Vitamin D is a steroid hormone, which is either synthesised from pre-vitamin D in the skin during exposure to UV light or through dietary intake in terrestrial vertebrates( Reference Lips 1 ). Vitamin D has two major forms: 25-hydroxyvitamin D (25D3) and 1,25-dihydroxyvitamin D (1,25D3). 25D3 is the major storage form of vitamin D, which is catalysed by the enzyme 1α-hydroxylase (CYP27B1) to produce the main active metabolite 1,25D3( Reference Prosser and Jones 2 ). Vitamin D has been well-known for its role in bone mineralisation and Ca homoeostasis. Emerging evidence from basic research studies reveals that it also has an important role in regulating the immune system, including immune responses to bacterial infection in mammals( Reference Liu, Stenger and Tang 3 – Reference Thoma-Uszynski, Stenger and Takeuchi 5 ). Khoo et al. ( Reference Khoo, Chai and Koenen 6 ) reported that vitamin D3 down-regulated pro-inflammatory cytokine production induced by Mycobacterium tuberculosis in peripheral blood mononuclear cells (PBMC). Zhao et al. ( Reference Zhao, Yu and Mao 7 ) demonstrated that dietary vitamin D supplementation attenuates immune response of pigs challenged with rotavirus in pig. These results indicate that vitamin D mediates innate immune response. However, most research on the antibacterial action of vitamin D has been carried out in mammals, and there are limited studies in fish. To our knowledge, there is only one report on the involvement of vitamin D3 in the modulation of the fish immune system. Cerezuela et al. ( Reference Cerezuela, Cuesta and Meseguer 8 ) reported that diet supplementation with 0·94 mg/kg vitamin D3 for 2 or 4 weeks resulted in a significant increase in phagocytic ability and serum peroxidase content. Physiological studies have suggested that the vitamin D3 system in teleost is similar to that in other vertebrates( Reference Sundell, Bishop and Björnsson 9 – Reference Avioli, Sonn and Jo 11 ). However, the structure and form of the immune system is different between fish and mammals( Reference Press and Evensen 12 ). Whether vitamin D3 exerts protective effects against bacterial infection in fish is unclear.
The intestine is an important immune organ of fish( Reference Nakagawa, Sato and Gatlin 13 , Reference Rombout, Abelli and Picchietti 14 ). The intestinal epithelial cells are the first line of defence against pathogenic bacteria present in the lumen of the gut. Besides acting as a physical barrier, epithelial cells utilise a variety of innate immune mechanisms to reduce the risk of infection from invading foreign agents, including bacterial lipopolysaccharide (LPS)( Reference Pitman and Blumberg 15 , Reference Maaser and Kagnoff 16 ). The normal immune response of the intestine has been found to be correlated with its intestinal health in fish( Reference Zhao, Feng and Liu 17 , Reference Luo, Feng and Jiang 18 ). Pro-inflammatory cytokines have an important role in intestinal immunity( Reference Delcenserie, Martel and Lamoureux 19 ). To date, there is scarce information about the effect of vitamin D on intestinal immunity in fish. LPS is a cell wall component of gram-negative bacteria and a potent immunostimulant. Recently, LPS has been extensively used in studies of various aspects of induced immune responses in fish enterocytes( Reference Kawano, Haiduk and Schirmer 20 – Reference Jiang, Shi and Zhou 23 ). Mulder et al. ( Reference Mulder, Wadsworth and Secombes 22 ) reported that exposure to Aeromonas salmonicida induced the expression of TNF-α, IL-1β and IL-8 gene in the intestine of rainbow trout. Previous studies in our laboratory also demonstrated that LPS exposure improved TNF-α, IL-1β and IL-6 gene expression in the intestine of carp( Reference Jiang, Shi and Zhou 23 ). Therefore, we used LPS-induced inflammatory responses in isolated enterocytes and the intestine as a model to investigate vitamin D3’s anti-inflammatory effect in fish.
