Pregnancy has been suggested to be one of the causes for developing overweight and obesity in women(1–Reference Gunderson, Murtaugh and Lewis9). It is clear that obesity has a substantial adverse effect on health and various degenerative conditions including insulin resistance, hypertension, hyperuricaemia, atherosclerosis and cancer(Reference Scholl and Chen10, Reference Rooney, Schauberger and Mathiason11). Also, postpartum women almost universally desire to get back into shape after delivery. Factors associated with postpartum weight development were addressed by studies in Western populations but without conclusive results; overall it is surprisingly difficult to identify strong predictors for weight retention. Pregnancy-related weight gain has been suggested to be individual and multifaceted, with gestational weight gain, ethnicity, socio-economic status, smoking, parity and lactation, heritable characteristics, and changes in lifestyle factors, such as eating habits and physical activity, also being important contributors to postpartum weight development(Reference Linne, Barkeling and Rossner3, Reference Parker and Abrams5, Reference Harris, Ellison and Clement12, Reference Boardley, Sargent and Coker13). Considering practical recommendations, it has been difficult to give factual answers to postpartum women seeking weight control. From the viewpoint of early prevention of obesity, excess gestational weight gain has been suggested to probably play a significant role for weight retention in puerperium and needs to be considered in prenatal care education(Reference Keppel and Taffel14).
Therefore we attempted to investigate modifiable diet and lifestyle factors associated with pregnancy-related weight development by this prospective follow-up study from early pregnancy to 1 year postpartum. From our knowledge in the literature until now, relationships between dietary intake and weight development during and after pregnancy have not been examined in Asian women and not much information is available for influential factors regarding pregravid BMI, gestational weight gain, nutrition status and dietary intakes, parity, and lactation on postpartum weight development. The main purpose of the present prospective study was to examine dietary intakes, especially energy-related variables, and influences affecting weight development during and after pregnancy by participants living in an urban Asian city, Taipei, Taiwan.
Subjects and methods
The present study was a prospective study of women who participated in the longitudinal follow-up study of pregnant women and their newborn children. The eligible criteria included generally being in good health, over 20 years old, of Han ethnicity, in North Taiwan more than 10 years, and being less than 20 weeks pregnant with singleton gestations. We recruited pregnant women at the Taipei Municipal Women and Children Hospital between October and December 2002 at the obstetrical and gynecological (OBGYN) prenatal care clinics. For the eligible pregnant women, the participation rate of the recruitment was about 50 %. A total of 151 agreed to participate initially, with 51 % being of first pregnancy. At birth, fourteen women withdrew, and at 6 months and 12 months postpartum, there were a total of 130 and 122 subjects remaining, respectively. No severe pregnancy complications were reported from our interviews. We conducted face-to-face interviews by trained interviewers at the initial visit, at the last prenatal care visit and at 6 months postpartum to collect detailed family, lifestyle and dietary information including 24 h recalls, Chinese FFQ, weekly FFQ and also asked them to mail 3 d records back to us later.
The face-to-face interviews took 1–1·5 h to complete. After the initial interview, we conducted follow-up telephone interviews every month for about 20–30 min to collect information including a 24 h recall with lifestyle changes including physical activity until 1 year postpartum. For assessing physical activity levels, we asked questions for type of occupations, daily transportation and leisure activity during pregnancy and after delivery. After pooling these answers we categorised the subjects into low, medium or heavy, one of the three physical activity levels during pregnancy and postpartum. Postpartum data collection included a modified diet history (Chinese FFQ) and anthropometric measures at 6 months postpartum. Before and after 6 months postpartum, we collected monthly 24 h recalls and weight history until 1 year postpartum. We also collected information related to newborn care, growth and development and infant health by monthly telephone interviews after delivery.
