 5·0 g doses was not statistically different in terms of absorption. However, adding 7·5 g of any of the three algae (Ulva sp., Sargassum sp. or Porphyra sp.), significantly improved Fe absorption compared with the other two doses tested, except for the 5·0 g dose of Sargassum sp., in which absorption was no different from the bread containing 7·5 g of the same algae.
Table 4 Iron absorption in human subjects from wheat bread-based diets containing three concentrations of different species of marine algae (Ulva sp., Sargassum sp. and Porphyra sp.) (Mean values and standard deviations)
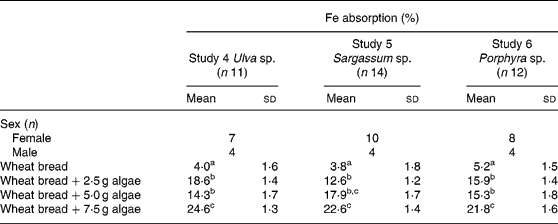
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
Experiments with wheat bread showed a similar percentage of absorption between the three algae tested, comparing the same doses. This is different from results obtained with rice(Reference Garcia-Casal, Pereira, Leets, Ramirez and Quiroga24) and maize bread, where absorptions, although without statistical significance, were higher for Porphyra sp. and Sargassum sp. and lower for Ulva sp.
When expressed as total dose of Fe absorbed from the wheat-bread meals containing algae, the Fe supplied was 0·21, 0·22 and 0·35 mg from Porphyra sp., 0·34, 0·35 and 0·76 mg from Ulva sp., and 0·39, 0·88 and 1·54 mg from Sargassum sp. It can be noticed that although the highest percentage of Fe absorption was achieved with Ulva sp., the highest Fe intake was obtained with Sargassum sp., due to its Fe content.
Discussion
Porphyra is one of the most consumed algae in Japan(Reference Nisizawa, Noda, Kikuchi and Watanabe1), while Ulva and Sargassum are widely consumed in Hawaii(Reference Abbott25). In Western countries algae consumption has been increasing for quite some time now. France uses at least ten species as food and condiments(Reference Mabeau and Fleurence26). In Mexico, Eucheuma isiforme is used as emulsifier in a local beverage called ‘atole’. In Chile the consumption of Durvillea antártica (cochanyuyo), Gracilariopsis and Ulva lactuca, fresh, cooked or canned, is common, while Ulva fasciata, Sargassum polyceratium, Sargassum partans and Turbinaria turbinata are common in Cuba. The most consumed algae in the Cariribbean is Gracilariopsis sp., especially as a refreshing drink know as ‘sea moss drink’(Reference Smith27, Reference Smith28).
On the other hand, algae overpopulation constitutes a serious problem in many regions of the world, but it is particularly severe in tropical regions were sun radiation and water temperature favour algae growth. The problem becomes more serious since these algae suffocate complete water bodies, becoming inactive due to the impossibility of light penetration and O2 depletion. There is not an economically feasible and simple way to control algae overgrowth in an environmentally friendly fashion. There are many reports and evidence about the excellent nutritional value of algae; therefore an alternative to control the massive growth in tropical regions could be their use as a source of nutrients for human or animal consumption.
It has been reported that some algae species contain important amounts of Fe, especially green algae such as the Ulva species that grow uncontrollably in some Caribbean coasts and are considered as waste for habitants of those regions(Reference Chapman and Chapman29). The chemical form of Fe contained in different algae has not been described, at least for the three species included in the present study. There are some interesting studies on Fe requirements in different soils, with organic and inorganic forms of added Fe, and like with earth vegetables, Fe content in algae varies according to geographical location and the time of year(Reference Garcia-Casal, Pereira, Leets, Ramirez and Quiroga24).
The three species tested in the present study showed a high Fe content, especially Sargassum sp. with 157 mg Fe/100 g dry weight. The present study also shows that this alga presented the highest content in polyphenols and antioxidant capacity, and as reported previously, also of ascorbic acid, reaching a concentration of 362·44 μg/g dry weight, compared with 37·8 in Ulva sp. and 232 in Porphyra (Reference Garcia-Casal, Pereira, Leets, Ramirez and Quiroga24).
Fe absorption from maize bread was significantly increased by the inclusion of algae in the amounts and species used in the present study. Of the doses tested, 7·5 g produced the highest Fe absorption, significantly different from the control bread but also from the breads prepared with 2·5 or 5·0 g of the same algae. The evaluation of the amount of Fe absorbed from each maize bread depending on the algae and the doses administered showed that Sargassum sp. not only increased the Fe content of the bread but also that the 7·5 g doses would cover daily Fe needs, achieving an absorption higher than 1 mg Fe. The absorption from maize breads prepared with 7·5 g of Ulva sp. or Porphyra sp. provided approximately 0·5 and 0·25 mg Fe in one serving.