Toll-like receptor 4 (TLR4) is an important mediator of the host inflammatory response to infection. LPS induces the interaction of TLR4 with adaptor molecule myeloid differentiation primary response gene 88 (MyD88), which activates downstream mitogen-activated protein kinases (MAPK) and NF-κB signalling pathways and subsequently causes inflammatory cytokine production such as TNF-α, IL-1, IL-6 and IL-12( Reference O’Neill, Golenbock and Bowie 24 – Reference Kawai and Akira 26 ). Our previous study also demonstrated that the TLR4 signalling pathway could be activated by LPS exposure in the intestine of Jian carp( Reference Jiang, Shi and Zhou 23 ). Khoo et al. ( Reference Khoo, Chai and Koenen 6 ) reported that 1,25D3 modulated the balance in cytokine production towards an anti-inflammatory profile by repression of TLR4 expression in PMBC. Sadeghi et al. ( Reference Sadeghi, Wessner and Laggner 27 ) also reported that vitamin D3 down-regulated monocyte TLR4 expression. These facts suggest that vitamin D3 might influence the TLR4-Myd88 signalling pathway against LPS-induced inflammatory response in the intestine of fish, which warrants investigation.
The present study was conducted to investigate the effects of vitamin D3 on LPS-induced inflammatory responses in vivo and in vitro and to explore whether the anti-inflammatory effect is mediated through TLR4-Myd88 signalling pathways in this experiment.
Methods
Chemicals
LPS, 1,25D3, insulin, collagenase, dispase, transferrin, benzyl penicillin and streptomycin sulphate were purchased from Sigma. Hank’s balanced salt solution (HBSS) and fetal bovine serum (FBS) were purchased from Hyclone. 1,25D3 stock solutions of 10−3 m were prepared in 100 % dimethyl sulfoxide, and further dilutions were performed using Dulbecco’s modified Eagle’s medium (DMEM). All 1,25D3 working solutions were stored in Eppendorf tubes at −80°C. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was purchased from Promega Corporation.
In vitro experiments
Primary enterocyte culture
Cell isolation and culture were performed according to the methods of Jiang et al. ( Reference Jiang, Zheng and Zhou 28 , Reference Jiang, Liu and Jiang 29 ). Briefly, healthy Jian carps (56·78 (sem 2·8) g) were maintained for approximately 24 h without feeding before the experiment, and killed by decapitation. The intestines were rapidly separated from the carcass, opened and rinsed with HBSS-containing antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Cells were isolated by enzymatic dissociation using collagenase and dispase, followed by physical disaggregation. Then, cells were suspended in DMEM (containing 2 % d-sorbitol, S-DMEM) and washed with S-DMEM five times to remove any undigested material and single cells according to Booth and O’Shea( Reference Booth and O’Shea 30 ) with slight modifications. Isolated enterocytes were cultured in DMEM supplemented with 5 % FBS, 0·02 mg transferrin/ml, 0·01 mg insulin/ml and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Cultures were kept at 26 (sem 0·5)°C in twenty-four-well culture plates (Falcon) that had been coated with collagen I (Sigma), as previously described by Jiang et al. ( Reference Jiang, Kuang and Zhou 31 ). The cells were allowed to attach to plates for 72 h.
Lipopolysaccharide-induced cytotoxicity and inflammatory response in enterocytes
The cells were stimulated for 24 h with 10 mg LPS/l; this concentration was chosen because the previous experiment showed that 10 mg LPS/l of medium could induce inflammatory response in carp enterocytes( Reference Jiang, Shi and Zhou 23 ). Cell viability was quantified by MTS assay. Cytotoxicity was assessed by determining the release of lactate dehydrogenase (LDH) from enterocytes. The TNF-α and IL-1β mRNA expression levels were detected in cell lysates.
