Accurate measurement of food and energy intake (EI) is the basis of studies that focus on associations between diet and health( Reference Livingstone and Black 1 ). Dietary assessment on its own is associated with several concerns regarding validity, mostly because it relies on self-report and/or proxy-reported measurements( Reference Willett 2 ). No gold standard for the evaluation of reported dietary intake exists( Reference Lafay, Basdevant and Charles 3 ); many diet studies are faced with the reporting of implausible EI in both directions, under-reporting (UR) and over-reporting (OV)( Reference Livingstone and Black 1 ), a widely acknowledged limitation of dietary assessment methods.
EI misreporting is characterised by reports of habitual EI, which are implausibly low or high, that is, UR or OV, respectively, when compared with the energy requirements estimated using objective methods of energy expenditure, such as the doubly labelled water (DLW) technique or prediction equations( Reference Mendez 4 ). This implies the presence of systematic bias and differential misreporting in dietary intake assessments, which may attenuate, or even reverse, the directions of the associations under study( Reference Lioret, Touvier and Balin 5 ). This problem may also lead to an inadequate estimation of the prevalence of nutrient deficiencies. For that reason, intentional dietary misreporting represents a major concern in studies that monitor dietary intake at the population level and/or evaluate diet–health associations( Reference Macdiarmid and Blundell 6 , Reference Nielsen and Adair 7 ).
Identification of misreporting in any of its forms, that is, UR and/or OV, and its characteristics is thus crucial to the appropriate interpretation of nutritional data( Reference Lafay, Basdevant and Charles 3 ). Different studies have investigated the factors associated with intentional misreporting; however, a consensus is still lacking for various reasons( Reference Lioret, Touvier and Balin 5 ). BMI in particular has been repeatedly linked to misreporting in adults( Reference Livingstone and Black 1 ), but also among children and adolescents( Reference Lioret, Touvier and Balin 5 ). Other factors found to be associated with EI misreporting include age, sex, socio-economic status (SES), health consciousness, proxy-reporting, cultural variations and psychological differences( Reference Livingstone and Black 1 ).
As with adults, inaccurate energy reporting also occurs among young populations( Reference Lioret, Touvier and Balin 5 , Reference Börnhorst, Huybrechts and Ahrens 8 ). Adolescents, unlike children, have full cognitive capability to provide self-reported dietary data; however, adolescence is characterised by increasingly greater food requirements, unstructured eating patterns, rapidly changing food habits and more frequent out-of-home eating( Reference Livingstone and Robson 9 ). These factors, along with a possibly reduced level of interest to recall their own intake, might lead to less motivation, forgetfulness and lack of compliance, and thus to a reduced reporting accuracy( Reference Livingstone and Robson 9 ).
As has been the case for similar surveys, the Healthy Lifestyle in Europe by Nutrition in Adolescence Cross-Sectional Study (HELENA-CSS) is also susceptible to EI misreporting( Reference Moreno, Gonzalez-Gross and Kersting 10 ). Therefore, the present study assessed UR and OV among European free-living adolescents participating in this study. Available information on misreporting and its correlates in European adolescents is still scarce. Therefore, several factors potentially associated with dietary misreporting according to previous literature – that is, socio-demographic indicators, lifestyle variables, weight status and weight- and diet-related attitudes – were investigated in this sample of European adolescents to shed light on this under-investigated topic in youth populations.
Methods
Subjects and study design
The HELENA-CSS obtained standardised, reliable and comparable data from a random sample of European adolescents on a broad battery of relevant nutrition and health-related parameters( Reference Moreno, Gonzalez-Gross and Kersting 10 , Reference Moreno, De Henauw and Gonzalez-Gross 11 ). Data collection took place during 2006 and 2007 in ten European cities: Athens (inland city) and Heraklion (Mediterranean island city) in Greece, Dortmund in Germany, Ghent in Belgium, Lille in France, Pécs in Hungary, Rome in Italy, Stockholm in Sweden, Vienna in Austria and Zaragoza in Spain. A detailed description of the sampling and recruitment approaches, standardisation and harmonisation processes, data collection, analysis strategies and quality control activities has been published elsewhere( Reference Moreno, Gonzalez-Gross and Kersting 10 – Reference Beghin, Castera and Manios 12 ). Written informed consent was obtained from all adolescents and their parents or guardians. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects/patients were approved by the Human Ethics Committees of the centres involved( Reference Beghin, Castera and Manios 12 ).
A total of 3528 adolescents (52·3 % females) aged 12·5–17·5 years were recruited from randomly selected schools in each city. The mean participation rate within the HELENA-CSS was 67 %, which can be considered acceptable, given the demanding nature of this epidemiological study. The response for all the questionnaires included in the database was more than 80 %. For the purposes of this study, only those adolescents who completed two 24-h dietary recalls (24-HDR) and had objectively measured accelerometer data were included (n 1512). Owing to logistical reasons, energy and nutrient intake information from the participants from Greece and Hungary was available for only 1 d. Therefore, only eight HELENA-CSS centres were included in this study. Excluded adolescents (n 2016) weighed significantly more (60·1 v. 57·8 kg; P<0·05) and had significantly higher BMI mean (21·7 v. 21·0 kg/m2) compared with those included in this study (n 1512).
Socio-economic status, educational level and parental weight perception
A self-reported questionnaire was used to collect data on living conditions, family structure and employment status, occupation and educational level of both parents( Reference Iliescu, Beghin and Maes 13 ). The Family Affluence Scale was used as an indicator of affluence, based on the concept of material conditions of the household in which adolescents lived( Reference Currie, Molcho and Boyce 14 ). Family affluence was determined from a set of items including car ownership, bedroom occupancy, home computers and Internet access. The adolescents reported the educational level of their parents (dummy: low/medium v. high). Their perception of parental weight (dummy: overweight/obese v. normal weight/thin/very thin) was also recorded.
