INTRODUCTION
Five to eight million people in Latin America are infected with the protozoan parasite Trypanosoma cruzi, the aetiologic agent of Chagas disease (Hashimoto and Yoshioka, Reference Hashimoto and Yoshioka2012; Bern, Reference Bern2015). Infections are spread primarily by blood-sucking triatomine bugs, although other means of transmission include the congenital route, contaminated food and drink, organ transplantation and blood transfusion. Chagas disease is also becoming a global public health problem, with significant numbers of symptomatic cases now being detected within migrant populations, particularly in the USA and Europe, where the estimates of those infected are 300 000 and 100 000, respectively (Bern et al. Reference Bern, Kjos, Yabsley and Montgomery2011; Pérez-Molina et al. Reference Pérez-Molina, Norman and López-Vélez2012; Requena-Méndez et al. Reference Requena-Méndez, Aldasoro, de Lazzari, Sicuri, Brown, Moore, Gascon and Muñoz2015).
Chagas disease has been divided into three discrete phases. The initial ‘acute’ stage, which occurs in the first 4–6 weeks post-infection, usually manifests as a mild and transient febrile condition, and in many cases, is asymptomatic. However, in children it can be more severe and sometimes fatal. With the development of a vigorous adaptive immune response, in which CD8+ IFN-γ + T cells play a major role (Cardillo et al. Reference Cardillo, de Pinho, Antas and Mengel2015; Tarleton, Reference Tarleton2015), the infection is suppressed, but sterile immunity is not achieved. This ‘indeterminate’ or ‘asymptomatic chronic’ stage is characterized by an intermittent and extremely low-level parasitaemia. However, ~30% of infected individuals eventually proceed to the ‘symptomatic chronic’ stage, often decades after the primary infection. Cardiomyopathy develops in the majority of these individuals, whilst a minority (approximately 10% of those infected) suffer digestive tract megasyndromes (Ribeiro et al. Reference Ribeiro, Nunes, Teixeira and Rocha2012; Cunha-Neto and Chevillard, Reference Cunha-Neto and Chevillard2014). Chagas disease is a major cause of premature death in many areas of South America.
The front-line drugs used to treat T. cruzi infections are the nitroheterocyclic compounds benznidazole and nifurtimox (Wilkinson and Kelly, Reference Wilkinson and Kelly2009; Gaspar et al. Reference Gaspar, Moraes, Freitas-Junior, Ferrari, Costantino, Costi, Coron, Smith, Siqueira-Neto, McKerrow and Cordeiro-da-Silva2015). Both have been in use for almost 50 years, despite widespread evidence of treatment failures (Molina et al. Reference Molina, Gómez i Prat, Salvador, Treviño, Sulleiro, Serre, Pou, Roure, Cabezos, Valerio, Blanco-Grau, Sánchez-Montalvá, Vidal and Pahissa2014; Morillo et al. Reference Morillo, Marin-Neto, Avezum, Sosa-Estani, Rassi, Rosas, Villena, Quiroz, Bonilla, Britto, Guhl, Velazquez, Bonilla, Meeks, Rao-Melacini, Pogue, Mattos, Lazdins, Rassi, Connolly and Yusuf2015, Reference Morillo, Waskin, Sosa-Estani, Del Carmen Bangher, Cuneo, Milesi, Mallagray, Apt, Beloscar, Gascon, Molina, Echeverria, Colombo, Perez-Molina, Wyss, Meeks, Bonilla, Gao, Wei, McCarthy and Yusuf2017). Other drawbacks include the long treatment period (often 60 –90 days), the frequency and severity of side-effects, and the potential for cross-resistance, which arises from the requirement of these nitroheterocyclic agents to be activated by the same parasite mitochondrial nitroreductase, TcNTR-1 (Wilkinson et al. Reference Wilkinson, Taylor, Horn, Kelly and Cheeseman2008; Mejia et al. Reference Mejia, Hall, Taylor, Gómez-Palacio, Wilkinson, Triana-Chávez and Kelly2012). Although benznidazole has proven to be effective at curing some acute and chronic T. cruzi infections, the extent to which it can prevent or alleviate chronic cardiac pathology remains uncertain (Molina-Berríos et al. Reference Molina-Berríos, Campos-Estrada, Lapier, Duaso, Kemmerling, Galanti, Leiva, Ferreira, López-Muñoz and Maya2013; Villar et al. Reference Villar, Perez, Cortes, Riarte, Pepper, Marin-Neto and Guyatt2014; Gruendling et al. Reference Gruendling, Massago, Teston, Monteiro, Kaneshima, Araújo, Gomes, Barbosa and Toledo2015; Morillo et al. Reference Morillo, Marin-Neto, Avezum, Sosa-Estani, Rassi, Rosas, Villena, Quiroz, Bonilla, Britto, Guhl, Velazquez, Bonilla, Meeks, Rao-Melacini, Pogue, Mattos, Lazdins, Rassi, Connolly and Yusuf2015). The only new compound recently advanced into clinical trials has been the anti-fungal agent posaconazole, which blocks ergosterol biosynthesis through inhibition of lanosterol 14α-demethylase (CYP51). Unfortunately, posaconazole proved to have limited efficacy against chronic infections (Molina et al. Reference Molina, Gómez i Prat, Salvador, Treviño, Sulleiro, Serre, Pou, Roure, Cabezos, Valerio, Blanco-Grau, Sánchez-Montalvá, Vidal and Pahissa2014; Francisco et al. Reference Francisco, Lewis, Jayawardhana, Taylor, Chatelain and Kelly2015; Morillo et al. Reference Morillo, Waskin, Sosa-Estani, Del Carmen Bangher, Cuneo, Milesi, Mallagray, Apt, Beloscar, Gascon, Molina, Echeverria, Colombo, Perez-Molina, Wyss, Meeks, Bonilla, Gao, Wei, McCarthy and Yusuf2017), despite some initially promising outcomes in experimental animal models (Molina et al. Reference Molina, Martins-Filho, Brener, Romanha, Loebenberg and Urbina2000; Ferraz et al. Reference Ferraz, Gazzinelli, Alves, Urbina and Romanha2007).