Prevention of lipopolysaccharide-induced inflammatory response by 1,25-dihydroxyvitamin D in enterocytes
To investigate the effect of 1,25D3 on cytokine levels in LPS-treated cells, enterocytes seeded into twenty-four-well plates were pre-treated with different concentrations of suggesting potential 1,25D3 for 72 h, and then cultured for 24 h with 10 mg LPS/l in a 27°C incubator. There were six groups (1,25D3 pre-treatment+LPS exposure): Ctrl+Ctrl (1,25D3 and LPS free), Ctrl+LPS (1,25D3 free+LPS), 1 pm-1,25D3+LPS, 10 pm-1,25D3+LPS, 100 pm-1,25D3+LPS and 200 pm-1,25D3+LPS. At the end of the experiment, media were collected to analyse LDH release. Cell lysates were collected to detect mRNA expressions of TNF-α, IL-1β, IL-6, IL-8, IL-10, TLR4, Myd88, NF-κBp65 and MAPKp38.
In vivo experiments
The Animal Care and Use Committee of Sichuan Agricultural University approved all experimental procedures.
Feeding trial
A total of 300 fish with an average initial weight of 12·58 (sem 0·23) g from the acclimatisation aquarium were randomly assigned into two groups of three replicates, each of sixty fish. The groups were respectively fed the Ctrl diet (non-supplemented vitamin D3) and the VD3 diet (supplemented 0·06 mg/kg vitamin D3) for 60 d. Experimental diets were formulated in our laboratory (Table 1). Vitamin D3 was added in the form of cholecalciferol. For this, vitamin D3 was first dissolved in ethanol in the appropriate doses and then dissolved in cod oil, which was sprayed on the pellets before feeding fish. The Ctrl diet was sprayed with cod oil only. Procedures for diet preparation and storage were the same as those described by Cerezuela et al. ( Reference Cerezuela, Cuesta and Meseguer 8 ). The experimental conditions were the same as in our previous study( Reference Yang, Zhou and Jiang 32 ).
Table 1 Feed formulation and proximate composition of the experimental diets (air-dry basis)
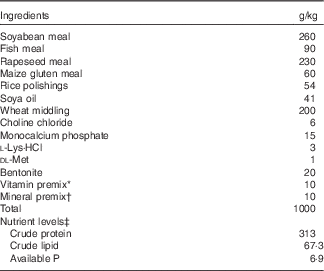
* The vitamin premix provides for per kg of diet: retinyl acetate 275 mg/g, 0·80 g; cholecalciferol 12·5 mg/g, 0 g for control and 0·48 g for VD3; dl-α-tocopherol acetate (50 %), 20·00 g; menadione (23 %), 0·22 g; cyanocobalamin (1 %), 0·10 g; d-biotin (2 %), 5 g; folic acid (96 %), 0·52 g; thiamine hydrochloride (90 %), 0·13 g; ascorhyl acetate (93 %), 7·16 g; niacin (99 %), 2·58 g; inositol (99 %), 52·33 g; calcium-d-pantothenate (98 %), 3·07 g; riboflavin (80 %), 0·99 g; pyridoxin (81 %), 0·75 g.
† Mineral premix provides for per kg of diet: ZnSO4·7H2O (22·5 % Zn), 21·64 g; MgSO4·H2O (15 % Mg), 230·67 g; FeSO4·7H2O (19·7 % Fe), 69·695 g; CuSO4.5H2O (25 % Cu), 1·201 g; MnSO4 H2O (31·8 % Mn), 3·774 g; KI (3·8 % I), 2·895 g; NaSeO3 (1 % Se), 2·50 g.
‡ Available P was calculated according to National Research Council (1993), whereas the others were measured according to the method of the Association of Official Analytical Chemists (1998).
Lipopolysaccharide exposure trial
After a 60 d feeding trial, the fish were weighed and collected for LPS exposure trial. There were three different groups: that is, control group (Ctrl/Ctrl), LPS exposure alone group (Ctrl/LPS) and VD3+LPS exposure group (VD3/LPS). There were thirty-six fish in each group, with three replicates per group and twelve fish per replicate. The fish of Ctrl/Ctrl and Ctrl/LPS groups came from the fish fed the Ctrl diet, and the fish of VD3/LPS group were from the fish fed VD3 diet in the feeding trial. Each fish of the Ctrl/Ctrl group was injected intraperitoneally with 100 μl of sterile PBS. Each fish of Ctrl/LPS and VD3/LPS groups was injected intraperitoneally with 100 μl of Escherichia coli LPS serotype 0111:B4 (3 mg of LPS/kg of fish) diluted in sterile PBS. The LPS concentration used in this study was according to our previous study, which has been proven to induce inflammatory response( Reference Jiang, Shi and Zhou 23 ). After 48 h of exposure, the intestines were quickly removed, frozen in liquid nitrogen and stored at −70°C for further analysis.