The dietary information and nutrient intakes were coded by standard procedures and calculated by National Normal University Food System (NUFOOD) consisting of three interactive data management and calculation systems to process 24 h recalls, 3 d food records, weekly FFQ and Chinese FFQ(Reference Lyu, Chi and Huang15). In the present analysis, we used data from 24 h recalls and the Chinese FFQ. Because the nutrient densities per 4184 kJ (per 1000 kcal) from the Chinese FFQ during 6 months postpartum did not show any significant associations with weight retention, we did not include data of nutrient densities in the following analyses. The selective nutrients from 24 h recalls included in the analyses were total energy, protein, fat, carbohydrate, vitamin A, vitamin E, vitamin B1, vitamin B2, vitamin B12, vitamin C, folate, Na, K, Ca, Fe, dietary fibre, cholesterol and the polyunsaturated:saturated ratio. The average intakes for a total of 12 d of monthly 24 h recalls for each subject were calculated and used in the following analyses. Since the diet during the first month after delivery has been considered to be important and with a very different meal composition due to the local ‘doing the month’(Reference Chien, Huang and Hsu16) cultural practice, we reported the nutrient intakes separately. The correlation coefficient was 0·37 (P < 0·05) between daily energy intake in the first month and the average energy intake for the total 12 d. Moreover, energy intake per kg body weight was calculated as a measure of long-term habitual physical activity suggested by Sopko et al. (Reference Sopko, Jacobs and Taylor17).
Background and lifestyle factors regarding weight retention considered were maternal age, education level, family socio-economic status, family income, maternal height, physical activity levels, occupational physical activity, lactation status and parity. For statistical analyses, we used the software packages SPSS 11.0 (SPSS, Inc., Chicago, IL, USA) and STATA 8.0 (StataCorp LP, College Station, TX, USA). Testing for measures were reported to be significant by using P < 0·05. One-way ANOVA was performed to report the comparisons among subclasses. Bivariate and multivariate analyses were applied and results from normal transformation and non-parametric analyses were compared and reported. Multiple regression models by SPSS and generalised estimating equations by STATA were performed to estimate the magnitude of effects from various covariates. Specifically, generalised estimating equations by STATA were used to calculate the average weight of participating women with regards to the longitudinal time effect, with and without adjusting various covariates including age, pre-pregnancy BMI, gestational weight gain and parity. The generalised estimating equation method improved the calculation for longitudinal study in that individuals are measured repeatedly through time with a cluster method to solve the problem for time-dependent data(Reference Liang and Zeger18). The multiple regression models by SPSS were performed to examine the effects from variances which could predict 6-month and 1-year weight retention. The variations that could be explained by weight and energy-related dietary factors were calculated by general linear models.
Results
Table 1 shows the physical characteristics of the pregnant participants. The average maternal age was 30 years, the means of height and pre-pregnant body weight were 159·6 cm and 53·4 kg, respectively, with an average BMI of 21 kg/m2 for the total 130 study subjects. The average of nulliparous body weight was 51·5 kg, with 51 % of the participants being in their first pregnancies. These subjects represent a well-nourished, middle-class pregnant population with an average of 14 years of school education. The weight changes were from − 8 to 12 kg between pre-pregnancy and 6 months postpartum, with an average of 2·4 kg, and from − 12 to 21 kg at 12 months postpartum, with a mean value of 1·3 kg. The gestational weight gain was from 2·0 to 24·5 kg, with a mean of 14·1 kg. Figure 1 illustrates the four pre-pregnancy BMI subgroups ( < 20 kg/m2, 20–22 kg/m2, 22–24 kg/m2, >24 kg/m2) having parallel patterns from pre-pregnancy to 1 year postpartum, with average weight retentions of 1·2, 1·1, 0·1 and 3·1 kg, respectively. The maternal weight-change patterns by gestational weight-gain subgroups are shown in Fig. 2. The group of women gaining more than 17 kg during gestation had the highest average weight retention of 4·2 kg; the second highest was the subgroup gaining 14–17 kg, having an average weight retention of 1·3 kg. Interestingly, Fig. 2 also shows that the two subgroups with gestational weight gains over 17 kg and less than 10 kg had a higher body weight at 1 year postpartum compared with the three other subgroups of 10–12, 12–14 and 14–17 kg.