Sedentary and sleep behaviours
The average minutes per day in which the adolescents were engaged in two sedentary behaviours (TV viewing and playing with video games) were estimated by means of a self-administered questionnaire previously found to demonstrate good reliability( Reference Rey-Lopez, Vicente-Rodriguez and Ortega 15 ). Habitual sleep time (h/d), as estimated by a self-reported questionnaire, was defined as the average of sleep duration during weekdays and weekend days.
Physical activity
Physical activity (PA) assessment in the HELENA-CSS study is described elsewhere( Reference Ruiz, Ortega and Martinez-Gomez 16 ). Uni-axial accelerometers (Actigraph MTI, model GT1M; Manufacturing Technology Inc.) were used to objectively measure PA. Adolescents were asked to wear the accelerometer for 7 consecutive days during all waking hours, except for water-based activities. At least 3 d of recording, with a minimum of 8 h of registration/d, was set as an inclusion criterion. The time-sampling interval was set at 15 s, and bouts of ≥20 min of consecutive zero counts were deleted from the data sets. Total PA was expressed as total counts recorded, divided by total daily registered time (counts per min). The time spent at moderate-to-vigorous PA (MVPA) (>3 metabolic equivalents) was calculated on the basis of the following cut-off points – ≥2000 counts per min for moderate PA and ≥4000 counts per min for vigorous PA( Reference Ruiz, Ortega and Martinez-Gomez 16 ) – and was presented as the average time per day (min/d). Total energy expenditure (TEE, kJ/d or kcal/d) was estimated from activity counts using the equation of Ekelund et al. ( Reference Ekelund, Sjostrom and Yngve 17 ) already validated in youth:
where sex was coded as 0 in boys and 1 in girls. This formula from the study of Ekelund et al. ( Reference Ekelund, Sjostrom and Yngve 17 ) was derived from multiple stepwise regression analyses where the best regression equation explained 60 % of the variation in TEE and included sex, activity counts and body weight. The cross-validation study showed no significant differences between predicted and measured TEE by the developed prediction equation; thus, it was judged to provide valid data for assessing TEE in youth.
Physical examination
Anthropometric measurements were obtained following a standardised protocol( Reference Nagy, Vicente-Rodriguez and Manios 18 ). Weight and height were measured in underwear and barefoot using an electronic scale (type SECA 861; Seca Ltd) and a stadiometer (type SECA 225; Seca Ltd). BMI was calculated as body weight in kilograms divided by the square of height in metres and was additionally categorised according to Cole et al. ( Reference Cole, Bellizzi and Flegal 19 , Reference Cole, Flegal and Nicholls 20 ).
Diet-related attitudes
The validated ‘eating attitudes and weight problems inventory’ designed for adolescents( Reference Diehl 21 ) was used to allow cross-cultural comparisons in dietary attitudes and behaviours, as well as to link the data to food habits( Reference Kersting, Sichert-Hellert and Vereecken 22 ). The following statements were entered in the analysis: ‘I’m very worried about gaining weight’, ‘I dread being fat’, ‘I am constantly aware that I weigh too much’, ‘I often eat less than I would like to not gain weight’, ‘I deliberately have small portions to not gain weight’, ‘I try to eat as little as possible so that I do not gain weight’, ‘I’m content with my figure’ and ‘My parents think I’m too fat’. Responses were re-coded into two categories (dummy: does not apply/seldom v. occasionally/always applies). Breakfast consumption was assessed by the ‘food choices and preferences’ questionnaire( Reference Gilbert, Hall and Hegyi 23 ). The statement ‘I often skip breakfast’ was also assessed (dummy: strongly/moderately/slightly disagree v. strongly/moderately/slightly agree).
Dietary intake
Following recommendations of the ‘European Food Consumption Survey Method’ project, two non-consecutive 24-HDR, within a time span of 2 weeks, were completed by the adolescents( Reference Biro, Hulshof and Ovesen 24 ). Assessment was performed by a computer-based tool for self-reported 24-HDR, HELENA-Dietary Assessment Tool (DIAT), based on a previous version developed for Flemish adolescents, shown to provide valid measurements of dietary intake compared with an interview by a dietitian( Reference Vereecken, Covents and Sichert-Hellert 25 ). Dietary intake referred to the day before the administration and was divided into six meal occasions. For each occasion, the user was invited to select all the consumed food items and beverages from a standardised food list. Information on quantities was collected using household measurements or pictures of portion sizes. The self-administration took place during school time in a computer classroom where the pupils completed the programme autonomously while fieldworkers were present to give assistance if necessary( Reference Vereecken, Covents and Sichert-Hellert 25 ). No information on Fridays and Saturdays was available.
The German Food Code and Nutrition Database (Bundeslebensmittelschlüssel (BLS), version II.3.1, 2005) was used to calculate energy and nutrient intakes; the BLS is the most complete food composition database across Europe in terms of nutrients and food items( Reference Dehne, Klemm and Henseler 26 , Reference Julian-Almarcegui, Bel-Serrat and Kersting 27 ). EI was estimated in kJ/d or kcal/d and macronutrient intakes (fat, protein and carbohydrate) were expressed in g/d. Subsequently, intakes of each macronutrient were converted into percentage of total EI.