Analysis and measurement
Cell viability assays
After enterocytes were stimulated for 24 h with 10 mg LPS/l, cell viability was quantified using the CellTiter 96® AQueous One Solution cell proliferation assay kit. In brief, at the time of experimental termination, 40 µl of MTS working solution was added to each well. After incubation for 2 h at 27°C in a humidified atmosphere, the amount of formazan was estimated by optical density at 490 nm on a plate reader (Wellscan MK3; Labsystems).
Lactate dehydrogenase activity measurement
LPS-induced cytotoxicity was quantified by measuring the amounts of LDH released into the culture medium from injured cells( Reference Ahn, Hong and Park 33 , Reference Tang, Wu and Wu 34 ). The amount of LDH released was measured using the method of Mulier et al. ( Reference Mulier, Rahman and Watchorn 35 ).
RNA extraction and quantitative real-time PCR analysis
The RNA extraction and quantitative real-time PCR analysis were identical to those described in our previous study(
Reference Wu, Jiang and Liu
36
). Total RNA was isolated using RNAiso Plus (TaKaRa) followed by DNAse I treatment, and then 1 μg of total RNA was used to synthesise cDNA using the PrimeScript™ RT reagent Kit (TaKaRa). The RT products (cDNA) were stored at −80°C. Specific primers for the TNF-α, IL-1β, IL-6, IL-8, IL-10, TLR4, Myd88, NF-κBp65 and MAPKp38 genes were designed with Primer Premier software (Premier Biosoft International) based on the carp sequences (Table 2). The PCR mixture consisted of l μl of the first-strand cDNA sample, 0·5 μl of each of forward and reverse primers from 10 μm-stocks, 3 μl of RNase-free dH2O and 5 μl of 2×Ssofast EvaGreen Supermix (Bio-Rad). Cycling conditions were 98°C for 10 s, followed by forty cycles of 98°C for 5 s, annealing at a different temperature (Table 2) for each gene for 10 s and 72°C for 15 s. Target gene mRNA concentration was normalised to the mRNA concentration of the reference gene EF1а. After verification that the primers were amplified with an efficiency of approximately 100 %, the results were analysed using the
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
37
). Target and housekeeping gene amplification efficiencies were calculated according to the specific gene standard curves that were generated from 10-fold serial dilutions.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method(
Reference Livak and Schmittgen
37
). Target and housekeeping gene amplification efficiencies were calculated according to the specific gene standard curves that were generated from 10-fold serial dilutions.
Table 2 Primers and annealing temperature used for in real-time quantitative PCR

MAPK, mitogen-activated protein kinase; Myd88, myeloid differentiation primary response gene 88; TLR, Toll-like receptor 4.
Statistical analysis
Results are presented as means with their standard errors. Data were subjected to one-way ANOVA followed by the Duncan’s multiple-range test to determine significant differences among treatments using SPSS 13.0 (SPSS Inc.). A t test was used for comparisons between two groups. P<0·05 was considered to be statistically significant.
Results
Lipopolysaccharide-induced cytotoxicity and inflammatory response in enterocytes
To assess LPS-induced cytotoxicity in carp enterocytes, cells were incubated with 10 mg LPS/l. The cell viability and LDH activity were measured 24 h later. The result indicated that cells exposed to LPS resulted in a significant loss of viability (Fig. 1(a)). LDH release could be a good indicator of cellular damage. LPS exposure significantly increased LDH activity in medium (P<0·05) (Fig. 1(a)). The expression of TNF-α and IL-1β mRNA in enterocytes with LPS treatment was measured by RT-PCR (Fig. 1(b)). The results indicated that TNF-α and IL-1β mRNA levels were significantly increased by LPS exposure compared with the unexposed group (P<0·05).

