Introduction
Energy use and production are without a doubt the main causes behind the pollution of our cities and current climate change. Decreasing emissions from the transport sector as well as from the production of electricity, simultaneously with the continual increase in energy consumption and the need for mobility—not the least in emerging economies—is the biggest challenge we face today. Not surprisingly, there is a strong effort in the development of sustainable technologies for clean electricity production and energy conversion. In the short term, progress can be made with improved efficiency of processes. One example is the strong trend today with small and highly efficient combustion engines in new cars, which help to cut fuel consumption, in some cases, by more than 50% compared to standard models sold 10 years ago. Another example is the improved efficiency of thermal power plants by optimizing the combustion process (e.g., increasing the temperature). However, to achieve true sustainability in the long term, the only solution is to move to renewable energy sources and to go from combustion engines to electric drive trains.
Several steps have already been taken on the path to sustainability (also see the April 2012 special issue of MRS Bulletin on “Materials for sustainable development”)—installation of wind farms, solar cells in private houses, and an increasing number of hybrid vehicles. The principal concepts are there, but in many cases, the key components for large-scale implementation are lacking. To take the next step, performance, cost, reliability, and safety need to be improved. As for many other technology areas, the lack of sufficiently good materials is one of the main limiting factors. In the transport sector, the major hindrance for large-scale implementation of electric vehicles is the lack of safe and low-cost large capacity energy storage systems. Li batteries are the main candidate being considered, but capacity and safety issues, the latter mainly due to the presence of a volatile electrolyte, are slowing down progress. Fuel cells, converting hydrogen to electricity, were demonstrated in automotive applications some 17 years ago,Reference Coppel1 but also in this case, an optimal electrolyte is lacking. The target here, from the automotive industry, is an operating temperature of around 110–130°C. The US Department of Energy (DOE) target for automotive applications is 120°C to allow efficient electrode reactions and to move away from expensive and unsustainable noble metal catalysts.
In this issue of MRS Bulletin, we highlight the potential of ionic liquids (ILs) in energy applications that can contribute significantly to the transition to sustainable production of electricity and use of energy. In this article, we provide an introduction to ILs and their properties, with these serving as the basis for the other topical articles in the issue on efficient and environmentally friendly synthesis methods for new ILs (Passerini and Appetecchi), Li batteries (Navarra), supercapacitors (Brandt et al.), fuel cells (Yasuda and Watanabe), and the synthesis of new materials and structures in ILs (Endres).
Only ions
As the name implies, ILs consist only of ions. Compounds consisting only of ions are normally known as salts, and indeed ILs are salts with very low melting points. A generally accepted definition of ILs is that the melting point should be below 100°C.Reference Wilkes2 On many occasions, it is desired to also have the liquid phase at ambient conditions, and room-temperature ILs are defined as having a melting point below room temperature. For a low melting point, ILs commonly consist of bulky and asymmetric ions with a large degree of charge delocalization. Increasing the size of the ions decreases ion-ion interactions and prevents efficient packing of the ions into a crystal structure. The influence of size and charge delocalization on the melting point is shown in Figure 1 for several salts, systematically changing the cation and anion.

Figure 1. Increasing the size and charge delocalization of the ions decreases the electrostatic interactions and prevents efficient packing into a crystal structure, effectively lowering the melting point of the salt. BMIm, butyl-methylimidazolium; TFSI, bis(trifluoromethylsulfonyl)imide; and IL, ionic liquid.