Energy intake misreporting: under-reporting and over-reporting
UR and OV were calculated according to the approach proposed by Huang et al. ( Reference Huang, Roberts and Howarth 28 ). The method relies on the direct comparison of reported EI and predicted TEE, based on the principle that EI is equal to TEE, assuming weight stability. The approach uses ±1 sd cut-off points to statistically compare reported EI with predicted TEE. A report is excluded if %EI/TEE is outside the ±1 sd range. A %EI/TEE outside this range indicates that EI is too low (<−1 sd) or too high (>+1 sd) to represent the habitual intake and that the reported EI is therefore implausible. The ±1 sd cut-off points were calculated by means of the following equation:
The equation accounts for intra-individual variation in EI reporting (CVrEI) over the number of days (d) of intake, the error in the equations for predicted TEE (CVpTEE, which includes the errors of the parameters in those equations, including physical activity level) and measurement error and day-to-day biological variation in TEE (CVmTEE)( Reference Huang, Roberts and Howarth 28 ). Number of days were d=2 for the mean of two interviews and d=1 when the cut-off points were calculated for one interview day. The CVrEI and the CVpTEE were calculated separately by sex based on the HELENA-CSS data( Reference Garriguet 29 ). The CVmTEE was set to 8·2 %, as estimated from DLW measurements( Reference Black and Cole 30 ). Adolescents were classified as under-reporters, plausible reporters or over-reporters according to these cut-off values. All values and sex-specific cut-off points to estimate UR and OV are presented in Table 1.
Table 1 Reference and cut-off values used to identify misreporting among adolescents
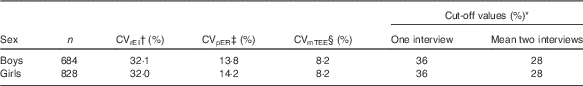
EI, energy intake; ER, energy requirement; TEE, total energy expenditure.
* Cut-off values calculated according to Huang et al. ( Reference Huang, Roberts and Howarth 28 ).
† Intra-individual variation of EI.
‡ Error in predicted energy expenditure requirements.
§ Day-to-day variation and measurement error for TEE based on the doubly labelled water technique( Reference Black and Cole 30 ).
Statistical analysis
The statistical software package Stata version 12.0 (StataCorp LP), was used to perform the analyses, and the threshold for statistical significance was set at P≤0·05. Characteristics of the study sample are presented as medians and percentiles for continuous variables and as percentages for categorical variables. Multilevel logistic regression analysis, with study centre as the random intercept, was performed to investigate factors associated with misreporting, considering UR and OV as the outcome variables (reference category: plausible reporters). Analysis was conducted at a first step for each potential correlate of UR and OV, adjusting for age and sex. At a second step, those variables with P<0·20 were entered simultaneously in the same model with UR and OV as the outcome variables.
Results
Energy intake misreporting
Table 2 shows the degree of misreporting on day 1 and day 2, separately. The degree of UR was higher for the second 24-HDR (29·5 %) than for the first one (26·5 %), whereas adolescents over-reported more on the first 24-HDR (14·6 v. 14·0 %). When assessing misreporting for mean EI of both interview days, 33·3 and 15·6 % of the adolescents were categorised as under-reporters and over-reporters, respectively (Table 3).
Table 2 Energy reporting for 24-h dietary recalls on interview days 1 and 2 (Numbers and percentages)
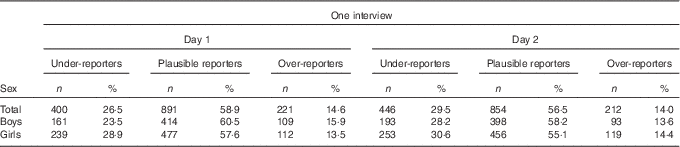
Table 3 Energy reporting by sex and weight status (Numbers and percentages)
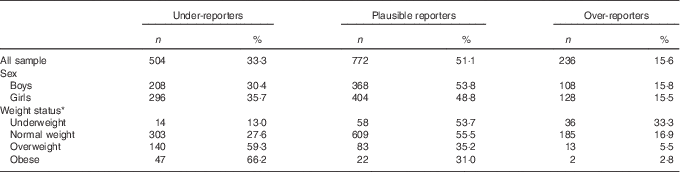
* BMI categories as described by Cole et al. ( Reference Cole, Bellizzi and Flegal 19 , Reference Cole, Flegal and Nicholls 20 ).
The degree of UR and OV separately by sex and weight status is displayed in Table 3. The percentage of UR was higher for girls (35·7 %) than for boys (30·4 %). However, boys over-reported slightly more (15·8 %) than girls (15·5 %). The degree of UR was higher among overweight (59·3 %) and obese (66·2 %) adolescents compared with normal-weight (27·6 %) and underweight adolescents (13·0 %) and increased with increasing BMI categories. On the contrary, underweight adolescents over-reported more (33·3 %), followed to a lesser extent by normal-weight (16·9 %), overweight (5·5 %) and obese (2·8 %) adolescents.
Characteristics of the study sample
Characteristics of the study sample are presented in Table 4 for the total study sample and stratified by reporting group (UR, plausible reporting and OV). In the UR and OV groups, a higher percentage of adolescents were females. Adolescents classified as under-reporters stated more that they were worried about gaining weight, dreaded being fat and skipped breakfast. Compared with plausible reporters and over-reporters, adolescents in the UR group had higher median BMI, MVPA, TEE and contribution of protein and carbohydrate intakes to EI. Over-reporters showed higher median of EI, screen time engagement and contribution of fat to EI.
Table 4 Descriptive characteristics of the sample by reporting group (Medians and 25th–75th percentiles)

MVPA, moderate-to-vigorous physical activity; TEE, total energy expenditure; EI, energy intake.
* Based on objective physical activity measurements
† BMI categories as described by Cole et al.( Reference Cole, Bellizzi and Flegal 19 , Reference Cole, Flegal and Nicholls 20 ).
