Introduction
Periprosthetic joint infections (PJIs) are rare complications of prosthetic device surgery [Reference Zimmerli, Trampuz and Ochsner1]. These infections are associated with high morbidity, mortality and health costs [Reference Darouiche2]. The incidence rate of PJIs for knee or hip prosthesis is 1–3%. The most frequent bacteria found in these infections are staphylococci. Specifically, in chronic PJIs, the most frequent bacteria involved are coagulase-negative staphylococci (CNS), which cause 19–40% of infections [Reference Stefani and Varaldo3]. CNS strains are often resistant to many antibiotics, especially to anti-staphylococcal beta-lactams, with methicillin resistance observed in 60–70% of all isolates [Reference Stefani and Varaldo3]. This resistance is often associated with resistance to fluoroquinolones, clindamycin and rifampicin, the first line of orally available antibiotics for use in bone and joint infections [Reference Becker, Heilmann and Peters4]. New agents, including linezolid, daptomycin and tigecycline, have been developed as alternatives to glycopeptides against multi-resistant strains.
CNS, especially S taphylococcus epidermidis, are able to produce a biofilm and remain in a nongrowing phase [Reference Schoenfelder5]. Antibiotic combination treatments must consider biofilm penetration and the frequent adaptations for resistance to antimicrobials. Many CNS with various antibiotic susceptibility profiles can be involved in PJIs. Defining the most frequent profiles associated with these species is of interest. In literature, a few data have been reported on antibiotic susceptibilities profiles of CNS causing PJIs.
The aim of this study was to determine the distribution of all the species of CNS involved in PJIs and compare their antimicrobial susceptibility.
Material and methods:
We conducted a retrospective, multi-centre study, which included three hospitals: two university hospitals and a clinic in the south of France, from 2011 to 2015.
All patients were >18-years-old, living in the south-west area of France, diagnosed with a CNS PJIs. PJIs diagnosis was based on multidisciplinary criteria.
The design of the study was the same in all the centres: diagnosis criteria and microbiological analysis. Treatments and follow-up were performed by the same infectious diseases specialist.
Patients and samples
Diagnosis of PJIs was suspected based on clinical, biological, microbiological, histopathological and radiological arguments [Reference Osmon6, Reference Parvizi, Gehrke and Chen7].
Microbiological PJIs diagnosis was established by the presence of at least two positive periprosthetic cultures with the same species and antibiotic susceptibility profile.
Intraoperative bone tissue, synovial membranes and articular fluid samples were used to perform the microbiological assessments and diagnoses.
At least three deep intraoperative samples were collected per patient. After collection, the samples were transferred to the microbiological laboratory in less than 1 h.
All patients were managed in the orthopaedic unit of the hospitals by a multi-disciplinary team, which included an orthopaedic surgeon, an infectious diseases specialist, a radiologist and a microbiologist. The type of surgery was determined by the common advice of the orthopaedic surgeon and the infectious disease specialist. Three types of surgery were used: irrigation and debridement, one- or two-stage exchange of the implant, or resection arthroplasty.
Bacteriological culture
For each suspicious site, solid and tissue specimens were collected in sterile ball vials; articular fluids were inoculated in blood culture bottles. All samples were incubated with CO2 and in an anaerobic atmosphere for 15 days. Gram staining was performed for each sample on day 1. Solids and tissues were then crushed by vortexing for 10 min in 1 ml of saline solution. Standard cultures were performed on Columbia blood agar, polyvitex chocolate agar and thioglycolate solution (Oxoid®, Dardilly, France). Media were observed daily for microbial growth.
In the case of positive culture, identification was performed by an automatised technique, using Vitek2 Staphylococci cards (Biomérieux®), or in case of failure manually, using ApiStaph (Biomérieux®, Marcy l'Etoile, France).
From 2015, identification was performed by MALDI-TOF (Brucker) and all the strains previously found were identified by this technic.
Antimicrobial susceptibilities were tested on Vitek2 cards (Biomérieux®) according to the recommendations of the Committee of Antibiotic Susceptibility from the French Society of Microbiology [Reference Bonnet8]. Methicillin resistance was interpreted from oxacillin minimal inhibitory concentrations (MIC). Staphylococci strains were considered as susceptible when the MIC between 0.5 and 2 mg/l were included. Discordant methicillin susceptibility results were verified by cefoxitin and moxalactam disks according to the Comity of Antibiotic susceptibility from the French Society of Microbiology recommendations (Bio-Rad®, Marnes-la-Coquette, France) [Reference Bonnet8].
Glycopeptides susceptibilities were interpreted from MIC tested by broth microdilution. The CNS strains were considered as susceptible when vancomycin MIC were under 2 mg/l and teicoplanin MIC under 4 mg/l [Reference Bonnet8].
Antibiotic therapy
The empirical intravenous antibiotic prescribed was vancomycin or daptomycin, in combination with ceftriaxone or piperacillin-tazobactam, for at least 7 days.
Antibiotic therapy was adapted when microbiological results were obtained. Oral antibiotic treatment was planned for at least 6–8 weeks, according to French and International guidelines [Reference Osmon6, Reference Dupont and Dutronc9].
Follow-up
The outcome was evaluated after a follow-up of 24 months for all the patients.