The urgent need to develop more effective therapy against Chagas disease is now being tackled by large international, multidisciplinary teams (Katsuno et al. Reference Katsuno, Burrows, Duncan, Hooft van Huijsduijnen, Kaneko, Kita, Mowbray, Schmatz, Warner and Slingsby2015; Chatelain, Reference Chatelain2016). These have introduced a more systematic framework to drug development by bringing together expertise from both the academic and commercial sectors. However, it is clear that progress is being limited by gaps in our knowledge of parasite biology and disease pathogenesis, and that further technical innovations are required to accelerate the pathway that stretches from lead compound optimization to pre-clinical testing. Below, we highlight these major biological questions and discuss various approaches that could help to streamline the drug development process.
DOES PARASITE DIVERSITY IMPACT ON DRUG EFFICACY?
Trypanosoma cruzi is a highly diverse species with genetic distances between major lineages greater than those between members of the Trypanosoma brucei species complex (Franzén et al. Reference Franzén, Ochaya, Sherwood, Lewis, Llewellyn, Miles and Andersson2011). Our understanding of parasite taxonomy has been complicated further by evidence of genetic exchange and widespread detection of putative hybrid strains (Machado and Ayala, Reference Machado and Ayala2001; Brisse et al. Reference Brisse, Henriksson, Barnabé, Douzery, Berkvens, Serrano, De Carvalho, Buck, Dujardin and Tibayrenc2003; Gaunt et al. Reference Gaunt, Yeo, Frame, Stothard, Carrasco, Taylor, Mena, Veazey, Miles, Acosta, de Arias and Miles2003; Lewis et al. Reference Lewis, Llewellyn, Yeo, Acosta, Gaunt and Miles2011). The geographical range of T. cruzi extends from southern Chile and Argentina, through Central America, into wide areas of the southern USA (Brenière et al. Reference Brenière, Waleckx and Barnabé2016). The parasite can be transmitted by more than 100 species of triatomine vector and is capable of infecting most, if not all, mammalian species that it encounters (Messenger et al. Reference Messenger, Miles and Bern2015). The taxonomic categorization of T. cruzi has been subject to long, and at times vigorous, debate. Currently, the species is divided into six discrete typing units (DTUs) designated TcI–TcVI (Zingales et al. Reference Zingales, Miles, Campbell, Tibayrenc, Macedo, Teixeira, Schijman, Llewellyn, Lages-Silva, Machado, Andrade and Sturm2012) (Fig. 1), although a seventh, Tcbat, has recently been proposed (Marcili et al. Reference Marcili, Lima, Cavazzana, Junqueira, Veludo, Maia Da Silva, Campaner, Paiva, Nunes and Teixeira2009; Pinto et al. Reference Pinto, Kalko, Cottontail, Wellinghausen and Cottontail2012). TcI is the most geographically dispersed DTU, with a range stretching from the USA to Argentina, and although the other lineages are more localized, there is considerable overlap (for review, Miles et al. Reference Miles, Llewellyn, Lewis, Yeo, Baleela, Fitzpatrick, Gaunt and Mauricio2009; Brenière et al. Reference Brenière, Waleckx and Barnabé2016). This extensive diversity has prompted speculation as to whether there are correlations between parasite lineage, host preference, disease pathology and drug sensitivity.

Fig. 1. Key features of the Trypanosoma cruzi major genetic lineages. The phylogenetic tree was reconstructed using multi-locus microsatellite genotype data, adapted from Lewis et al. (Reference Lewis, Llewellyn, Yeo, Acosta, Gaunt and Miles2011). Haplotype diversity is based on mitochondrial gene sequences for COII and ND1 reported in Lewis et al. (Reference Lewis, Llewellyn, Yeo, Acosta, Gaunt and Miles2011), and the values shown indicate the probability that two randomly selected haplotypes will be different. The percentage of human infections is estimated from metadata compiled by Brenière et al. (Reference Brenière, Waleckx and Barnabé2016), which encompass all isolates derived from human infections (n = 1902) and typed to each lineage. These values may reflect historical variation in sampling intensities between endemic areas.
All six DTUs are capable of infecting humans (Fig. 1); overall TcI and TcV infections are the most commonly identified, although other lineages predominate in some specific endogenous areas, such as TcII in parts of Brazil. Despite some circumstantial data, there has been no unequivocal evidence of a causative link between parasite diversity and disease outcome in humans. For example, suggestions that the absence of gastrointestinal megasyndromes in Venezuela compared with Brazil might be associated with genetic differences in the populations of circulating parasites have yet to be validated (Messenger et al. Reference Messenger, Miles and Bern2015). There is substantial intra-lineage genetic diversity, particularly within TcI, II, III and IV (Lewis et al. Reference Lewis, Llewellyn, Yeo, Acosta, Gaunt and Miles2011); however, this has rarely been taken into account, because in most studies, genotyping is only conducted at the lineage level. Studies using experimental animal models do show that there can be important differences in virulence between individual strains (Schlemper et al. Reference Schlemper, Avila, Coura and Brener1983; Postan et al. Reference Postan, Bailey, Dvorak, McDaniel and Pottala1987; Espinoza et al. Reference Espinoza, Rico, Sosa, Oaxaca, Vizcaino-Castillo, Caballero and Martínez2010; Rodriguez et al. Reference Rodriguez, Guerrero, Fortes, Santi-Rocca, Gironès and Fresno2014; Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Jayawardhana and Kelly2016), although no candidate genetic factors have been identified. The increasing availability of genomic technologies should enable progress to be made in delineating the extent to which parasite genetics contributes to disease outcome.