Fig. 1 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) optical density (OD) and lactate dehydrogenase (LDH) release (a) and expression of TNF-α and IL-1β (b) in carp enterocytes in response to lipopolysaccharide (LPS) challenge. The primary cultured carp enterocytes were stimulated with 10 mg/l LPS for 24 h. Values are means (n 6) with their standard errors represented by vertical bars. *Mean values are significantly different (P<0·05). ![]() , TNF-α;
, TNF-α; ![]() , IL-1β.
, IL-1β.
Effect of 1,25-dihydroxyvitamin D on lipopolysaccharide-induced cytokine production in enterocytes
To determine whether 1,25D3 could exert an anti-inflammatory effect in vitro, we assessed the effect of 1,25D3 on LPS-induced inflammatory response by measuring TNF-α, IL-1β, IL-6, IL-8, and IL-10 mRNA expression in cells treated with LPS with or without 1,25D3. Cells with LPS alone resulted in significant increases in TNF-α, IL-1β, IL-6, IL-8 and IL-10 mRNA expression as compared with Ctrl/Ctrl treatment (P<0·05) (Table 3). Pre-treatment of enterocytes with 1,25D3 inhibited the LPS-induced TNF-α, IL-1β, IL-6 and IL-8 mRNA expression in a dose-dependent manner. Treatment with 10–200 pm-1,25D3 led to a statistically significant decrease in TNF-α, IL-1β and IL-6 mRNA expression when compared with Ctrl/LPS (P<0·05) (Table 3). The addition of 1,25D3 (100–200 pm) to cells significantly down-regulated IL-8 mRNA expression (P<0·05) (Table 3). In contrast, the IL-10 mRNA expression was increased significantly at 200 pm-1,25D3 pre-treatment (P<0·05) (Table 3).
Table 3 mRNA expression of TNF-α, IL-1β, IL-6, IL-8 and IL-10 in carp enterocytes in response to 1,25-dihydroxyvitamin D (1,25D3) and lipopolysaccharide (LPS) challengeFootnote * (Mean values with their standard errors; n 6)
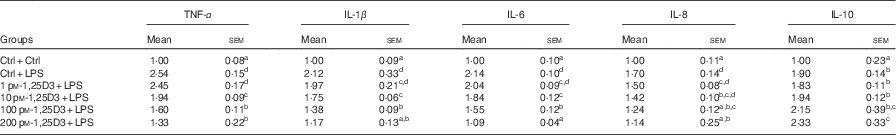
* The cells were pre-treated with different concentrations (0, 1, 10, 100, 200 pm) of 1,25D3 for 72 h before stimulation with 10 mg/l LPS for 24 h.
a,b,c,dValues with unlike letters within the same columns are statistically different (P<0·05).
Effects of 1,25-dihydroxyvitamin D on Toll-like receptor 4-myeloid differentiation primary response gene 88 signalling pathways in lipopolysaccharide-stimulated enterocytes
The present results have shown 1,25D3 to have anti-inflammatory effect in carp enterocytes. We determined whether the involvement of TLR4-Myd88 signalling pathways in 1,25D3-mediated inhibition of pro-inflammatory cytokine. The regulation of TLR4, Myd88, NF-κBp65 and MAPKp38 mRNA expression during LPS exposure with and without 1,25D3 treatment was investigated in carp enterocytes (Table 4). As shown, TLR4, Myd88, NF-κBp65 and MAPKp38 mRNA expression increased markedly after 24 h of stimulation with LPS (P<0·05) and 1,25D3 markedly inhibited LPS-induced TLR4, Myd88 and NF-κBp65 mRNA expression (P<0·05). However, the addition of 1,25D3 did not alter MAPKp38 mRNA levels (P>0·05).