Correlates of under-reporting
The results of the first step of the multilevel logistic regression analysis are shown in Table 5, where the model was only adjusted for sex and age. No significant associations were found between UR and OV and either age or sex. Under-reporters were less likely to be underweight and reported a higher contribution of fat intake to EI. On the other hand, under-reporters were more likely to be overweight or obese, perceived their own mothers as overweight/obese, had higher MVPA levels, reported higher contributions of protein and carbohydrate intakes to EI, were worried about gaining weight, dreaded being fat, were constantly aware that they weighed too much, reported eating less often than they liked, reported eating deliberately small portions and trying to eat as little as possible to not gain weight. Under-reporters skipped breakfast more often, and were more likely to report that their parents perceived them as being too fat. Lower risk of UR was observed among those adolescents who were content with their figure. Over-reporters were more likely to be underweight and had higher screen time, whereas they were less likely to be overweight or obese and reported lower contribution of protein intake to EI. Those who were worried about gaining weight, dreaded being fat, were constantly aware that they weighed too much and tried to eat as little as possible to not gain weight were less likely to over-report.
Table 5 Multilevel logistic regression (Adjusted odds ratios and 95 % confidence intervals for age- and sex, including random effects for study centre)

MVPA, moderate-to-vigorous physical activity; EI, energy intake.
* Statistically significant OR.
† BMI categories as described by Cole et al. ( Reference Cole, Bellizzi and Flegal 19 , Reference Cole, Flegal and Nicholls 20 ).
Most of the associations found in the previous model did not remain significant when the model was simultaneously adjusted for other covariates potentially related to UR or OV (Table 6). Overweight (OR 3·25; 95 % CI 2·01, 5·27) and obese (OR 4·31; 95 % CI 1·92, 9·65) adolescents were more likely to under-report and underweight adolescents were more likely to over-report (OR 1·67; 95 % CI 1·01, 2·76). Increasing screen time reduced the odds for UR (OR 0·997; 95 % CI 0·995, 0·999) and increased the odds for OV (OR 1·003; 95 % CI 1·001, 1·005). The associations observed previously between MVPA and UR (OR 1·02; 95 % CI 1·01, 1·02) remained significant. Besides, OV and MVPA levels were significantly associated (OR 0·991; 95 % CI 0·983, 0·999). The same applied for the contribution of protein intake to EI for both UR (OR 1·08; 95 % CI 1·02, 1·14) and OV (OR 0·87; 95 % CI 0·82, 0·93) and for the contribution of carbohydrate intake to EI (OR 1·06; 95 % CI 1·03, 1·09) among under-reporters. Considering diet-related attitudes, hardly any of the associations previously observed persisted in the second model. Those who were content with their figure were less likely to under-report (OR 0·61; 95 % CI 0·41, 0·89), and those who skipped breakfast were more likely to under-report (OR 2·14; 95 % CI 1·53, 2·99). On the other hand, those who were very worried about gaining weight were less likely to over-report (OR 0·55; 95 % CI 0·33, 0·92).
Table 6 Multilevel logistic regressionFootnote † (Multivariable odds ratios and 95 % confidence intervals including random effects for study centre)

MVPA, moderate-to-vigorous physical activity; EI, energy intake.
* Statistically significant OR.
† OR corresponds to a multivariable multilevel logistic regression analysis adjusted for sex, age and all the variables with P<0·20 in previous analyses entered simultaneously (Table 5).
‡ BMI categories as described by Cole et al. ( Reference Cole, Bellizzi and Flegal 19 , Reference Cole, Flegal and Nicholls 20 ).
Discussion
To the best of our knowledge, this is the first study assessing misreporting and its correlates in a large sample of adolescents from several cities across Europe, offering the opportunity to examine these factors among culturally diverse populations. A review on implausible EI in children and adolescents revealed that the prevalence of misreporting among studies ranged from 2 to 85 % for UR and from 3 to 46 % for OV( Reference Forrestal 31 ). As already acknowledged by Börnhost et al. ( Reference Börnhorst, Huybrechts and Ahrens 8 ), the large variability observed in the occurrence of UR can be explained by different methodologies (dietary assessment tools and number of assessment days), cut-off values applied, respondent characteristics such as age group (children v. adolescents) and status (self-report v. proxy). The degree of UR markedly increased (3 %) for the second interview in comparison with the first one. An increase of UR rates with the number of collected days in adolescents has been previously reported( Reference Moreno, Kersting and de Henauw 32 ). It is known that increasing the number of recording days provides more precise estimates of the individual dietary intake and reduces the within-person variability( Reference Thompson and Subar 33 ); however, long recording periods tend to reduce the accuracy of recording by increasing fatigue and boredom( Reference Gibson 34 ).
Sex
The association of sex with the occurrence of misreporting is not conclusive yet, at least in young populations( Reference Lioret, Touvier and Balin 5 , Reference Forrestal 31 , Reference Rangan, Flood and Gill 35 , Reference Murakami, Miyake and Sasaki 36 ). We failed to detect a significant association between sex and misreporting in any of its forms in our sample. Overall, studies with DLW did not find significant differences in energy reporting between male and female adults( Reference Livingstone and Black 1 ). Livingstone & Black( Reference Livingstone and Black 1 ) questioned the validity of these results, showing a higher proportion of UR among females as a result of the application of a single cut-off point for EI/BMR. Males usually have higher TEE than women, which involves higher EI values. Therefore, the use of a single cut-off point for all individuals would identify more girls as under-reporters. This hypothesis could explain other reported findings( Reference Lioret, Touvier and Balin 5 , Reference Rangan, Flood and Gill 35 , Reference Murakami, Miyake and Sasaki 36 ).