There have been multiple reports of wide divergence in the drug susceptibility of different T. cruzi strains. In a survey which encompassed representatives of DTUs I–VI, significant differences in benznidazole sensitivity were identified, but there was no correlation with parasite lineage (Villarreal et al. Reference Villarreal, Barnabé, Sereno and Tibayrenc2004). These results were consistent with data obtained from a panel of 28 parasite isolates from Colombia, where in vitro EC50 values against benznidazole ranged from 1 to 35 µ m (Mejia et al. Reference Mejia, Hall, Taylor, Gómez-Palacio, Wilkinson, Triana-Chávez and Kelly2012). Again, there was no obvious correlation between sensitivity and lineage (the panel contained DTU I and II strains), or with the biological origin of the parasites, either insect vector, small mammal or human. This extensive natural variation in benznidazole sensitivity was independent of TcNTR-1 sequence, implying that it must be associated with additional factors. In another report, parasites belonging to each of the DTUs were tested in vitro against several nitroheterocylic drugs and other lead compounds (Moraes et al. Reference Moraes, Giardini, Kim, Franco, Araujo-Junior, Schenkman, Chatelain and Freitas-Junior2014). Although parasite strains exhibited a range of susceptibilities to individual drugs (e.g. up to 8-fold in the case of nifurtimox), there was no evidence that any of the lineages were intrinsically more resistant to drugs in general. In vivo studies have been inconsistent in terms of linking drug susceptibility to parasite lineage. For example, while Toledo et al. (Reference Toledo, Bahia, Carneiro, Martins-Filho, Tibayrenc, Barnabé, Tafuri and de Lana2003) found that TcI-infected mice were less frequently cured by benznidazole or itraconazole than TcII- or TcV-infected mice, they also observed extensive heterogeneity in drug sensitivity between strains within these lineages. Similar intra-lineage variations were also reported when the curative potential of benznidazole was assessed with a number of Brazilian strains (Teston et al. Reference Teston, Monteiro, Reis, Bossolani, Gomes, de Araújo, Bahia, Barbosa and Toledo2013). In a study with the other front line drug nifurtimox, no association was found between therapeutic effectiveness and parasite lineage (Oliveira et al. Reference Oliveira, Branquinho, Alessio, Mello, Nogueira-de-Paiva, Carneiro, Toledo, Reis, Martins-Filho and Lana2017).
In summary, although natural T. cruzi isolates can display large variations in drug susceptibility, there is little evidence to link this with their taxonomic designation at the DTU level. As a general observation, intra-lineage differences seem to be as extensive as those between lineages. This highlights that assessment of the ability of lead compounds to display in vivo activity against a wide panel of isolates, reflecting the diverse phylogeny and geographical range of T. cruzi, must be considered an integral step in the drug development pathway. The importance of this is emphasized further by the commonality of mixed infections (Bontempi et al. Reference Bontempi, Bizai, Ortiz, Manattini, Fabbro, Solari and Diez2016).
ARE ALL PARASITE LIFE-CYCLE STAGES EQUALLY SUSCEPTIBLE TO CHEMOTHERAPY?
From the drug development perspective, there are a number of important questions relating to the T. cruzi life-cycle that need to be addressed: (i) Is it necessary to kill all developmental forms to produce a curative outcome? (ii) Are all developmental forms equally susceptible to trypanocidal compounds? (iii) Is there a point in the life-cycle during chronic stage infections where the parasites enter a biochemically quiescent or dormant phase? The T. cruzi life-cycle involves a series of differentiation steps, in which the parasite passes through both replicative and non-replicative stages. In the classical text-book version of the mammalian life-cycle, which has been established for more than a century, insect-transmitted non-replicating metacyclic trypomastigotes invade host cells, differentiate into small round-shaped, non-flagellated intracellular amastigotes, and then divide by binary fission in the cytosol. When they reach a threshold level, which may be several hundred per infected cell, they differentiate into non-dividing flagellated trypomastigotes, which are released following lysis of the host cell. The trypomastigotes can then re-invade other cells, or be taken up in a bloodmeal by a feeding triatomine bug.
Further research has revealed that this established view of the life-cycle is rather superficial and that in reality, the process is almost certainly more complex (Fig. 2). For example, evidence for an intracellular epimastigote-like stage has been intermittently reported (for review, Tyler and Engman, Reference Tyler and Engman2001), although it is unclear whether this enigmatic form is simply an intermediate in the amastigote to trypomastigote transition, or represents an obligate intracellular stage of the life-cycle, with a distinct role in vivo. Similarly, amastigote-like forms with short flagella, termed sphaeromastigotes, have also been widely reported, although these probably represent intermediate forms in the transition to epimastigotes, rather than distinct life-cycle stages (Tyler and Engman, Reference Tyler and Engman2001). Recently, trypomastigotes have also been shown to have the capacity to differentiate into an epimastigote-like morphological form, after transition through an amastigote-like intermediate. These recently differentiated epimastigotes (rdEpi) display a distinct proteomic fingerprint, are complement-resistant, able to invade phagocytic and cardiac cells (but not fibroblasts or epithelial cells), and can initiate an infection in mice (Kessler et al. Reference Kessler, Contreras, Marliére, Guarneri, Villamizar Silva, Mazzarotto, Batista, Soccol, Krieger and Probst2017). It has also been demonstrated that the initial differentiation from the metacyclic trypomastigote involves an asymmetric cell division (Fig. 2), which results in one amastigote and one ‘zoid’ – a cell with a kinetoplast, but no nucleus. The zoid quickly dies and is degraded by the host cell machinery with some of its antigens being presented on the infected cell surface (Kurup and Tarleton, Reference Kurup and Tarleton2014). The role(s) of the intermediate in vivo forms are not well defined, and it is unclear whether their sensitivity (or otherwise) to test compounds is adequately captured using current in vitro screening systems. Efficacy testing against trypomastigotes is now routinely incorporated into drug screening protocols (Cortes et al. Reference Cortes, Castro, Pesce, Maya, Ferreira, Castro-Castillo, Parra, Jara and López-Muñoz2015; Guedes-da-Silva et al. Reference Guedes-da-Silva, Batista, Meuser, Demarque, Fulco, Araújo, Da Silva, Da Silva, Patrick, Bakunova, Bakunov, Tidwell, Oliveira, Britto, Moreira and Soeiro2016). However, the sensitivity of other intracellular and/or intermediate stages is intrinsically difficult to establish, and it is unknown whether it is necessary to target each of these morphological forms to eradicate an infection.