Table 1 Physical characteristics of study subjects
(Mean values, standard deviations and ranges)
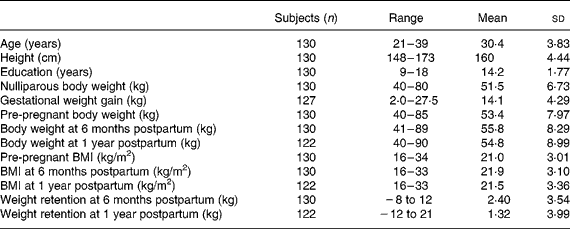

Fig. 1 Maternal weight changes by pre-pregnancy BMI subgroups: (–♦–), BMI ≤ 20 kg/m2; (–■–), BMI >20 to ≤ 22 kg/m2; (–▲–), BMI >22 to ≤ 24 kg/m2; (– × –), BMI >24 kg/m2.

Fig. 2 Maternal weight changes by gestational weight-gain (GWG) subgroups: (–♦–), GWG ≤ 10 kg; (–■–), GWG >10 to ≤ 12 kg; (–▲–), GWG >12 to ≤ 14 kg; (– × –), GWG >14 to ≤ 17 kg; (–![]() –), GWG >17 kg.
–), GWG >17 kg.
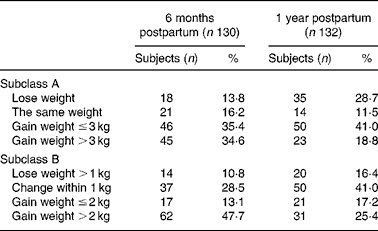
Figure 3 shows the maternal weight patterns from pre-pregnancy to 1 year postpartum by 1 year postpartum weight-retention subgroups. The subgroup gaining more than 2 kg after a year had moderate pre-pregnancy weight, but gained more weight during gestation. In addition, Fig. 3 suggests that pre-pregnant weights were not the important factors to determine the weight retention from the third trimester to 1 year postpartum; however, the crucial timing for weight retention over 2 kg at 1 year seems to be between 3 months and 6 months postpartum. Table 2 shows the frequencies of maternal retention distribution by two categorical subclass systems (the four groups for subclass A: loss weight, the same, under 3 kg and over 3 kg; subclass B: loss of weight over 1 kg, weight change within 1 kg, weight gain under 2 kg and over 2 kg). Subclass A demonstrates at both 6 months and 1 year postpartum that there were about 60–70 % of subjects remaining with some weight gain; however, the subclass B system suggests that 48 % of subjects retained more than 2 kg at 6 months postpartum, but decreased to 25 % at 1 year postpartum.

Fig. 3 Maternal weight changes by 1-year postpartum weight-retention (PPWR) subgroups: (–♦–), PPWR < 2 kg; (–■–), PPWR > − 2 to ≤ 2 kg; (–▲–), PPWR >2 kg.
Table 2 Frequencies of maternal weight retention at 6 months postpartum and 1 year postpartum for the 130 subjects
Since we collected dietary information every month, Table 3 shows the selective nutrient intakes calculated from 24 h recalls at 1 month, 6 months and 12 months and also the average intakes from the total 12 months. The sources of energy remained similar during and after delivery (pregnant data not shown): about 15 % from protein, 33 % from fat and 52 % from carbohydrate. The energy intakes per kg body weight were 134 kJ/kg (32 kcal/kg), 134 kJ/kg (32 kcal/kg) and 159 kJ/kg (38 kcal/kg) at 1 month, 6 months and 12 months, respectively, with an average of 142 kJ/kg (34 kcal/kg) during 12 months. The slight increases of many micronutrients including vitamin A, vitamin B1, vitamin B2, niacin, vitamin B6, cholesterol and polyunsaturated:saturated ratio at 1 month compared with at 6 months and 12 months were probably due to the ‘doing-the-month’ cultural practice after delivery(Reference Sopko, Jacobs and Taylor17) that would increase consumption of chicken and organ meats (kidney and liver) with sesame oil for this population. Because we did not find any correlation between nutrient densities per 4184 kJ (per 1000 kcal) calculated from FFQ with weight retentions, we used the average nutrient intakes by 24 h recalls in 12 months in our analyses.