Age
Unlike sex, the inverse association between increasing age and plausible reporting has been consistently reported( Reference Lioret, Touvier and Balin 5 , Reference Forrestal 31 , Reference Rangan, Flood and Gill 35 , Reference Murakami, Miyake and Sasaki 36 ). Despite adolescents being able to report their own dietary intake, they usually show less interest, motivation and cooperation than children or younger adolescents – that is, 12–15-year-old individuals( Reference Collins, Watson and Burrows 37 ). They might find the task of recalling their dietary intake irritating and tedious, thus decreasing the level of compliance, and subsequently increasing the reporting error( Reference Collins, Watson and Burrows 37 ). No association with age, however, was observed in our study. Likewise, the above-mentioned theory formulated by Livingstone & Black( Reference Livingstone and Black 1 ) could also explain the finding of higher levels of misreporting among older individuals; similar to males, younger individuals have higher TEE than older people, and consequently higher EI.
Socio-economic indicators
Our findings did not show any association between misreporting and the SES indicators examined. The existing literature is inconclusive on the association of misreporting with education levels or SES. Some studies reported no significant association( Reference Lioret, Touvier and Balin 5 , Reference Hare, Sherrill-Mittleman and Klesges 38 , Reference Lanctot, Klesges and Stockton 39 ), whereas others reported a positive association with either low( Reference Murakami, Miyake and Sasaki 36 ) or higher education levels( Reference Garriguet 40 ). Poor literacy skills might account for the higher misreporting in less-educated groups; however, several studies have indicated an association of misreporting, mainly as UR, with higher SES, potentially due to higher awareness for socially desirable responding( Reference Livingstone and Black 1 ). Methodological differences in the assessment of SES across studies could account for these conflicting results( Reference Forrestal 31 ).
Sedentary behaviours and physical activity
The association between dietary misreporting and sedentary behaviours in adolescents has not been evaluated yet by other studies. We observed that adolescents with higher screen time were less likely to under-report and more likely to over-report. Lioret et al. ( Reference Lioret, Touvier and Balin 5 ) reported a positive association of UR with sedentary behaviours among children aged 3–10 years, with data reported mainly by the parents. Misreporting could be affected by difficulties of the individual to perform the task, inattention to eating and/or social desirability bias, among other factors( Reference Collins, Watson and Burrows 37 ). Inattentive respondents or those concerned about any social judgement may misreport EI, screen time or both at the same time, which could have occurred in our sample, but also, to certain extent, in the sample from Lioret et al. ( Reference Lioret, Touvier and Balin 5 ). In addition, these factors affecting energy reporting could be more or less prevalent depending on the respondent status – that is, parents/proxy-reported data v. adolescents/self-reported data – and therefore have an influence on the direction of the observed associations.
Consistent with previous literature( Reference Garriguet 40 ), our results showed that those engaging in higher MVPA levels were more likely to under-report their EI and less likely to be over-reporters. Similar findings were observed when moderate PA and vigorous PA were assessed separately (data not shown). One potential explanation is that those engaging in more exercise tend to have generally higher health consciousness, and may therefore be more prone to provide socially desirable responses. This could additionally explain a lower engagement in sedentary activities as a result of a major concern to have a healthy lifestyle. Higher PA performance together with lower levels of sedentary behaviours could also reflect attempts to lose weight. Hare et al. ( Reference Hare, Sherrill-Mittleman and Klesges 38 ), in contrast, observed that minutes of MVPA were lower among under-reporters, although they did not manage to give a strong explanation for these findings.
BMI
The most robust finding in a comprehensive review of characteristics associated with misreporting( Reference Livingstone and Black 1 ) was the positive association with BMI that was already reported in a number of studies( Reference Lioret, Touvier and Balin 5 , Reference Rangan, Flood and Gill 35 , Reference Murakami, Miyake and Sasaki 36 , Reference Hare, Sherrill-Mittleman and Klesges 38 – Reference Garriguet 40 ). Forrestal( Reference Forrestal 31 ) also observed that weight status was consistently associated with UR in both children and adolescents. Likewise, the probability of UR among the HELENA-CSS adolescents increased as BMI increased. In agreement with Rangan et al. ( Reference Rangan, Flood and Gill 35 ), over-reporters were more likely to be underweight. These results might be explained by social desirability bias, which could be most marked among overweight and/or obese adolescents, but might also indicate a poor ability or denial for self-monitoring of dietary intake within this group( Reference Rangan, Flood and Gill 35 ). Another plausible hypothesis could be that overweight/obese people are on a diet, and thus they are truthful when reporting low EI. Weight status is indisputably a factor that needs to be taken into consideration when assessing diet–disease associations. Nonetheless, it should be noted that there are overweight and obese individuals who do not under-report, thus being reliable reporters, whereas there are under-reporters among those within normal weight range( Reference Livingstone and Black 1 ). Although UR was most prevalent among overweight and obese adolescents, we noted that normal-weight and underweight adolescents accounted for 62·9 % of the individuals within our UR group. On the other hand, the degree of OV among overweight and obese adolescents was extremely low (6·3 %).