Regarding wheat breads, absorption patterns were similar to the results with maize breads, except that percentages of absorption were higher from wheat. Absorption increased significantly by the addition of any of the three algae tested, and differences were not statistically significant between algae species, for the same doses. When results were calculated as net amount of Fe absorbed, it was noticed that 7·5 g of Sargassum sp. or Ulva sp. included in a wheat bread will provide 1·5 and 0·8 mg Fe, while, from a bread containing 7·5 g Porphyra sp., the Fe absorbed was approximately 0·4 mg. Trying to establish a relationship between algae consumption and anaemia, we analysed recent data on prevalence of anaemia provided by the WHO, in countries known by a high algae consumption(30). It was not possible to draw conclusions since some countries such as Chile, Japan and UK showed prevalences of about 8 % in adult women, while for the same age and sex group, anaemia prevalence was about 20 % in Peru and China, indicating that other factors in diet and living styles should be taken into account.
The antioxidant properties of marine algae have been investigated in recent years, by the identification and quantification of capacities and compounds such as polyphenols, Fe-reducing power and ascorbic acid content, among many others(Reference Jiménez-Escrig, Jiménez-Jiménez, Pulido and Saura-Calixto31–Reference Yuan and Walsh33). Cinnamic acid and phenolic compounds act additively and/or synergistically with carotenoids, ascorbic acid and Se probably by dismutating superoxide radicals, inactivating hydroxyl groups and chelating Fe(Reference Kuda, Tsunekawa, Goto and Araki3, Reference Vidal, Fallarero, Silva, De Oliveira, De Lima, Pavan, Vuorela and Mancini-Filho34, Reference Le Tutour, Benslimane, Gouleau, Gouygou, Saadan and Quemeneur35). There are other hydroxyl radical-scavenging products, such as dimethylsulfoniopropionate and dimethylsulfide, that have been described in algae(Reference Sunda, Kiebert, Kene and Huntsman36). In the present study, Sargassum sp. presented the highest polyphenol and ascorbic acid content of the algae tested (data for ascorbic acid not shown), and also the highest ferric-reducing capacity. Besides their effect as antioxidants, polyphenols and ascorbic acid have opposite effects on Fe absorption, which under the experimental conditions used in the present study resulted in a highly bioavailable Fe. Of the three species studied, Sargassum sp. presented the highest concentrations of Fe, polyphenols, antioxidant capacity and Fe bioavailability. The high net Fe absorption obtained from this alga was the result of a high availability but also of the high Fe content found in this alga. Ulva sp. and Porphyra sp. contained similar concentrations of polyphenols, antioxidant power and Fe absorption, but different Fe contents, which resulted in significant differences in doses of Fe absorbed.
The high polyphenol content found in Sargassum sp. could be partly responsible for the antioxidant power reported here, and apparently did not significantly affect Fe absorption, probably due to the high ascorbic acid content reported in this alga, which not only improves Fe absorption, but also further increases the antioxidant capacity.
The rapid growth of the population and poverty that affects many countries worldwide have produced important malnutrition problems which have increased the need and the search for new food alternatives. In Venezuela, for example, in spite of a rich and exuberant marine flora, there have been limited efforts for the study and use of this resource. The present study shows the possibility of including marine algae as staple foods or as food fortificants for Latin American populations, since the inclusion of 7·5 g of Sargassum sp., Ulva sp. or Porphyra sp., included in a maize or wheat bread, will cover between 50 to more than 100 % of the daily needs for Fe.
With the results shown in the present study and in a previous one using rice as a vehicle for algae consumption, it would be interesting to continue studies to develop an algae-containing product of massive consumption in a country or a complete region. This product would be delivered as a social programme, to increase Fe and ascorbic acid intakes and to combat Fe deficiency and anaemia.
Acknowledgements
The present study was partially supported by the Fondo Nacional para Ciencia y Tecnología (FONACIT project number 2000001345) and IVIC-Locti funding. Algae were supplied by Biotecmar CA, maize flour was donated by Alimentos Polar Comercial, PROMESA, Venezuela and wheat flour by Cargill de Venezuela. There are no conflicts of interest for the authors. M. N. G. C. did the experimental design, collection of data, analysis of data and writing the manuscript. A. C. P., I. L., J. R. and M. F. Q. did the sample processing, quantification and analysis. J. R. did the field work and sample acquisition.