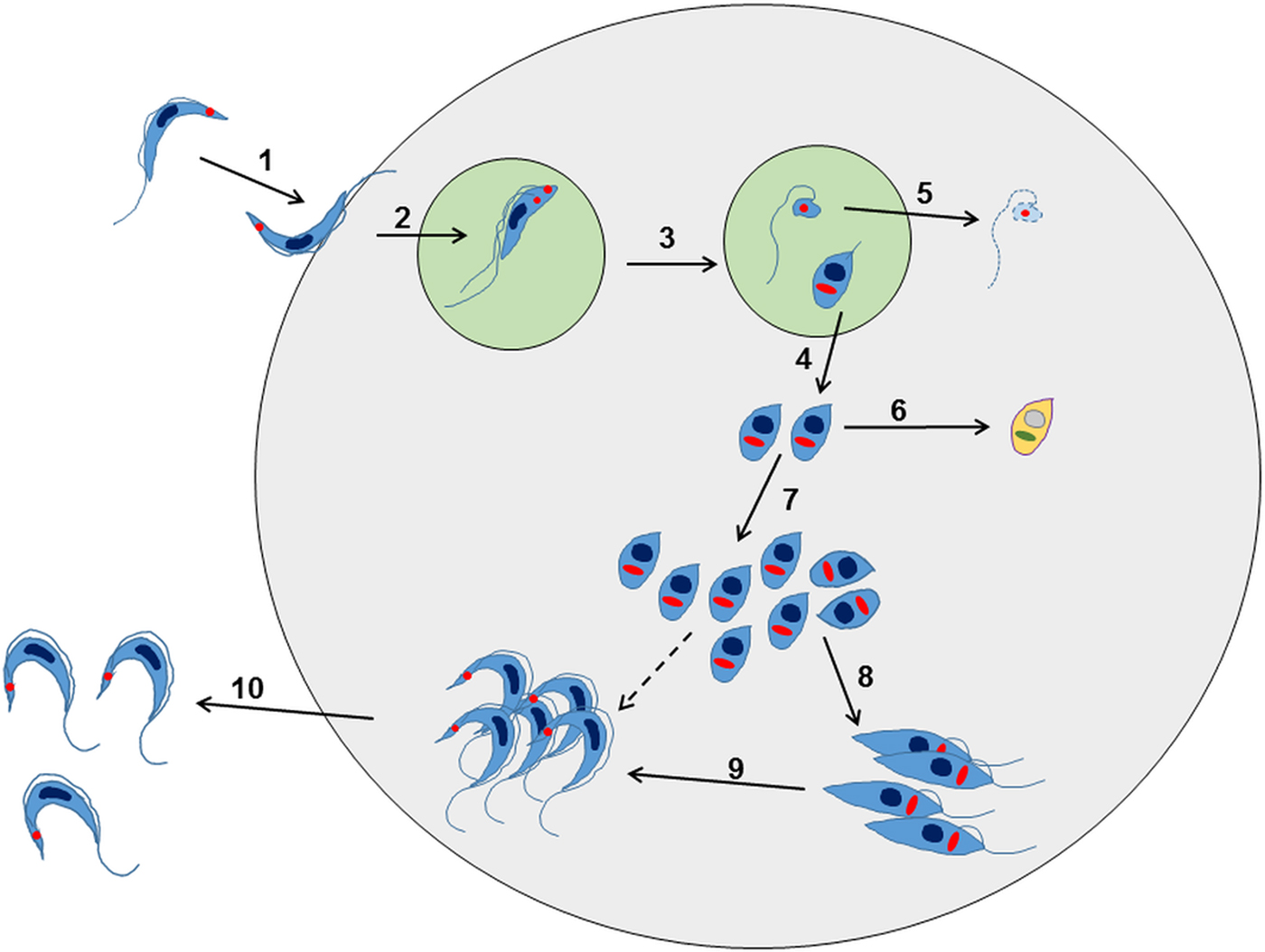
Fig. 2. Overview of the intracellular life cycle of Trypanosoma cruzi in the mammalian host. (1) The metacyclic trypomastigote binds to receptors on the host cell surface resulting in the parasite being taken up into a parasitophorous vacuole. This occurs regardless of whether or not the host cell is phagocytic. (2) The parasite undergoes an asymmetric cell division following replication of the kinetoplast (red circle) and flagellum, but not the nucleus (Kurup and Tarleton, Reference Kurup and Tarleton2014). (3) This results in one daughter cell being a replication competent amastigote with a short flagellum, and the other being a dysnuclear flagellated cytoplasmic fragment. (4) The amastigote escapes into the cytoplasm and begins replication by binary fission. (5) The remaining parasite component is degraded by the proteasome and its antigens are presented on the surface. (6) Some amastigotes may become metabolically quiescent, although this is yet to be proven. Such amastigotes could reside long term in chronically infected tissue. (7) The amastigotes continue to replicate. (8) Amastigotes differentiate into an intracellular epimastigote-like form. It is not clear whether this is an obligate stage, or if they can go straight from amastigotes to trypomastigotes (dashed arrow). (9) The parasites finally differentiate into the flagellated bloodstream trypomastigotes, lyse the host cell and escape into the bloodstream or tissue fluids (10).