Figure perception and diet-related attitudes
As already acknowledged by previous studies( Reference Lioret, Touvier and Balin 5 , Reference Macdiarmid and Blundell 6 ), UR may not exclusively reflect UR but also real under-eating as an attempt to lose or not to gain weight. In addition, adolescents are at an age when growth spurts occur and might indeed consume large amounts of foods; this would reflect real over-eating rather than OV. Owing to the short period of dietary recording, we could not distinguish between under-reporters/over-reporters and under-eaters/over-eaters. Our initial multilevel regression analysis showed that those who were more frequently concerned about gaining weight and being fat and more prone to dietary restraints by eating less to avoid gaining weight were more likely to UR and less likely to OV. In addition, those with figure dissatisfaction were more likely to under-report. Previous findings in adolescents have already illustrated that under-reporters exhibited greater weight consciousness and dieting( Reference Lioret, Touvier and Balin 5 , Reference Livingstone, Robson and Wallace 41 , Reference Ventura, Loken and Mitchell 42 ). Nevertheless, significant associations with weight concern and dietary restraint did not remain significant when all the variables were entered into the same model, apart from the inverse association between being concerned about gaining weight and OV and between figure satisfaction and UR. That might be partly explained by the fact that other covariates – which could be confounding the initially observed associations – that is, weight status and/or body image satisfaction – were entered into the model. This suggests that overweight/obese adolescents and/or those with poor body image could be more concerned about their weight and/or might engage in dietary restrictive behaviours more often, resulting in intentional alteration of the diet by eating less or avoiding certain food items (under-eating), and therefore less likely to OV, together with higher PA levels and lower engagement in sedentary behaviours.
Livingstone & Robson( Reference Livingstone and Robson 9 ) hypothesised that obese adolescents may feel even more stigmatised about their fatness than obese adults, given the widespread and excessive obsession with body weight and image among adolescents. Consistent with the findings of Lioret et al. ( Reference Lioret, Touvier and Balin 5 ), breakfast skipping was also more prevalent among under-reporters, which might reflect intentional dieting and further emphasise the need of differentiating among those who are truly under-reporting and those who are under-eating( Reference Lioret, Touvier and Balin 5 ).
Our study contributes to the existing evidence that errors in EI reporting may reflect pressure to meet cultural expectations( Reference Livingstone and Black 1 , Reference Rennie, Siervo and Jebb 43 ). It is of concern that this phenomenon might be more marked among adolescents as they are sensitive to context and social norms( Reference Borgers, de Leeuw and Hox 44 ). Attitudes towards food consumption are affected by a number of factors such as weight status and consciousness, body image, social desirability and dietary restraint( Reference Baranowski, Cullen and Baranowski 45 ), which cannot be ignored when evaluating energy reporting. Thus, inaccurate reporting is not simply a nutritionists’ issue; a multidisciplinary approach (including psychology, sociology and physiology) is required to further understand misreporting in dietary intake studies( Reference Macdiarmid and Blundell 6 ).
Macronutrient intakes
Misreporting is not limited only to EI but may also affect the macronutrient composition of the diet. Our results showed that under-reporters had a higher contribution of protein to EI, whereas it was lower among over-reporters, as described in other studies conducted in both adolescents( Reference Lioret, Touvier and Balin 5 , Reference Kobe, Krzisnik and Mis 46 ) and adults( Reference Livingstone and Black 1 , Reference Lafay, Basdevant and Charles 3 , Reference Macdiarmid and Blundell 6 ). A lower percentage of fat contribution to EI has also been reported, whereas the contribution of carbohydrates to EI has been observed to be variable( Reference Livingstone and Black 1 ). No significant associations, however, were observed in our study between percentage of EI from fat and misreporting, whereas adolescents with higher contribution of carbohydrates to EI were more likely to under-report. A potential explanation is that under-reporters may omit certain foods that are considered unhealthy such as sugar-rich products and/or that they restrain themselves from eating them( Reference Macdiarmid and Blundell 6 ). All these data suggest that misreporting may introduce bias in the assessment of macronutrient composition of the diet in adolescents and that should be considered when addressing relationships with macronutrient intake.
Bias in estimating nutrient intakes and in reporting meal patterns and foods eaten are the consequences of dietary misreporting, mainly due to UR, which is more widespread than OV( Reference Forrestal 31 ). This bias has a number of implications for the interpretation of descriptive analyses and diet–disease links. Misreporting, more specifically UR, can overestimate the number of subjects with deficient nutrient intakes, attenuate or even reverse associations between diet and disease and seriously hinder the derivation of food-based dietary guidelines due to selective reporting of foods( Reference Livingstone and Black 1 ). A common practice to deal with these concerns is to exclude UR from the analysis; however, this might exclude individuals who report low EI as a result of intentional dietary restraint or dieting – that is, under-eating( Reference Mendez 4 ). As exclusion of under-reporters introduces unknown bias, under-reporters should therefore be identified and corrected for( 47 ). Nevertheless, it would be advisable to perform the analysis including all the subjects available in the sample with and without adjustment for either reporting status or variables associated with misreporting. Running the analysis with and without under-reporters and comparing the obtained results with each approach could be another alternative. Unfortunately, there is no formal procedure to handle misreporting in the analysis and disadvantages are present in all the approaches. However, misreporting needs to be accounted for, at least in the interpretation and discussion of results. A similar approach could also be followed with over-reporters, although among adolescents OV should be treated cautiously as higher EI may represent real over-eating to cover the high energy costs due to growth.
In addition, the factors identified as major misreporting correlates in this study – that is, weight status, dietary restraint attitudes and weight dissatisfaction – could assist researchers in identifying individuals more prone to provide biased dietary intake reports; taking them into account during study design and data collection could minimise the error associated with self-reported dietary data. Future research lines including dietary intake estimates and/or addressing associations in which obesity indicators and/or dietary attitudes play an essential role will mainly benefit from these findings.