Table 3 Selective nutrient intakes at 1 month, 6 months, 12 months and average during the first year of puerperium
(Mean values and standard deviations)
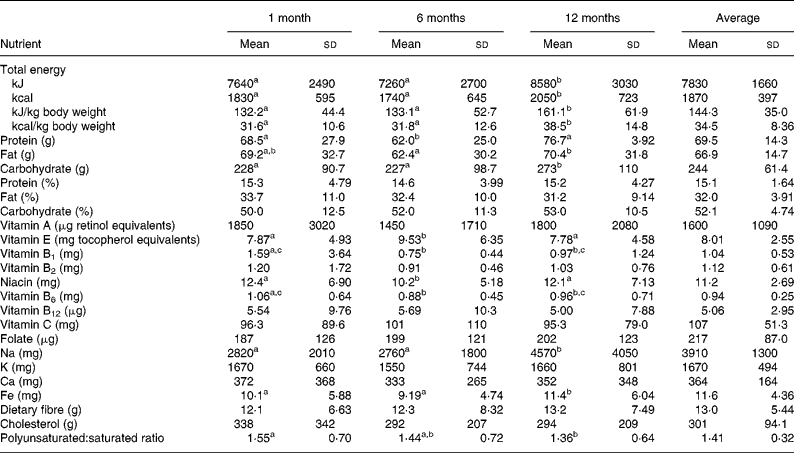
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
We performed both Spearman and Pearson correlation coefficients between weight retention and selective variables. For the skewed-to-the-left distributions of fat-related nutrients including fat, cholesterol and polyunsaturated:saturated ratio, vitamin A, vitamin E, vitamin B1 and vitamin B2, the transformed data showed similar results to non-parametric analyses; therefore we report the Spearman correlation coefficients in Table 4. The bivariate analyses showed that age and breast-feeding duration were not related to postpartum weight retention, but gestational weight gain had significant positive correlations (r 0·49 from Spearman and 0·54 from Pearson at 6 months; r 0·32 from Spearman and 0·44 from Pearson at 1 year postpartum; P < 0·05). Moreover, the total parity was surprisingly associated with weight retention negatively (P < 0·05). The total energy intake at 1 month postpartum (doing-the-month culture practice) was significantly associated with 6-month weight retention, but not 1-year weight retention. In addition, pre-pregnancy BMI and body weight were not related to postpartum weight retention both at 6 months and 1 year postpartum, with negative correlation coefficients (P>0·05). Together with evidence provided by Fig. 3, these data agree that pre-pregnancy BMI and pre-pregnancy weight had no association with 1-year weight retention.
Table 4 Spearman correlation coefficients of variables with 6-month and 1-year postpartum weight retention

Using generalised estimating equations, the average weight before pregnancy, at 6 months postpartum and at 1 year postpartum was 53·35 kg, 55·75 kg (weight retention 2·36 kg; P < 0·01) and 54·75 kg (weight retention 1·48 kg; P < 0·01), respectively (data not shown). After controlling for age, pre-pregnancy BMI, gestational weight gain and parity, we found at 6 months that the adjusted weight retention at postpartum was 0·79 kg (P < 0·01), but at 1 year was − 0·08 kg (P>0·05) (data not shown). Table 5 demonstrates various multivariate models to predict 6-month and 1-year postpartum weight retention variations. At 6 months postpartum, model 1 and 2 show that after adjusting for age, socio-economic status, breast-feeding, parity, physical activity and pre-pregnancy BMI, total gestational weight gain explains 28 % of variation (R 2 0·31 for model 1), and 1-month postpartum energy intake explains another 6 % of variation (R 2 0·37 for model 2). In addition, model 3 shows that after controlling for basic variables, pre-pregnancy BMI, gestational weight gain, postpartum average energy intakes and average energy consumption per kg (kJ/kg) could explain 54 % of variation for 6-month postpartum weight retention. For 1 year postpartum, with comparison with model 1, model 2 shows that 6-month weight retention explained 37 % of variation and dietary-related variables could explain an additional 15 % of variation by model 3. This model consisting of age, socio-economic status, breast-feeding, parity, physical activity, pre-pregnancy BMI, gestational weight gain, 1-month postpartum energy intakes, 6-month weight retention, postpartum average energy intakes and energy consumption per kg (kJ/kg) could explain 61 % of variation for 1-year postpartum weight retention. In general, dietary and weight-related variables shown in model 3 both at 6 months and 1 year postpartum could predict 50 to 60 % of postnatal weight changes.
