1. Introduction
Among the group II elements, magnesium (Mg) is the only efficient species used as p-type dopant in MOVPE grown GaN Reference Nakamura, Senoh and Mukai[1] Reference Goetz, Kern, Chen, Liu, Steigerwald and Fletcher[2] . Incorporation of Mg in GaN is typically achieved via bis-cyclopentadienyl magnesium Cp2Mg Reference Sheu, Su, Chi, Pong, Chen, Huang and Chen[3] Reference Kozodoy, Xing, DenBaars, Mishra, Saxler, Perrin, Elhamri and Mitchel[4]. The alternative precursor bis-methylcyclopentadienyl magnesium, (MeCp)2Mg, was in comparison less employed Reference Blaauw, Bruce, Miner, Howards, Emmerstorfer and Springthorpe[5] Reference Beaumont, Vaille, Lorenzini, Gibart, Boufaden and el Jani[6] Reference Haffouz, Beaumont, Leroux, Laugt, Lorenzini, Gibart and Hubert-Pfalzgraf[7] although it offers advantages over the Cp2Mg source because of its lower melting point and higher vapour pressure.
In this article, we report on a comparative study of Mg incorporation in GaN, using Solution Cp2Mg and (MeCp)2Mg. A solution of the solid Cp2Mg source was employed to increase transport efficiency and reliability of delivery for this precursor.
2. Experimental
The Mg concentration in GaN was measured by SIMS (CAMECA IMS4F) using a O2 + primary ion beam. Calibration was made with a Mg-implanted standard of known magnesium content. The sample's structure consisted of a 1.5 µm thick undoped GaN deposited on sapphire (0001), a thin insulating layer of AlN (80 nm), and a 0.8 µm Mg-doped GaN. The growth process was carried out in a home-made vertical reactor operating at 30 KPa Reference Vennegues, Beaumont, Haffouz, Vaille and Gibart[8].The growth temperature was 1090°C. Trimethylgallium (TMG), and either Solution Cp2Mg or (MeCp)2Mg, manufactured by Epichem, were used as the Ga and Mg sources. These elements were transported with a N2 carrier gas at a flow rate of 2 slm. A mixture of NH3 (2 slm) and N2 (2 slm) was introduced in the reactor by a separate inlet. The V/III ratio was 2200. Mg-doped GaN films were grown with a constant TMG flow rate of 40 µmol/min, while the Mg sources flow rates were varied between 80 nmol/min and 0.4 µmol/min. These values were determined from the equilibrium vapour pressures and from the input flux of hydrogen flowing through the metalorganic bubblers. The temperature of the bubblers was kept at 0°C for TMG and 26°C for the Mg sources.
The growth chamber was equipped with a laser (HeNe) reflectometry setup. As the film growth proceeds, constructive and destructive interferences of the reflected light across the film occur periodically. The growth rate G was measured by following the periodic variation of the reflected light intensity, according to:
where λ is the laser wavelength (632.8 nm), n is the refractive index of GaN at the growth temperature, and τ is the oscillation period (difference between extrema of two fringes).
3. Results and Discussions
Figure 1 shows the variation of the atomic fraction of magnesium [Mg]/[Ga] ([Ga] = 4.4 1022 cm−3) in the lattice as a function of the flux ratio ϕMg/ϕTMG in the vapour phase. [Mg] is the Mg concentration determined by SIMS and the precursor fluxes ϕMg and ϕTMG are given in mol/min. It turns out that the curves follow a linear trend, with the slopes yielding a dopant incorporation efficiency of 0.13 for the (MeCp)2Mg and 0.26 for the Cp2Mg. This two fold difference in the incorporation efficiency may have a variety of origins:
-
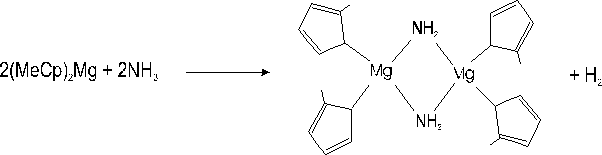
i) The occurrence of a parasitic reaction between NH3 and (MeCp)2Mg in the reactor. It has been reported elsewhere Reference Chang, Lec, Her, Lec, Peng and Wang[9] that amines Et2NH strongly interact (nucleophilic attack) with the Mg atoms located near methyl groups of aluminium-magnesium complexes. These reactions yield solid compounds at room temperature in which Et2N acts as an assembling ligand with Mg. A similar reaction could occur between NH3 and (MeCp)2Mg according to :