Table 4 mRNA expression of Toll-like receptor 4 (TLR4), myeloid differentiation primary response gene 88 (Myd88), NF-κB p65 and mitogen-activated protein kinases (MAPKp38) in carp enterocytes in response to 1,25-dihydroxyvitamin D (1,25D3) and lipopolysaccharide (LPS) challengeFootnote * (Mean values with their standard errors; n 6)
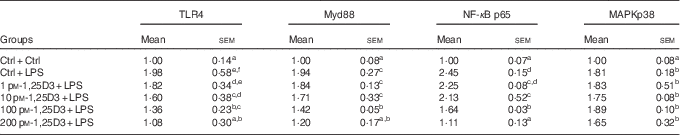
* The cells were pre-treated with different concentrations (0, 1, 10, 100, 200 pm) of 1,25D3 for 72 h before stimulation with 10 mg/l LPS for 24 h.
a,b,c,d,e,fValues with unlike letters within the same column were statistically different (P<0 05).
Vitamin D3 decreases lipopolysaccharide-induced cytokine production in vivo
Dietary vitamin D3 supplements administered for 60 d significantly increased the growth of carp when compared with the Ctrl group; the final weight was 48·8 (sem 1·9) v. 42·1 (sem 1·2) g (P<0·05). The effects of dietary supplementation with vitamin D3 on TNF-α, IL-1β, IL-6, IL-8 and IL-10 gene transcript abundance in the intestine of juvenile Jian carp after LPS exposure are presented in Fig. 2. The result indicated that the expression levels of the TNF-α, IL-1β, IL-6, IL-8 and IL-10 genes were increased by LPS exposure alone compared with the unexposed control group (P<0·05). Vitamin D3 pre-supplementation significantly depressed the TNF-α, IL-1β, IL-6 and IL-8 mRNA levels (P<0·05). Fish exposed to LPS showed an increase in IL-10 mRNA expression of intestine as compared with the Ctrl/Ctrl group (P<0·05). IL-10 mRNA expression in fish pre-feeding with vitamin D3 was significantly up-regulated (P<0·05).

Fig. 2 Expression of TNF-α, IL-1β, IL-6, IL-8, IL-10, Toll-like receptor 4 (TLR4), myeloid differentiation primary response gene 88 (Myd88), NF-κB p65 and mitogen-activated protein kinases (MAPKp38) mRNA in the intestine of juvenile Jian carp fed diets containing different vitamin D3 (VD3) levels for 60 d, followed by exposure to 3 mg lipopolysaccharide (LPS)/kg of fish for 2 d. Values are means (n 6) with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P<0·05). ![]() , TNF-α;
, TNF-α; ![]() , IL-1β;
, IL-1β; ![]() , IL-6;
, IL-6; ![]() , IL-8;
, IL-8; ![]() , IL-10;
, IL-10; ![]() , TLR4;
, TLR4; ![]() , MyD88;
, MyD88; ![]() , NF-κB p65;
, NF-κB p65; ![]() , MAPKp38.
, MAPKp38.
The effects of dietary supplementation with vitamin D3 on TLR4, Myd88, NF-κBp65 and MAPKp38 mRNA expression in the intestine of fish following LPS exposure are shown in Fig. 2. The results showed that, compared with Ctrl/Ctrl treatment, Ctrl/LPS caused a significant increase in TLR4, Myd88, NF-κBp65 and MAPKp38 mRNA expression levels in the intestine (P<0·05). Pre-feeding with vitamin D3 significantly prevented the up-regulation of TLR4, Myd88 and NF-κBp65 mRNA expression in the intestine. However, pre-feeding with vitamin D3 did not affect MAPKp38 mRNA expression compared with the results obtained from the Ctrl/LPS group.