Cations in ILs are large organic ions. Perhaps, the best-known example is the group of ILs based on the imidazolium cation, as indicated in Figure 1, but there is a whole range of cation families based on, for instance, pyrrolidinium, piperidinium, or ammonium cations. Anions, on the other hand, are commonly inorganic and range from the quite small and charge-localized halides (Cl– or Br–) to more bulky and weakly coordinating ions, with hexaflourophosphate (PF6–) and bis(trifluoromethylsulfonyl)imide (known as TFSI or NTf2) being two examples shown in Figure 1. In Figure 2, the structures of some common IL anions and cations are shown. The large variety of base structures and the relative ease to modify these, for instance by changing the length and type of side chains, leads to an enormous number of possible ILs, and thus the possibility to tailor properties.Reference Earle and Seddon3 As a result, ILs find application in a range of areas, including as lubricants in tribology,Reference Minami4 as solvents in biomass processing,Reference Brandt, Gräsvik, Hallet and Welton5 in catalysis,Reference Hallett and Welton6 in the stabilization of proteins,Reference Fujita, MacFarlane and Forsyth7,Reference Noritomi, Minamisawa, Kamiya and Kato8 and in electrochemical applications. In addition to Li batteries, fuel cells, supercapacitors, and electrochemical synthesis, ILs have also been applied in two other areas of relevance for new energy systems: in solar cells, as a solvent and as a source of redox couples in dye-sensitized solar cells,Reference Papageorgiou, Athanassov, Armand, Bonhote, Pettersson, Azam and Grätzel9–Reference Wang, Zakeeruddin, Comte, Exnar and Grätzel11 and as an electrolyte in biofuel cells.Reference Masuda, Motoyama, Kuwahara, Nakamura and Ohno12
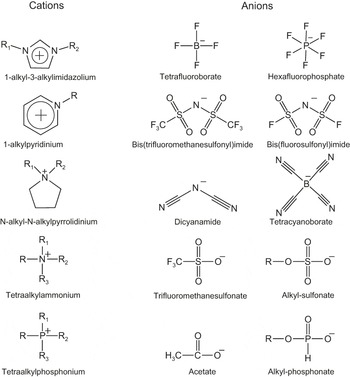
Figure 2. Structures of some common anions and cations in ionic liquids. R, R1, and R2 represent side groups, most commonly an alkyl chain.
Properties
Interest in ILs took off some 20 years ago, but low melting salts were already reported in the literature in the 1880s.Reference Gabriel and Weiner13 This interest arises from several generic properties of ILs that can be utilized in applications. Generally, ILs have a low vapor pressure, high ionic conductivity, large temperature range for the liquid phase, thermal and electrochemical stability, and large flexibility in molecular design.Reference Earle and Seddon3,Reference Welton14 The low vapor pressure is due to the ionic nature of the liquid. It has been shown that the species in the evaporation of ILs are ion pairs that are not present in the liquid.Reference Armstrong, Hurst, Jones, Licence, Lovelock, Satterley and Villar-Garcia15 The low vapor pressure also results in a very high boiling point for ILs, where thermal decomposition is commonly found to occur before boiling. Thus, the upper temperature limit for the use of ILs is determined by the decomposition, resulting in the large liquid range commonly observed.
For the applications featured in this issue, ionic conductivity is one of the most important properties. It is obviously related to the high density of ions in the liquid and to the fact that the ions are dissociated. Even though there can be clusters of ions present in the liquid, the ions are not present in the form of neutral ion pairs, which would destroy the low vapor pressure characteristic of the liquid. The ionic conductivity is also closely related to the viscosity of the liquid, with the ions being transported by viscous flow. In general, ILs are found to follow the Walden ruleReference Walden16,Reference Xu, Cooper and Angell17 relating the molar conductivity, Λ, to the inverse of the viscosity, η
However, the slope of the line in the Walden plot is slightly below one, expected for ideal behavior.Reference Xu, Cooper and Angell17 This can be related to the clustering of ions or to how ionic the liquid actually is—the iconicity of the liquid.Reference Tokuda, Tsuzuki, Susan, Hayamizu and Watanabe18 There are some examples where ILs are found above the ideal Walden line.Reference Walden16 These are usually protic ILs, where a mobile proton can contribute with a hopping mechanism to the conductivity in excess of the viscous flow (see the article by Yasuda and Watanabe in this issue).
Whereas a low melting point is central for an IL, it is not directly related to a low viscosity. The viscosity is instead related to the glass transition temperature of the liquid. The lower the glass transition temperature, the lower the viscosity of the liquid. The temperature dependence of the viscosity of ILs, the so-called fragility,Reference Xu, Cooper and Angell17,Reference Leys, Wübbenhorst, Menon, Rajesh, Thoen and Glorieux19 is often found to be similar, and as long as one stays within a given anion family, it is very similar.Reference Leys, Wübbenhorst, Menon, Rajesh, Thoen and Glorieux19 Thus, the absolute value of the viscosity at a certain temperature is, to a large extent, dictated by the value of the glass transition temperature.