-
i) We had already noticed Reference Beaumont, Vaille, Lorenzini, Gibart, Boufaden and el Jani[6] Reference Haffouz, Beaumont, Leroux, Laugt, Lorenzini, Gibart and Hubert-Pfalzgraf[7] that, under large (MeCp)2Mg fluxes (> 4 µmol/min) and at atmospheric pressure, i.e at low gas velocity, a parasitic reaction with NH3 occurred, leading to the formation of solid particles, evidenced by a bright diffuse emission visible along the path of the laser beam used for reflectivity measurements. Although we do not observe such a phenomenon in our present experiments, owing to the low pressure and low Mg fluxes used, we cannot discard the possibility that a certain amount of (MeCp)2Mg gas reacts with NH3 to form particles, however undetectable on the laser beam pathway.
It is interesting to note that, under the growth conditions of references Reference Beaumont, Vaille, Lorenzini, Gibart, Boufaden and el Jani[6] Reference Haffouz, Beaumont, Leroux, Laugt, Lorenzini, Gibart and Hubert-Pfalzgraf[7], where a parasitic reaction is clearly evidenced, the dopant incorporation efficiency was only 10−2.
-
ii) The (MeCp)2Mg should form a stronger adduct with NH3 due to the extra donating power of the methyl group. This adduct could be more volatile than the equivalent Cp2Mg adduct due to the effect of the Me group causing a more random structure and less chance of molecular interactions. Early works on amine complexes of dialkylmagnesiums Reference Zakharkin[10] have demonstrated the volatile character of such compounds. An increased volatility would enhance the desorption of Mg from the surface and thus decrease the amount available for incorporation in the growing layer.
-
iii) Alternatively, the more bulky (MeCp)2Mg adduct may also have a weaker binding energy to the surface and again be desorbed more easily than Cp2Mg.

Figure 1. Atomic fraction of Mg in GaN as a function of the Mg flux ratio in the gas phase, using Solution Cp2Mg or (MeCp)2Mg as as dopant sources. The slope gives the dopant incorporation efficiency.
Each of the three hypotheses described above has the consequence of reducing the effective Mg surface concentration and thus the incorporation efficiency.
An other interesting feature is the variation of the growth rate of GaN in the presence of Mg precursors in the vapour phase. This behaviour is illustrated in Figure 2. The growth rate decreases linearly with increasing Mg fluxes. This trend is observed for both Mg precursors but is more pronounced for the Cp2Mg. At the growth temperature of 1090°C, the TMG and NH3 undergo full dissociation to Ga and N atoms at the surface. The growth rate results from the balance between the incorporation of the Ga and N atoms into the bulk crystal and from the desorption of atoms from the surface. Under N-rich conditions (high V/III ratio), the growth rate is proportional to the Ga surface coverage, as the Ga atoms binding with the excess of N are readily incorporated into the lattice. The decrease of the growth rate with increasing Mg fluxes can be explained by the decrease of the Ga surface coverage due to the competing capture of Mg atoms into chemisorbed III sites. The difference in the growth rate between (MeCp)2Mg and Cp2Mg (Figure 2) can again be attributed to a reduced surface concentration of MeCpMg adduct and a resulting lower incorporation rate of Mg on III sites. Our explanation, based on the competition for III sites occupancy between Mg and Ga, implies that the growth rate depends only on the MgIII concentration and thus on Mg in the crystal lattice, regardless of the Mg precursor source used. This is verified in Figure 3, where the growth rate is found to follow a linear relationship with the bulk Mg concentration, as measured by SIMS. This curve can provide a useful estimate of the Mg content in the film, from the growth rate measured by reflectometry.

Figure 2. Dependence of the Mg flux ratio on the growth rate G. G was measured by reflectometry.

Figure 3. Growth rate as a function of Mg concentration, measured by SIMS. We find a unique linear relationship, regardless of the Mg precursor used.
4. Conclusion
In conclusion, we have observed a two fold lower Mg incorporation efficiency using the (MeCp)2Mg source instead of Solution Cp2Mg. The obvious difference between both sources is the presence of methyl groups, which can strongly affect interactions with NH3, leading to increased precipitate formation in the growth chamber, or by forming more volatile Mg adducts at the surface. These effects tend to reduce the effective Mg surface concentration. The decrease of the growth rate with the addition of Mg indicates that Mg incorporates at the surface on III sites in place of the Ga atoms. Since the surface concentration of Mg adducts is lower for (MeCp)2Mg, the amount present for incorporation to the growing layer is reduced, and consequently the growth rate dependence with the Mg flux in Figure 2 is less pronounced.
This study was supported by the European Contract RAINBOW BRPR-CT96-0340