Table 5 Multivariate regression models for predicting 6-month and 1-year postpartum weight retention

* P < 0·05, ** P < 0·01.
Discussion
In this Asian population, compared with pre-pregnancy, the 6-month and 1-year postpartum weight increased significantly by 2·36 and 1·48 kg. One study of 602 Taiwanese women, reported by Huang & Dai(Reference Huang and Dai19), with an average pre-pregnancy BMI of 21·5 kg/m2 showed that the average weight retention was 2·42 kg at 6 months postpartum. Surprisingly, the present study also showed a similar average weight retention of 1·5 kg increase at 1-year postpartum to those reported from a US population(Reference Olson, Strawderman and Hinton20) and a Swedish population(Reference Ohlin and Rossner4). After controlling for age, pre-pregnancy BMI, gestational weight gain and parity, the adjusted weight at 6 months postpartum increased significantly by 0·79 kg (P < 0·01), but not at 1 year postpartum by − 0·08 kg. These data are similar to a British study from London. Harris et al. in 1997 reported from a retrospective, repeat-pregnancy study for 243 mothers and showed no significantly long-term increase in mean maternal body weight following the first pregnancy after considering the ageing effect, and 70·8 % of mothers gained less than 1·0 kg before the next pregnancy(Reference Harris, Ellison and Holliday21). Our data show that 40 and 62 % of women gained weight less than 1 kg at 6 months and 1 year postpartum, respectively; but with 48 % of subjects gaining more than 2 kg at 6 months postpartum, and about 25 % at 1 year postpartum.
Regarding the influence of pregravid BMI, our bivariate analyses did not show significant relationships between pre-pregnancy BMI and postpartum weight retention both at 6 months and 1 year postpartum, with negative correlation coefficients. Moreover, Fig. 3 suggests that pre-pregnant weights were not the important factors to determine weight retention from the third trimester to 1 year postpartum and shows that the crucial timing for weight retention over 2 kg at 1 year seems to be between 3 months and 6 months postpartum. However, our descriptive data observed the subgroup with pre-pregnancy BMI over 24 kg/m2 to have the highest weight retention of 3·1 kg at 1 year postpartum compared with lower BMI subgroups. Because we followed these women only until 1 year postpartum with a small sample size, our data cannot demonstrate conclusive results. In the literatures, both studies from the UK(Reference Harris, Ellison and Holliday21) and USA(Reference Gunderson, Abrams and Selvin22) suggested that pre-pregnancy body weight may determine long-term weight gain for postpartum women(Reference Huang and Dai19). For example, the US study conducted in the San Francisco area with 985 healthy women from four race groups (Asian, Hispanic, black and white) found that early postpartum weight loss does not vary by maternal pregravid BMI group, but does vary on late postpartum weight change by a median of 2 years(Reference Gunderson, Abrams and Selvin22).