The importance of understanding the interplay between drug activity and the parasite life-cycle has been highlighted by studies with CYP51 inhibitors, such as posaconazole and ravuconazole. Although these drugs have low nanomolar EC50 values against a range of T. cruzi strains, they seem unable to completely clear parasites from infected mammalian cells in culture (Moraes et al. Reference Moraes, Giardini, Kim, Franco, Araujo-Junior, Schenkman, Chatelain and Freitas-Junior2014). Consistent with this, studies in murine models have shown that although posaconazole is highly effective at reducing the parasite burden when administered at 10 mg kg−1 day−1 for 25 days, it does not eliminate the infection, even when the dose is increased 10-fold, or when the treatment period extended to 40 days (Khare et al. Reference Khare, Liu, Stinson, Rivera, Groessl, Tuntland, Yeh, Wen, Molteni, Glynne and Supek2015). One possibility is that treatment could induce resistance by promoting higher level expression of the lanosterol 14α-demethylase target. Alternatively, there could be a sub-population of dormant, metabolically quiescent parasites within infected host cells, which have a reduced requirement for ergosterol biosynthesis. Addressing if this is the case, must be considered a major research goal, particularly because the existence of such parasites might have broader relevance for many other classes of drug.
In addition to the above, it could be that metabolically dormant parasites exist in some tissue niches during chronic infections. Investigating this is a major technical challenge, since parasites are present in extremely low numbers during the chronic stage. As outlined below, bioluminescence imaging allows parasites to be localized to specific organs and tissues in murine models, with the colon and/or stomach identified as the major reservoirs during the chronic stage (Fig. 3) (Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Burrell-Saward, McLatchie, Miles and Kelly2014,Reference Lewis, Fortes Francisco, Taylor, Jayawardhana and Kelly2016). However, the technology is not sufficiently sensitive or applicable to allow the microscopic detection of individual infected cells. One strategy currently being developed involves the generation of parasites that express dual bioluminescence:fluorescence reporters, so that infected foci in tissues can be pinpointed, excised and sectioned, and then intracellular parasites visualized by fluorescence microscopy (Taylor et al. unpublished). This type of approach will be essential if the phenotype of the reservoir host cells is to be determined, the metabolic and replicative status of the residual parasites defined, and their response to trypanocidal drugs assessed.
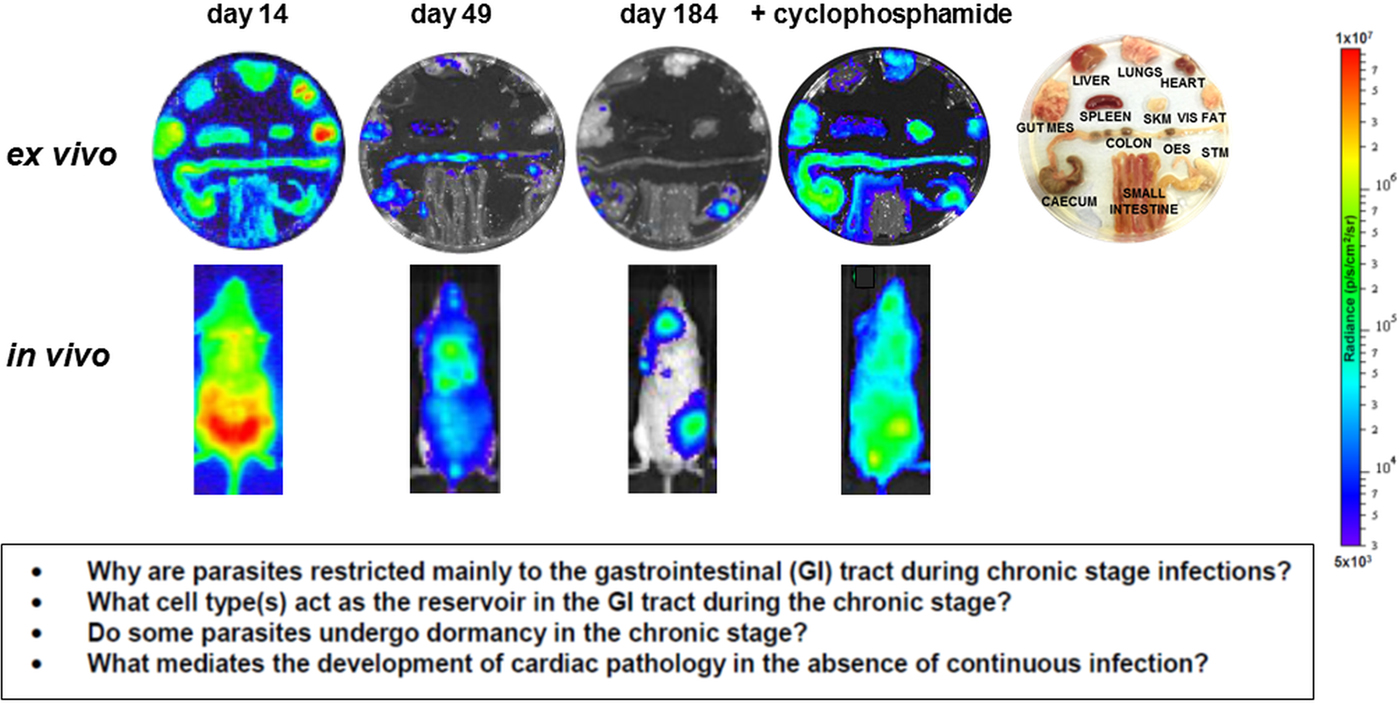
Fig. 3. Parasite tropism during Trypanosoma cruzi infections in a mouse model. BALB/c mice infected with bioluminescent T. cruzi strain CL Brener (Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Burrell-Saward, McLatchie, Miles and Kelly2014) were imaged at various stages post-infection, as indicated. Upper; ex vivo imaging of organs/tissues removed from mice and soaked in D-luciferin. The identity of each organ/tissue is indicated (right). Lower; in vivo imaging of infected mice. The bioluminescence images on the right-hand side are of a chronically infected mouse which has been immunosuppressed by cyclophosphamide treatment (Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Burrell-Saward, McLatchie, Miles and Kelly2014). Heat-maps are on log10 scales and indicate intensity of bioluminescence from low (blue) to high (red). Inset: summary of the major unanswered questions.