Limitations and strengths
The limitations of our study should be acknowledged. Diet was assessed by self-reported 24-HDR, which is prone to portion size estimation errors and recording bias. Dietary intake was estimated based on two non-consecutive 24-HDR, which are not sufficient to characterise individuals’ usual intakes. Collection of dietary data for >2 d would have been desirable to capture usual intakes and to account for day-to-day variability( Reference Thompson and Subar 33 ). However, the HELENA-DIAT has already been shown to provide reliable estimates of dietary intake among European adolescents( Reference Vereecken, Covents and Sichert-Hellert 25 , Reference Julian-Almarcegui, Bel-Serrat and Kersting 27 ).
Another limitation is that the applied cut-off points assumed stable body weight, which might not always be true in growing adolescents. However, as energy costs for growth in adolescence are small, at approximately 1 % of TEE( 48 ), we presume that this assumption does not significantly affect the study outcomes.
Reports of EI are always subject to the possibility of under-eating and over-eating. Similar to the Goldberg equation, the approach applied does not account for differences between respondents who are on a diet (under-eating) or those limiting their reported intake (UR) and among those who are intentionally eating more (over-eating) or reporting higher EI (OV). Nevertheless, aspects on the association between misreporting, mainly UR, and diet-related attitudes were also assessed to investigate under-eating in our sample.
Another factor to consider is the role of body composition, which was not investigated in our large sample of youths. In adults, free-living EI was found to be related to fat-free mass rather than to BMI or fat mass( Reference Blundell, Caudwell and Gibbons 49 ). Therefore, at any given BMI, a fatter person, that is, one with lower percentage of fat-free mass, might actually have a lower EI and not be UR. Nevertheless, studies evaluating the validity of dietary assessments using the DLW found that the likelihood of UR in adolescents was most strongly predicted by higher percent body fat( Reference Bratteby, Sandhagen and Fan 50 , Reference Perks, Roemmich and Sandow-Pajewski 51 ).
The cross-sectional nature of this study provides a transversal perspective of correlates of dietary misreporting and cannot be used to establish causation. Finally, these findings should be interpreted from an explorative point of view, given that the level of statistical significance was set at P≤0·05 despite performing multiple comparisons.
Our study has several strengths. First, the sample guaranteed a large geographical spread all over Europe and all measurements followed standardised procedures throughout the different study centres. Cut-off values to identify misreporting were calculated for each individual based on his or her own PA levels, which resulted in a more accurate classification in reported EI. Although there is no simple approach to deal with reporting error, the method suggested by Huang et al.( Reference Huang, Roberts and Howarth 28 ) offered a simpler and more individualised alternative than other existing methods. In addition, the use of the calculated cut-off points made it possible to overcome some of the limitations associated with the use of the Goldberg cut-off point, such as the error linked to the estimation of BMR( Reference McCrory, McCrory and Hajduk 52 ).
Conclusions
Our study showed that EI misreporting in adolescents seems to be associated with several characteristics, specifically weight status, being worried about gaining weight, body image dissatisfaction and skipping breakfast. Our results also confirm the general finding that overweight/obese people are more likely to report implausibly low EI than normal-weight people. To the degree that this reflects UR of actual EI, it might be due to a tendency to provide socially desirable answers. This interpretation is supported by our findings that adolescents with dietary restraint and self-image dissatisfaction were more likely to under-report, which emphasises the need to identify these individuals in epidemiological studies. The potentially limited ability of the adolescents to accurately report their own dietary intakes could also explain part of our findings. Another possibility is that overweight/obese people may be dieting to control weight and may be accurately reporting low EI. Therefore, it is difficult to distinguish under-eating from UR. Similarly, higher EI could reflect over-eating due to growth spurts instead of OV. Therefore, factors influencing misreporting, as the ones identified in our study, should be assessed in young populations to improve the interpretation of potentially biased findings, more in particular when addressing diet–obesity associations.
Acknowledgements
The authors gratefully acknowledge all participating children and adolescents and their parents and teachers for their collaboration.
The writing group takes sole responsibility for the content of this article. The content of this article reflects the views of the authors only, and the European Community is not liable for any use that may be made of the information contained therein. The HELENA-CSS study was carried out with the financial support of the European Community Sixth RTD Framework Programme (contract FOODCT-2005-007034). This work was also partially supported by the Swedish Council for Working Life and Social Research (FAS), and by grants from the Spanish Ministry of Education (EX-2007-1124), the Spanish Ministry of Health: Maternal, Child Health and Development Network (number RD08/0072). Funders had no role in the design, analysis or writing of this article.
M. G.-G., M. K., F. G., D. M., J. D., K. W., L. A. M., Y. M., S. D. H., C. L. and I. H. conceived and designed the study; S. B.-S., E. G., L. H., M. P. and R. R. conducted the research and collected data; S. B.-S. analysed the data; C. J.-A. and I. H. contributed to the analysis; S. B.-S. and I. H. wrote the paper; S. B.-S. and I. H. participated in data interpretation; S. B.-S., C. J.-A., M. G.-G., T. M., C. B., E. G., M. K., M. C.-G., F. G., D. M., L. H., J. D., M. P., R. R., K. W., L. A. M., Y. M., S. D. H., C. L., S. V., S. L., B. G. and I. H. critically reviewed the manuscript. B. G. edited the manuscript for English language usage. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Appendix: HELENA study group
Coordinator
L. A. M.
Core group members
L. A. M., F. G., S. D. H., M. G.-G. and C. Gilbert.
Steering committee
A. Kafatos (President), L. A. M., C. Libersa, S. D. H., J. Sánchez, F. G., M. K., M. Sjöstrom, D. M., M. G.-G., J. D., C. Gilbert, G. Hall, L. Maes and L. Scalfi.