Gestational weight gain is the net effect of fetal growth, maternal organ adjustments and energy balance during pregnancy. The gestational weight gain in this Asian population with an average pre-pregnancy BMI of 21 kg/m2 was from 2·0 kg to 27·5 kg with an average of 14·1 kg. In the USA, the actual gestational weight gain increased from an average of 10 kg in the 1960 s to 15 kg by the late 1980s(Reference Gunderson and Abrams8). In a Brazilian cohort follow-up to 9 months postpartum of 405 women, the average pre-pregnancy BMI was 22·7 kg/m2, the average gestational weight gain being 13 kg (range from − 6 kg to 33 kg)(Reference Kac, Benicio and Velasquez-Melendez23). Even though our Asian urban population is relatively slender in pre-pregnancy, both cohorts showed a consistent strong positive association between gestational weight gain and postpartum weight retention. In addition, both cohorts showed the negative associations between pre-pregnancy BMI and postpartum weight retention, but our data were not statistically significant. Our data showed that at 6 months postpartum, gestational weight gain explained 28 % of variation for weight retention and 17 % at 1 year postpartum. One Taiwanese study demonstrated that the significant predictors of 6-month postpartum weight retention (gestational weight gain, perceived body-image satisfaction and pre-pregnancy weight) together explained 34·5 % of variation(Reference Huang and Dai19). Moreover, our multiple regression data were similar to a those of a large-sample (n 7116) US study; the American study showed that gestational weight gain in the first pregnancy alone explained 21 % of the variance in weight change between pregnancies(Reference Greene, Smiciklas-Wright and Scholl24). In addition, Rooney and colleagues reported from 795 Wisconsin women who were followed at 6 months, 4, 10 and 15 years after pregnancy and demonstrated that excess gestational weight gain and failure to lose weight after pregnancy were strong predictors of long-term obesity, and also diabetes, hypertension and dyslipidaemia(Reference Rooney, Schauberger and Mathiason11, Reference Rooney and Schauberger25). Overall, the present results agree with various studies from European and American populations that gestational weight gain is significantly associated with maternal postpartum weight development(Reference Lederman2, Reference Parker and Abrams5, Reference Scholl and Chen10, Reference Boardley, Sargent and Coker13, Reference Keppel and Taffel14, Reference Olson, Strawderman and Hinton20, Reference Harris, Ellison and Holliday21, Reference Kac, Benicio and Velasquez-Melendez23, Reference Greene, Smiciklas-Wright and Scholl24).
There were many agreements and disparities between our analyses and published information provided from Western populations. Parity was suggested to be an important determinant of female obesity(Reference Heliovaara and Aromaa26–Reference Wolfe, Sobal and Olson28). However, the present results showed that total parity had a negative relationship with weight retention (r − 0·23 at 6 months postpartum, r − 0·24 at 1 year postpartum; P < 0·05). From further analyses, total parity had a non-significant positive association (r 0·13; P>0·05) with pre-pregnancy weight and a non-significant negative association with non-gravid weight (r − 0·12; P>0·05). Therefore, the present results suggest that parity was a confounding factor to weight retention and could not clarify the relationship with long-term weight retention. The parity issue in this analysis is impossible to solve because we did not have proper comparison groups of women with various parity, about 50 % of our subject being in their first pregnancy. Furthermore, the Stockholm Pregnancy and Weight Development study (SPAWAN) has suggested that women who have increased considerably in weight during their first pregnancy or retained weight after delivery should be advised about obesity prevention(Reference Linne and Rossner29). The relationship between parity and maternal weight retention needs further clarification and might not be crucial for long-term weight development.
Although breast-feeding was not a strong predictor for postpartum weight retention by multiple regression models in our analyses, the bivariate results showed that breast-feeding over 6 months was positively related to 6-month postpartum weight retention, but not weight retention at 1 year postpartum. Olson et al. reported from a prospective cohort study of 540 women in upstate New York and found that breast-feeding, along with gestational weight gain, exercise frequency and change in food intake were significantly related to postpartum weight retention, but not major weight retention(Reference Olson, Strawderman and Hinton20). In 1997, Janney et al. (Reference Janney, Zhang and Sowers30) published a study from USA and indicated that the pattern of postpartum weight retention differed between lactating and non-lactating women and was affected by gestational weight gain, age and marital status(Reference Janney, Zhang and Sowers30). In contrast to the present results, they showed that breast-feeding women were more likely to achieve their pre-pregnancy weights at an earlier time in the postpartum period. These disagreements could be explained by the different definition for lactation status in different studies. In addition, Lederman in 2004 reviewed the influence of lactation on body weight and suggested that early in lactation, fat mobilisation is physiological and gradual, so breast-feeding might not be good for losing weight in the short term in well-nourished women(Reference Lederman31). However, a comparison study in the US state of Georgia evaluated maternal weight and percentage body fat changes in exclusively breast-feeding v. mixed feeding mothers during the first 12 weeks postpartum and found that exclusive breast-feeding promotes greater weight loss(Reference Hatsu, McDougald and Anderson32). Moreover, data from a cohort of 405 Brazilian women showed that each month of breast-feeding contributed − 0·44 kg to 9-month postpartum weight retention(Reference Kac, Benicio and Velasquez-Melendez33). Whether long-term lactation has a role in the prevention of postpartum obesity is controversial and an unanswered question for further studies.