DOES PARASITE TROPISM DURING CHRONIC INFECTIONS HAVE THERAPEUTIC IMPLICATIONS?
In humans, infections with T. cruzi are considered to be life-long (Álvarez et al. Reference Álvarez, Fonseca, Borges da Silva, Marinho, Bortoluci, Sardinha, Epiphanio and D'Império Lima2014; Cardoso et al. Reference Cardoso, Reis-Cunha and Bartholomeu2016). However, during the chronic phase, parasites are highly focal, present at extremely low levels, and only sporadically detectable in the bloodstream. This can limit the accurate diagnosis of on-going infections, even with PCR-based methodology (Schijman et al. Reference Schijman, Bisio, Orellana, Sued, Duffy, Mejia Jaramillo, Cura, Auter, Veron, Qvarnstrom, Deborggraeve, Hijar, Zulantay, Lucero, Velazquez, Tellez, Sanchez Leon, Galvão, Nolder, Monje Rumi, Levi, Ramirez, Zorrilla, Flores, Jercic, Crisante, Añez, De Castro, Gonzalez and Acosta Viana2011), and is an important complicating factor in clinical trials. By necessity, most studies on parasite tropism and persistence during human chronic infections have focused on tissue samples retrieved at autopsy, or following organ transplantation. The degree to which these findings are relevant to the majority of infected people is uncertain, and as a result, parasite tropism in asymptomatic individuals is poorly understood.
Intracellular amastigotes have been detected in some chagasic heart samples using histology (Benvenuti et al. Reference Benvenuti, Roggério, Nishiya, Campos, Fiorelli and Levi2014; Kransdorf et al. Reference Kransdorf, Fishbein, Czer, Patel, Velleca, Tazelaar, Roy, Steidley, Kobashigawa and Luthringer2016), and more frequently using PCR or antibody-based techniques (Bellotti et al. Reference Bellotti, Bocchi, de Moraes, Higuchi, Barbero-Marcial, Sosa, Esteves-Filho, Kalil, Weiss, Jatene and Pileggi1996; Schijman et al. Reference Schijman, Vigliano, Viotti, Burgos, Brandariz, Lococo, Leze, Armenti and Levin2004; Burgos et al. Reference Burgos, Diez, Vigliano, Bisio, Risso, Duffy, Cura, Brusses, Favaloro, Leguizamon, Lucero, Laguens, Levin, Favaloro and Schijman2010). Evidence from transplantation-linked transmission, in both endemic and non-endemic regions (Kun et al. Reference Kun, Moore, Mascola, Steurer, Lawrence, Kubak, Radhakrishna, Leiby, Herron, Mone, Hunter and Kuehnert2009; Huprikar et al. Reference Huprikar, Bosserman, Patel, Moore, Pinney, Anyanwu, Neofytos, Ketterer, Striker, Silveira, Qvarnstrom, Steurer, Herwaldt and Montgomery2013), suggests that parasites can be present in a number of organs, with infections more common after heart transplants, than those involving kidney or liver. Parasite DNA has also been detected in the oesophagus (Vago et al. Reference Vago, Macedo, Adad, Reis and Corrêa-Oliveira1996; Lages-Silva et al. Reference Lages-Silva, Crema, Ramirez, Macedo, Pena and Chiari2001) and adipose tissue (Ferreira et al. Reference Ferreira, Segatto, Menezes, Macedo, Gelape, de Oliveira Andrade, Nagajyothi, Scherer, Teixeira and Tanowitz2011) during chronic infections, and parasites can become widely disseminated following reactivation of Chagas disease in immunosuppressed patients (see Fig. 3, as an example in a murine model), or those co-infected with HIV (for review, Lattes and Lasala, Reference Lattes and Lasala2014). CNS involvement leading to meningoencephalitis is a common outcome in these situations (Cordova et al. Reference Cordova, Boschi, Ambrosioni, Cudos and Corti2008; Diazgranados, et al. Reference Diazgranados, Saavedra-Trujillo, Mantilla, Valderrama, Alquichire and Franco-Paredes2009; Yasukawa et al. Reference Yasukawa, Patel, Flash, Stager, Goodman and Woc-Colburn2014).