Discussion
To our knowledge, the current study is the first evidence to demonstrate that vitamin D could inhibit LPS-induced inflammatory responses in enterocytes in vitro and in fish intestine in vivo. To study the anti-inflammatory effect of vitamin D against LPS-induced inflammatory cells, we first induced inflammatory response in carp intestinal epithelial cells. LPS is a major component of the cell wall of gram-negative bacteria implicated in the pathogenesis of bacterial infection, which is widely used as a toxicant to establish in vitro models of inflammatory response-induced injury in fish( Reference Jiang, Shi and Zhou 23 , Reference Teles, MacKenzie and Boltana 38 ). The cytotoxic effect has been assessed by markers such as cell viability and LDH release( Reference Legrand, Bour and Jacob 39 , Reference Fotakis and Timbrell 40 ). The present results demonstrated that exposure to LPS (10 mg/l) alone significantly increased LDH levels in the medium, indicating severe enterocyte damage. A colorimetric assay using the dye MTS can rapidly quantify the cell viability of European eel (Anguilla anguilla L.) PBMC( Reference Roland, Kestemont and Hénuset 41 , Reference Pierrard, Roland and Kestemont 42 ). Using this assay, the present study showed that cell viability was depressed by LPS exposure. This result was in good agreement with our previous report( Reference Jiang, Shi and Zhou 23 ). Cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, have a fundamental role in the regulation of the pro-inflammatory response in fish, having a pivotal role throughout the infection process( Reference Komatsu, Tsutsui and Hino 21 ). In the current study, the exposure of carp enterocytes to LPS caused a significant increase in IL-1β, TNF-α, IL-6 and IL-8 mRNA levels, indicating a stimulatory action upon pro-inflammatory processes. Therefore, to induce inflammatory response in carp enterocytes, cells were incubated for 24 h with 10 mg LPS/l.
In fish, protection of the digestive tract against pathogenic attack is crucial for maintaining health, as a large number of pathogenic microorganisms invade through its surface( Reference Ringø, Løvmo and Kristiansen 43 ). TNF-α and IL-1β are two of the most important pro-inflammatory cytokines; their inappropriate expression or overexpression can lead to the progression of inflammatory and autoimmune diseases( Reference Chiu and Yang 44 ). As a principal cytokine, IL-1β is a strong regulator of the expression of other cytokines, such as IL-6 and IL-8( Reference Brandolini, Bertini and Bizzarri 45 , Reference Ogilvie, Hack and Wagstaff 46 ). The present result clearly demonstrated that the expression levels of TNF-α, IL-1β, IL-6 and IL-8 were up-regulated in enterocytes in response to LPS exposure. 1,25D3 pre-treatment markedly inhibited LPS-induced up-regulation of TNF-α, IL-1β, IL-6 and IL-8 mRNA levels in enterocytes. This may indicate that 1,25D3 has a potential role in the inhibition of intestinal inflammation induced by LPS. Previously, 1,25D3 was demonstrated to inhibit pro-inflammatory cytokines in human corneal epithelial cells colonised with Pseudomonas aeruginosa ( Reference Xue, Zhu and Thakur 47 ). Our results are consistent with these reports. IL-10 is a pleiotropic cytokine with significant anti-inflammatory properties, which is a key regulator in the maintenance of immunological homoeostasis( Reference Fu, Ye and Lee 48 ). The present study showed that 1,25D3 treatment significantly up-regulated the expression of IL-10 mRNA. These results suggest that 1,25D3 could attenuate the intestinal inflammatory response in fish. To date, no study has been conducted to investigate the effect of 1,25D3 on the inflammatory cytokines gene expression in the intestine of fish.