In the literature, very few publications have documented the energy intakes from postpartum women(Reference Boardley, Sargent and Coker13, Reference Ohlin and Rossner34); also, few investigated exercise or physical activity(Reference Boardley, Sargent and Coker13, Reference Liang and Zeger18, Reference Lederman31). We reported that dietary and weight-related variables could predict 50 to 60 % of postnatal weight changes at 1 year postpartum. The multiple regression models agreed with the results from the Stockholm Pregnancy and Weight Development Study conducted in Sweden, which identified risk factors for postpartum weight retention. The research group reported in 1994 that the weight retention at 1 year postpartum was greater in women who increased their energy intake during and after pregnancy, increased snack eating after pregnancy and decreased lunch frequency. Women who had retained more than 5 kg at 1 year postpartum were more seldom physically active in their leisure time(Reference Ohlin and Rossner34). Oken et al. reported from a prospective cohort of 902 women in eastern Massachusetts, USA, that postpartum television viewing and walking were associated with 1-year postpartum weight retention and suggested that modifying these behaviours may help prevent obesity among women(Reference Oken, Taveras and Popoola35). In addition, analyses from the Cochrane database of six intervention trials suggested that dieting and exercise together appear to be more effective than diet alone at helping women to lose weight after childbirth(Reference Amorim, Linne and Lourenco36). These studies all support the suggestion that an active lifestyle and balanced energy and nutrient intakes are essential for weight control for postpartum women.
The strength of the present study was performing a longitudinal study with a natural lifestyle, observing and recording the dietary and related information, without interfering in the participants' lives. We also had a low rate for losing follow-ups. The 1-year postpartum weight history data are the first documented for Asians. However, there are weaknesses in the present study, including that the participants had a narrow distribution of education and socio-economic status in this volunteer population, and we also might not have had variant and wide-enough distributions for the obesity- and weight-related variables including BMI levels, dietary intakes and physical activity levels. Furthermore, we collected self-reported pre-pregnancy and postpartum weight data as in most observational studies(Reference Gunderson, Abrams and Selvin37–Reference Stevens-Simon, Roghmann and Mcnarney40). Also the precise data on gestational weight gain were impossible to obtain because the hospital only measured weights during prenatal care visits, as other researchers have noticed. Regardless of the mentioned methodology issue, the present study demonstrated that increased gestational weight gain is associated with increasing prevalence of overweight Asian women. Decreased energy intake and increased physical activity after delivery also are critical to minimise postpartum weight retention. Therefore, the present results suggest that pregnant women should be advised to control gestational weight gain, decrease energy intakes after child-bearing and maintain regular exercise in order to prevent postpartum obesity.
Acknowledgements
The present study was supported by the National Science Council (NSC) in Taiwan (grant no. NSC91-2320-B-003-003 and no. 92-2320-B-003-002).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the administration committee at Taipei Municipal Women and Children Hospital. Written informed consent was obtained from all subjects.
The contribution made by each author to the research is as follows: L.-C. L., study design and manuscript writing; C.-C. L., data collection and processing; H.-F. C., data collection and processing; C.-Y. W., data analyses; D.-M. L., subject recruitment.
There are no conflicts of interest.