Current knowledge on infection dynamics and parasite tropism during chronic T. cruzi infections in humans is insufficient to identify which tissue sites are important in terms of drug targeting and bioavailablity, or to determine if specific organs or tissues have a role in recrudescence. Given the practical difficulties in addressing these questions in infected patients, predictive experimental models have been at the forefront of the research effort. Animal models include dogs (Santos et al. Reference Santos, Mazzeti, Caldas, Gonçalves, Lima, Torres and Bahia2016), primates (Vitelli-Avelar et al. Reference Vitelli-Avelar, Sathler-Avelar, Mattoso-Barbosa, Gouin, Perdigão-de-Oliveira, Valério-Dos-Reis, Costa, Elói-Santos, Gomes, Amaral, Teixeira-Carvalho, Martins-Filho, Dick, Hubbard, VandeBerg and VandeBerg2017), chickens (Teixeira et al. Reference Teixeira, Hecht, Guimaro, Sousa and Nitz2011) and most commonly, mice, where several model systems are available which mimic aspects of disease pathology in humans (Eickhoff et al. Reference Eickhoff, Lawrence, Sagartz, Bryant, Labovitz, Gala and Hoft2010; Olivieri et al. Reference Olivieri, Molina, de Castro, Pereira, Calvet, Urbina and Araújo-Jorge2010; Molina-Berríos et al. Reference Molina-Berríos, Campos-Estrada, Lapier, Duaso, Kemmerling, Galanti, Leiva, Ferreira, López-Muñoz and Maya2013; Sbaraglini et al. Reference Sbaraglini, Bellera, Fraccaroli, Larocca, Carrillo, Talevi and Alba Soto2016). However, even in experimental models, difficulties in monitoring parasite burden and location during chronic infections have been a limiting step. With mice, these issues have been partially resolved by the development of highly sensitive bioluminescence imaging procedures, which for the first time enable chronic infections to be assessed in real time (Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Burrell-Saward, McLatchie, Miles and Kelly2014, Reference Lewis, Fortes Francisco, Taylor and Kelly2015). The system, which utilizes genetically modified parasites that express red-shifted luciferase (Branchini et al. Reference Branchini, Ablamsky, Davis, Southworth, Butler, Fan, Jathoul and Pule2010), has a limit of detection close to 100 parasites in inoculated mice and allows infections to be monitored in individual animals for more than a year (Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Burrell-Saward, McLatchie, Miles and Kelly2014). In the BALB/c mouse -T. cruzi CL Brener (DTU VI) model, the infection peaks on day 14 post-inoculation (Fig. 3). Following induction of an adaptive immune response, the parasite burden then decreases by two to three orders of magnitude over the next 30–40 days, as the infection progresses to the chronic stage. Long-term infections are characterized by a dynamic profile, in which bioluminescence foci appear and disappear over a period of hours, in an apparently stochastic manner (Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Burrell-Saward, McLatchie, Miles and Kelly2014). Similar patterns of infection occur with other parasite lineages and mouse strains, although with some differences in the precise timing of progression, presumably reflecting the influence of host and parasite genetics (Taylor et al. Reference Taylor, Lewis, Fortes Francisco, Wilkinson and Kelly2015; Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Jayawardhana and Kelly2016).
In the murine acute stage, the infection is pan-tropic, with parasites easily detectable by ex vivo imaging in all organs and tissues (Fig. 3). However, in the chronic stage, the colon and/or stomach are the primary sites of infection. Other organs, including the heart, are infected only sporadically, with the extent of this varying in different host:parasite strain combinations (Lewis et al. Reference Lewis, Fortes Francisco, Taylor, Jayawardhana and Kelly2016). Myocarditis and heart fibrosis can develop in the absence of end-point cardiac infection, implying that the continuous presence of the parasite in the heart is not a pre-requisite for chagasic pathology. These data suggest a model where the gut is a permissive immunological niche that tolerates continuous low-level infection, with periodic trafficking of parasites, or more likely, parasite-infected cells, from this reservoir to other sites, including the heart. This leads to the generation of intermittent inflammatory immune responses that eliminate the transient infections in non-gut sites, but can result in collateral damage to surrounding tissue (Lewis and Kelly, Reference Lewis and Kelly2016). It is implicit in this model that drug-mediated elimination of parasites from the gut reservoir sites should lead to parasitological cure in asymptomatic, immunocompetent individuals, with the immune system eradicating the remaining parasites from non-gut sites. While this model has yet to be proven, it does generate a number of questions with implications for drug development. First, what is the nature of the host cells in which parasites persist within the gut reservoir sites, and what is their immunological and metabolic status? Second, do parasites in these sites display dormancy, and if so, does this affect their sensitivity to therapeutic treatment? Third, do the transient bioluminescent foci in chronically infected mice represent infected cells undergoing trafficking, and what is their fate in an immunocompetent individual? Finally, can these insights into parasite tropism and persistence be extended from murine models to human patients?
The current inability to reliably cure chronic T. cruzi infections has led to speculation that the parasite might be able to survive in organs/tissues where drug access is limited. However, this appears not to be the case with nitroheterocyclic drugs, at least in mice. Treatment failures, in both acute and chronic infections, are not linked to a single or predominant site of recrudescence (Francisco et al. Reference Francisco, Lewis, Jayawardhana, Taylor, Chatelain and Kelly2015, Reference Francisco, Jayawardhana, Lewis, White, Shackleford, Chen, Saunders, Osuna-Cabello, Read, Charman, Chatelain and Kelly2016). Consistent with this, a detailed study of benznidazole pharmacokinetics has revealed that inadequate bio-distribution is unlikely to be responsible for therapeutic failure during chronic phase murine infections (Perin et al. Reference Perin, Moreira da Silva, da Silva Fonseca, Mirelle de Oliveira Cardoso, Mathias, Reis, Molina, Correa-Oliveira, de Abreu Vieira and Carneiro2017). With posaconazole, the situation is less clear-cut. Although adipose tissue has been identified as a frequent site of cyclophosphamide-induced relapse after non-curative treatment of acute stage infections (Francisco et al. Reference Francisco, Lewis, Jayawardhana, Taylor, Chatelain and Kelly2015), this was not observed in all mice. Adipose tissue has been implicated as a possible reservoir of recrudescence in other parasitic infections, including African trypanosomiasis (Trindade et al. Reference Trindade, Rijo-Ferreira, Carvalho, Pinto-Neves, Guegan, Aresta-Branco, Bento, Young, Pinto, Van Den Abbeele, Ribeiro, Dias, Smith and Figueiredo2016; Tanowitz et al. Reference Tanowitz, Scherer, Mota and Figueiredo2017); however, further work will be required before definitive conclusions can be drawn about the situation in T. cruzi.
DOES DRUG TREATMENT PREVENT OR ALLEVIATE CHRONIC DISEASE PATHOLOGY?