Project manager
P. Meléndez.
Universidad de Zaragoza (Spain)
L. A. M., J. Fleta, J. A. Casajús, G. Rodríguez, C. Tomás, M. I. Mesana, G. Vicente-Rodríguez, A. Villarroya, C. M. Gil, I. Ara, J. Revenga, C. Lachen, J. Fernández-Alvira, G. Bueno, A. Lázaro, O. Bueno, J. F. León, J. M. Garagorri, M. Bueno, J. P. Rey López, I. Iglesia, P. Velasco, S. B.-S., L. Gracia-Marco, T. M. and D. Jiménez-Pavón.
Consejo Superior de Investigaciones Científicas (Spain)
A. Marcos, J. Wärnberg, E. Nova, S. Gómez-Martínez, E. Ligia Díaz, J. Romeo, A. Veses, M. Angeles Puertollano, B. Zapatera and T. Pozo.
Université de Lille 2 (France)
L. Beghin, C. Libersa, F. G., C. Iliescu and J. Von Berlepsch.
Research Institute of Child Nutrition Dortmund, Rheinische Friedrich-Wilhelms-Universität Bonn (Germany)
M. K., W. Sichert-Hellert and E. Koeppen.
Pécsi Tudományegyetem (University of Pécs) (Hungary)
D. M., E. Erhardt, K. Csernus, K. Török, S. Bokor, Angster, E. Nagy, O. Kovács and J. Répasi.
University of Crete School of Medicine (Greece)
A. Kafatos, C. Codrington, M. P., A. Papadaki, K. Sarri, A. Viskadourou, C. Hatzis, M. Kiriakakis, G. Tsibinos, C. Vardavas, M. Sbokos, E. Protoyeraki and M. Fasoulaki.
Institut für Ernährungs- und Lebensmittelwissenschaften – Ernährungsphysiologie. Rheinische Friedrich-Wilhelms-Universität (Germany)
P. Stehle, K. Pietrzik, M. G.-G., C. Breidenassel, A. Spinneker, J. Al-Tahan, M. Segoviano, A. Berchtold, C. Bierschbach, E. Blatzheim, A. Schuch and P. Pickert.
University of Granada (Spain)
M. J. Castillo, A. Gutiérrez, F. B. Ortega, J. R. Ruiz, E. G. Artero, V. España, D. Jiménez-Pavón, P. Chillón, C. Sánchez-Muñoz and M. C.-G.
Council for Agricultural Research and Economics (CREA), Research Center for Food and Nutrition, Rome, Italy
D. Arcella, E. Azzini, E. Barrison, N. Bevilacqua, P. Buonocore, G. Catasta, L. Censi, D. Ciarapica, P. D’Acapito, M. Ferrari, M. Galfo, C. Le Donne, C. L., G. Maiani, B. Mauro, L. Mistura, A. Pasquali, R. Piccinelli, A. Polito, R. R., R. Spada, S. Sette and M. Zaccaria.
University of Napoli ‘Federico II’ Department of Food Science (Italy)
L. Scalfi, P. Vitaglione and C. Montagnese.
Ghent University (Belgium)
I. De Bourdeaudhuij, S. D. H., T. De Vriendt, L. Maes, C. Matthys, C. Vereecken, M. de Maeyer, C. Ottevaere and I. H.
Medical University of Vienna (Austria)
K. W., K. Phillipp, S. Dietrich, B. Kubelka and M. Boriss-Riedl.
Harokopio University (Greece)
Y. M., E. G., Z. Bouloubasi, T. Louisa Cook, S. Eleutheriou, O. Consta, G. Moschonis, I. Katsaroli, G. Kraniou, S. Papoutsou, D. Keke, I. Petraki, E. Bellou, S. Tanagra, K. Kallianoti, D. Argyropoulou, K. Kondaki, S. Tsikrika and C. Karaiskos.
Institut Pasteur de Lille (France)
J. D. and A. Meirhaeghe.
Karolinska Institutet (Sweden)
M. Sjöström, J. Ruiz, F. B. Ortega, M. Hagströmer, L. H., E. Patterson, L. Kwak, J. Wärnberg, N. Rizzo and A. Hurtig Wennlöf.
Asociación de Investigación de la Industria Agroalimentaria (Spain)
J. Sánchez-Molero, E. Picó, M. Navarro, B. Viadel, J. E. Carreres, G. Merino, R. Sanjuán, M. Lorente, M. J. Sánchez and S. Castelló.
Campden BRI (UK)
C. Gilbert, S. Thomas, E. Allchurch and P. Burguess.
SIK-Institutet foer Livsmedel och Bioteknik (Sweden)
G. Hall, A. Astrom, A. Sverkén and A. Broberg.
Meurice Recherche & Development asbl (Belgium)
A. Masson, C. Lehoux, P. Brabant, P. Pate and L. Fontaine.
Campden & Chorleywood Food Development Institute (Hungary)
A. Sebok, T. Kuti and A. Hegyi.
Productos Aditivos SA (Spain)
C. Maldonado and A. Llorente.
Cárnicas Serrano SL (Spain)
E. García.
Cederroth International AB (Sweden)
H. von Fircks, M. Lilja Hallberg and M. Messerer.
Lantmännen Food R&D (Sweden)
M. Larsson, H. Fredriksson, V. Adamsson and I. Börjesson.
European Food Information Council (Belgium)
L. Fernández, L. Smillie and J. Wills.
Universidad Politécnica de Madrid (Spain)
M. G.-G., J. Valtueña, U. Albers, R. Pedrero, A. Meléndez, P. J. Benito, D. Cañada, D. Jiménez-Pavón, A. Urzanqui, J. C. Ortiz, F. Fuentes, J. J. Gómez Lorente, R. Mardía Torres and P. Navarro.