TLR4 is a member of the TLR family of pattern recognition receptors that specifically mediates signalling by LPS. Classically, TLR4 recognises the microbial lipids in homodimer format, and thus activates various intracellular signalling pathways, such as the NF-κB and MAPK pathways( Reference Chow, Young and Golenbock 49 , Reference Medvedev, Kopydlowski and Vogel 50 ). Recently, a TLR4 sequence has been identified experimentally in Chinese rare minnow, and even two TLR4 genes were found in the zebra fish genome( Reference Su, Yang and Xiong 51 , Reference Jault, Pichon and Chluba 52 ). MacKenzie and Milston reported that teleost fish also display LPS responsiveness( Reference MacKenzie, Planas and Goetz 53 – Reference Milston, Vella and Crippen 55 ). Su et al. ( Reference Su, Yang and Xiong 51 ) demonstrated that the TLR4 signalling pathway can be triggered by grass carp reovirus and Aeromonas hydrophila infection in rare minnow. Thus, it is possible that piscine TLR4 gene was already implicated in LPS sensing. To clarify the cellular mechanisms that regulated the cytokine production after LPS exposure, we examined the effect of 1,25D3 pre-treatment of carp enterocytes on LPS-induced MyD88-dependent signalling. The MAPKp38 and NF-κBp65 are the family members of MAPK and NF-κB, respectively, and they are the main signalling molecules in the TLR4-Myd88 signalling pathway of their family( Reference Hsieh, Frink and Thobe 56 – Reference Yang, Zhou and Yang 58 ). Over-activation of this signalling pathway would aggravate inflammatory reaction exacerbating their negative effects on the fish. Our data indicate that LPS exposure up-regulated the expression of TLR4, Myd88, MAPKp38 and NF-κBp65 mRNA in enterocytes. Pre-treatment with 1,25D3 inhibited the up-regulation of TLR4, Myd88 and NF-κBp65 mRNA levels. Interestingly, 1,25D3 pre-treatment did not alter MAPKp38 mRNA expression. Studies in various cell types, including dendritic cells( Reference Dong, Craig and Xing 59 – Reference D’Ambrosio, Cippitelli and Cocciolo 61 ), pancreatic islet cells( Reference Giarratana, Penna and Amuchastegui 62 ) and kidney cells( Reference Deb, Chen and Zhang 63 ), indicated that vitamin D dampens NF-κB signalling. Our observations are in accordance with those reports, but how vitamin D3 interacts with the TLR4 signalling pathway is unknown. However, several mechanisms have been proposed, including a vitamin D-induced increase in the levels of IκBα ( Reference Sun, Kong and Duan 64 ), interference with the binding of NF-κB subunits to promoter regulatory areas( Reference D’Ambrosio, Cippitelli and Cocciolo 61 ) or both.
On the basis of the beneficial effects of 1,25D3 against LPS-induced inflammatory response in the enterocytes, it was reasonable to hypothesise that vitamin D can protect fish against LPS-induced inflammatory responses in vivo. The present study showed that inflammation induced by intraperitoneal injection of 3 mg LPS/kg fish was associated with increased expression of TNF-α, IL-1β, IL-6 and IL-8 mRNAs in the intestine. A previous study has looked at the effects of LPS on the immune system in fish and has demonstrated a high potential for mediating pro-inflammatory cytokine mRNA abundance( Reference Boltaña, Tridico and Teles 65 ). Vitamin D3 pre-supplementation decreased TLR4, Myd88 and NF-κBp65 mRNA expression. The results presented suggest that impaired inflammatory response to LPS in fish is, at least in part, because of TLR4 down-regulation. As TLR4 is a key component in pathogen (LPS) recognition and crucial mediators in the early inflammatory response to foreign microorganisms, down-regulation of TLR4 by vitamin D3 clearly represents an important and novel immune-modulating effect. This result was in agreement with this statement in vitro. Studies from monocytes also indicated that vitamin D3 downregulates TLR4 expression and triggers hyporesponsiveness to pathogen-associated molecular patterns( Reference Sadeghi, Wessner and Laggner 27 ). However, the mechanisms await further characterisation.
In conclusion, our present study demonstrated that LPS exposure could induce inflammatory response, resulting in up-regulation of TNF-α, IL-1β, IL-6 and IL-8 mRNA abundance in the intestine and in the enterocytes of fish. Dietary and medium pre-supplementation with vitamin D3 could inhibit LPS-induced immune damage in fish intestine and the enterocytes, respectively. The anti-inflammatory effect of vitamin D3 may associate with decreasing the expression of pro-inflammatory cytokines by downregulating TLR4, Myd88 and NF-κBp65 mRNA abundance.
Acknowledgements
The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
This study was financially supported by the Youth Foundation Program of the Education Department of Sichuan Province, China (grant number 14ZB0021) and the Applied Basic Research Programs of Science and Technology Commission Foundation of Sichuan Province, China (grant number 2015JY0067).
J. J. and D. S. conducted the trial, performed the RT-PCR experiments, and wrote the manuscript. Y. Z. and X. Z. contributed to the design of the study. L. F. and W. J. assisted in the manuscript preparation. Y. L. and L. T. assisted with all data analysis. L. Y. and P. W. assisted with the trail.
There are no conflicts of interest to disclose.