At a population level, the major health and economic burdens associated with Chagas disease result from chronic cardiac pathology. However, chronic stage studies, particularly on drug efficacy and disease pathogenesis, are made difficult by the scarce and highly focal nature of T. cruzi infection. As a result, the major research effort has focussed on the acute stage, where the monitoring of parasite load and the assessment of tissue tropism are more straightforward. There has been much discussion within the community on the underlying causes of chronic Chagas disease pathology (Gironès et al. Reference Gironès, Cuervo and Fresno2005; Kierszenbaum, Reference Kierszenbaum2005; Gutierrez et al. Reference Gutierrez, Guedes, Gazzinelli and Silva2009; Bonney and Engman, Reference Bonney and Engman2015). The context for this debate has been the inability to routinely detect parasites in the hearts of patients with cardiac damage and the non-specific (and sometimes autoreactive) polyclonal B-cell and T-cell responses that are characteristic of T. cruzi infection (Iwai et al. Reference Iwai, Juliano, Juliano, Kalil and Cunha-Neto2005; Bermejo et al. Reference Bermejo, Amezcua Vesely, Khan, Acosta Rodríguez, Montes, Merino, Toellner, Mohr, Taylor, Cunningham and Gruppi2011). The central question has been: does chronic stage cardiac pathology develop as a consequence of autoimmunity, or does it result from parasite persistence and the generation of aberrant inflammatory responses within target organs? There is now a strong consensus that the presence of the parasite is a pre-requisite for heart pathology (for review, Bonney and Engman, Reference Bonney and Engman2015), although as mentioned above, the mode of cardiac infection may be one of episodic re-invasion rather than continuous persistence (Lewis and Kelly, Reference Lewis and Kelly2016).
Cardiomyopathy develops in 20–30% of T. cruzi-infected patients. Progressive heart failure, thromboembolism, ventricular arrhythmia, stroke and sudden death are common outcomes (Rossi et al. Reference Rossi, Ramos and Bestetti2003; Carod-Artal, Reference Carod-Artal2010; Carod-Artal and Gascon, Reference Carod-Artal and Gascon2010). Patient management is based on standard protocols for treating progressive cardiac failure (Ribeiro et al. Reference Ribeiro, Nunes, Teixeira and Rocha2012), despite the lack of robust randomized clinical trials to validate their use in cases of chagasic heart disease. The ability of drug treatment to prevent or alleviate the development of cardiac pathology is unresolved, and controversial. Despite this, the consensus view is that treatment should be offered to patients infected with T. cruzi, irrespective of their disease status. The validity of this approach is one of the central debates in the Chagas disease field. There is reasonable evidence from experimental models that curative treatment of acute stage infections results in reduced disease pathology in the longer term (Davies et al. Reference Davies, Marino Cardozo, Sánchez Negrette, Mora, Chung and Basombrío2010; Molina-Berríos et al. Reference Molina-Berríos, Campos-Estrada, Lapier, Duaso, Kemmerling, Galanti, Leiva, Ferreira, López-Muñoz and Maya2013; Assíria Fontes Martins et al. Reference Assíria Fontes Martins, de Figueiredo Diniz, Mazzeti, da Silva do Nascimento, Caldas, Caldas, de Andrade, Ribeiro and Bahia2015; Gruendling et al. Reference Gruendling, Massago, Teston, Monteiro, Kaneshima, Araújo, Gomes, Barbosa and Toledo2015). With chronic stage infections, the data are less clear-cut (Villar et al. Reference Villar, Perez, Cortes, Riarte, Pepper, Marin-Neto and Guyatt2014). For example, in a recent large multi-centre, randomized clinical trial, no significant improvements in terms of cardiomyopathy were observed 5 years after benznidazole treatment (BENEFIT trial; Morillo et al. Reference Morillo, Marin-Neto, Avezum, Sosa-Estani, Rassi, Rosas, Villena, Quiroz, Bonilla, Britto, Guhl, Velazquez, Bonilla, Meeks, Rao-Melacini, Pogue, Mattos, Lazdins, Rassi, Connolly and Yusuf2015). However, because evidence of cardiomyopathy was a pre-requisite for enrolment in this trial, it has not been possible to draw conclusions about the type of outcomes that might be achievable by treating asymptomatic individuals.
Clinical trials to assess the link between anti-parasitic treatment and reductions in pathology present numerous challenges. These include the long-term and diverse nature of the human disease, the toxicity of current drugs, the resulting compliance issues, and difficulties in demonstrating parasitological cure. This is complicated further by other variables such as the severity or otherwise of the acute stage infection, the possibility of re-infection/co-infection, host and parasite genetics, environmental factors and immune status. With advances in imaging technology, which allow real-time monitoring of chronic stage infections, it should be feasible to better exploit predictive animal models to investigate the rationale for using anti-parasitic drugs to prevent or alleviate symptomatic Chagas disease. Data from such experiments will be invaluable for informing the design of clinical trials aimed at establishing, for example, if there is a post-infection time limit within which curative therapy has to be administered to significantly impact on the development of cardiac pathology. The outcome of such studies should have major implications for the 5–8 million people infected with T. cruzi. However, it should be noted that currently, only 1% of those infected have access to diagnosis and treatment (DNDi, 2015).
Concluding remarks
The development of more effective drugs against Chagas disease is a major challenge for the biomedical research community. The complexity of the infection, combined with our limited understanding of parasite biology and disease pathogenesis are major factors that inhibit progress in this area. Addressing these problems will require a twin track strategy; basic research to address the questions outlined in this review, and applied research, with input from the not-for-profit consortia and the commercial sector, to exploit the resulting opportunities and fast-forward the drug development pipeline.
FINANCIAL SUPPORT
JMK acknowledges financial support from the British Heart Foundation (PG/13/88/30556), the Australian Research Council (LP140100560) and the Drugs for Neglected Diseases Initiative (DNDi). MDL is supported by an EU Marie Curie Fellowship (625810).