In order to decrease the melting point, an increase in the ion size was shown to be beneficial, as discussed previously (see Figure 1). This is, to some extent, also valid for the glass transition temperature, T g. Angell and co-workersReference Xu, Cooper and Angell17 showed how this is valid as long as the ions are small and the interaction is dominated by electrostatic contributions. This is, for instance, the case when halides are used as anions. When the ions are large with long side chains, the contribution from van der Waals interactions needs to be taken into account, as shown in Figure 3. In this case, increasing the ion size can lead to an increase in the glass transition temperature.Reference Xu, Cooper and Angell17 Adding further interactions to the system, such as hydrogen bonding, will also lead to an increase in T g (when comparing two ILs of similar size). As an example, one can compare the glass transition temperatures of two ILs with the cations 1-ethyl-3-methyl imidazolium (C2MIM) and 1-ethanol-3methylimmidazolium (EtOHMIM) and with the TFSI anion. Here, the cations only differ in that the end group on the side chain is a CH3 group on C2MIM and C–OH on EtOHMIM. The glass transition temperature for C2MIMTFSI is considerably lower (T g = 181 K20) compared to that found for EtOHMIMTFSI (T g = 194 K21).

Figure 3. Depending on the structure of the ionic liquids, the interaction between the ions can have contributions from electrostatic, van der Waals, and hydrogen bonding forces. In the example with the cation C10MIM (1-decyl-3-methyl imidazolium), the charge is mainly localized to the imidazolium ring (pink shade), whereas the long side chain will contribute with a van der Waals component (blue shade). By exchanging the methyl group at the end of the side chain with an OH group, hydrogen bonding (green shade) will also contribute to the total interaction of the system. Note: k B, Boltzmann constant; T, temperature.
As for other liquids, the temperature dependence of the inverse of the viscosity, η–1, of ILs is well described by the empirical Vogel-Fulcher-Tamman (VFT) equation:
where ![]() ${{\rm{\eta }}_0}$ is the viscosity at infinite temperature (usually set to 10–4 Pa.s), B is a constant controlling the deviation from an Arrhenius behavior (inversely proportional to the fragility), and T 0 is a constant sometimes referred to as the ideal glass transition temperature.Reference Xu, Cooper and Angell17,Reference Tokuda, Hayamizu, Ishii, Susan and Watanabe22,Reference Castner and Wishart23 As a result of the Walden rule, the conductivity (σ) is also found to obey the (VFT) equation with
${{\rm{\eta }}_0}$ is the viscosity at infinite temperature (usually set to 10–4 Pa.s), B is a constant controlling the deviation from an Arrhenius behavior (inversely proportional to the fragility), and T 0 is a constant sometimes referred to as the ideal glass transition temperature.Reference Xu, Cooper and Angell17,Reference Tokuda, Hayamizu, Ishii, Susan and Watanabe22,Reference Castner and Wishart23 As a result of the Walden rule, the conductivity (σ) is also found to obey the (VFT) equation with
where ![]() ${{\rm{\sigma }}_0}$ in this case is the high temperature limit of the ionic conductivity. Thus, to obtain high conductivity, a low glass transition temperature is desired.Reference Tokuda, Hayamizu, Ishii, Susan and Watanabe22,Reference Castner and Wishart23 Figure 4 shows the temperature dependence of the ionic conductivity for a few selected ILs.Reference Pitawala, Kim, Jacobsson, Koch, Croce and Matic24,Reference Martinelli, Matic, Jacobsson, Börjesson, Fernicloa and Scrosati25 The deviation from Arrhenius behavior is quite obvious, and when comparing it with the glass transition temperature, it is clear that the magnitude of the ionic conductivity at a fixed temperature correlates well with T g. This is even more obvious when the same data are plotted with a T g-scaled temperature axis (Figure 4b). All conductivity curves now collapse onto a master curve as long as the material is in the liquid state.Reference Pitawala, Kim, Jacobsson, Koch, Croce and Matic24,Reference Martinelli, Matic, Jacobsson, Börjesson, Fernicloa and Scrosati25
${{\rm{\sigma }}_0}$ in this case is the high temperature limit of the ionic conductivity. Thus, to obtain high conductivity, a low glass transition temperature is desired.Reference Tokuda, Hayamizu, Ishii, Susan and Watanabe22,Reference Castner and Wishart23 Figure 4 shows the temperature dependence of the ionic conductivity for a few selected ILs.Reference Pitawala, Kim, Jacobsson, Koch, Croce and Matic24,Reference Martinelli, Matic, Jacobsson, Börjesson, Fernicloa and Scrosati25 The deviation from Arrhenius behavior is quite obvious, and when comparing it with the glass transition temperature, it is clear that the magnitude of the ionic conductivity at a fixed temperature correlates well with T g. This is even more obvious when the same data are plotted with a T g-scaled temperature axis (Figure 4b). All conductivity curves now collapse onto a master curve as long as the material is in the liquid state.Reference Pitawala, Kim, Jacobsson, Koch, Croce and Matic24,Reference Martinelli, Matic, Jacobsson, Börjesson, Fernicloa and Scrosati25

Figure 4. (a) Temperature (T) dependence of the ionic conductivity of some selected ionic liquids. The abrupt change in conductivity found for EMIMTFSI and PYR14TFSI is related to crystallization (i.e., in the low temperature regime, these two materials are solids). (b) Ionic conductivity shown with a scaled temperature axis. The scaling parameter is the glass transition temperature (T g). All data collapse onto one master curve as long as the material is in the liquid state. The ionic liquids in the figure are (BzMI)2C52Im—1,5 bis(3-benzyl-2-methylimidazolium)pentane di-bis(trifluoromethylsulfonyl)imide, (M2I)2C102Im—1,10 bis(2,3-dimethylimidazolium)decane di-bis(trifluoromethylsulfonyl)imide, PYR14TFSI—N-butyl-N-ethylpyrrolidinium bis(trifluoromethylsulfonyl)imide, and EMIMTFSI—1-ethyl-3-methyl imidazolium bis(trifluoromethylsulfonyl)imide. The original data can be found in References 24 and 25.
In order to further develop ILs, it is of interest to make a connection between the macroscopic properties discussed previously and the microscopic structure of the cations and anions as well as the coordination of anions and cations in the liquid. It has, for instance, been shown that there is a considerable degree of structural heterogeneities on mesoscopic length scales (the range of 1–10 nm) in ILs.Reference Salanne, Simon, Turq and Madden26–Reference Russina, Gontrani, Fazio, Lombardo, Triolo and Caminiti28 The heterogeneity is inferred from a well-defined peak, found in the range of a few nm–1 in the static structure factor, in small-angle x-ray scattering data, as well as in molecular dynamics simulations. The heterogeneities are suggested to originate from segregation of the alkyl chains into nonpolar domains embedded into a charged matrix. We have also recently shown how the Li-ion coordination changes as a function of Li-salt concentration in Li-salt-doped ILs, and this is reflected in changes in the glass transition temperature.Reference Pitawala, Kim, Jacobsson, Koch, Croce and Matic24 It is now of interest to link such knowledge, for instance, to transport properties to rationally design new materials with optimized properties for particular applications.
In this issue
We sample some of the research where ILs are applied to electrochemical devices. Passerini and Appetecchi show how synthesis routes for ILs can be developed to meet requirements for purity, high yield, and environmental friendliness for large-scale applications. Navarra shows how ILs can contribute to the safety of Li-ion and Li-metal batteries. Increasing safety while maintaining capacity is key for introducing Li-batteries in large-scale energy storage systems. Related to this is the example provided by Endres on the preparation of nanostructured electrodes from ILs. Brandt et al. provide insight into how ILs can contribute to both improved performance and safety in supercapacitors. Supercapacitors can provide very high power and very good cycle life that are important, for instance, for electric vehicles, where a combined supercapacitor/battery system might be an optimum solution. As a last example, Yasuda and Watanabe show the application of protic ILs to fuel cells. The possibility of operating fuel cells at intermediate temperatures, around 130°C and under non-humidified conditions, paves the way for large-scale applications, as precious metal catalysts can be avoided and the efficiency increased.
Acknowledgments
The guest editors are grateful to all authors contributing articles to this issue. Special thanks also to Dr. Jagath Pitawala for help with figures. A.M. wishes to acknowledge the Swedish Research Council and FORMAS for generous support for research on ionic liquids and their applications.