A multidisciplinary consult (surgeon and infectious disease physician) was performed, which included a clinical and radiological evaluation and a CRP blood analysis.
Regarding statistical analysis, all quantitative results were expressed in percentages.
Results
Between 2011 and 2015, 215 CNS strains causing PJIs were included from 179 patients.
The mean age of the patients was 69.5 years (43–94), 77.4% of the patients were men.
The mean number of samples collected was five samples per patient.
Regarding PJIs localisations, the knees were involved in 54.2% of the patients, the hips in 39.1% and other sites (ankles, shoulders) in 6.7% (Table 1).
Table 1. PJI sites and antibiotic resistance profiles of CNS in PJI
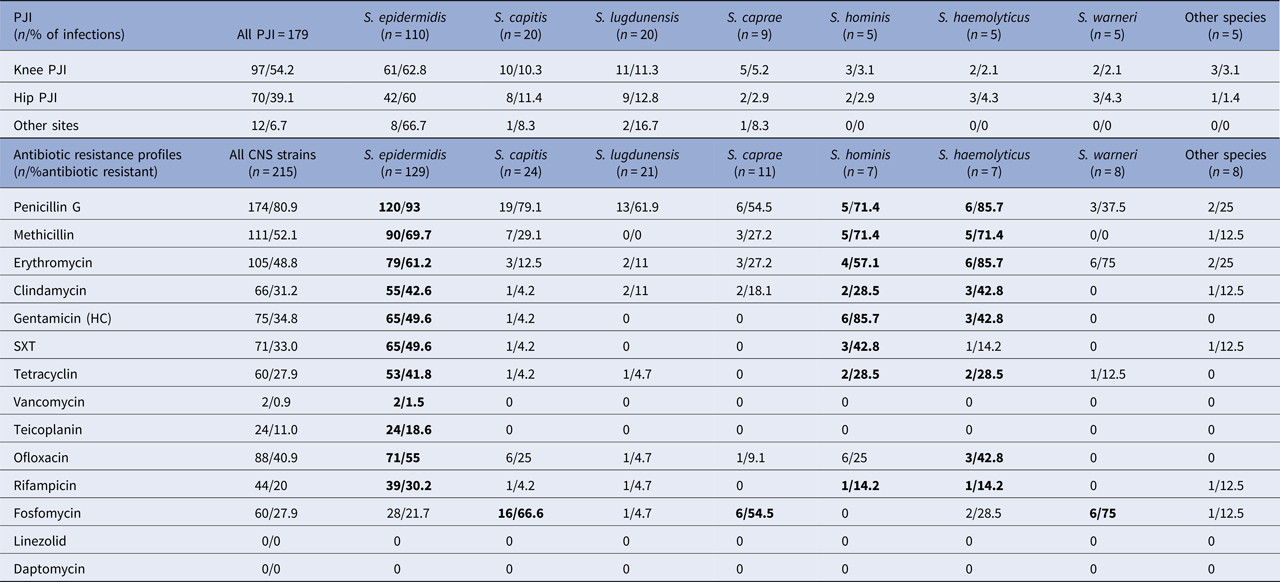
Other species: S. schleiferi. S. simulans. S. condimenti. S. intermedius. S. cohni; Antibiotics: SXT, trimethoprim + sulfamethoxazole (cotrimoxazole). Bold values mentioned as most resistant species of CNS.
CNS species in decreasing order are as follows: S taphylococcus epidermidis (SE) 129 (60%), S taphylococcus capitis 24 (11%), S taphylococcus lugdunensis (SL) 21 (10%), S taphylococcus caprae 11 (5%), S aphylococcus. warneri (SW) 8 (4%), S taphylococcus hominis (S. Ho) 7 (3%), S taphylococcus haemolyticus (S. Ha) 7 (3%) and other species 8 (4%).
SE, S. capitis and SL were the most frequent species found in CNS PJIs regardless of the PJI's site.
CNS global antibiotic susceptibilities
Eighty-one percent of the CNS strains were resistant to penicillin G and 52.1% to methicillin. Regarding oral antibiotic used in the treatment of PJI, 31.2%, 40.9%, 33%, 20% and 27.9% of the strains were resistant to clindamycin, ofloxacin, trimethoprim + sulfamethoxazole (SXT), rifampicin and tetracycline, respectively.
Species-specific susceptibilities of the CNS
Of all CNS species and for all classes, the most resistant species was SE.
Focusing on methicillin, the most resistant species were SE, S. Ho and S. Ha, with 70%, 71% and 71% resistance, respectively.
The oral antibiotics used in bone and joint infections were specifically analysed.
The most resistant species to clindamycin were SE, S. Ha and S. Ho, with 43%, 43% and 29% resistance, respectively.
For the fluoroquinolones, 55% of the SE, 43% of the S. Ha and 25% of the S. capitis and S. Ho strains were resistant to ofloxacin.
For the rifampicin, 30% of the SE, 14% of the S. Ho and 14% of the S. Ha strains were resistant.
For the SXT, 50% of the SE and 43% of the S. Ho strains were resistant.
For the glycopeptides, 1.5% of the SE strains were resistant to vancomycin (CMI>2 mg/l) and 18.6% were resistant to teicoplanin (CMI>4 mg/l). All other CNS species were susceptible to glycopeptides.
No CNS strain was resistant to linezolid (CMI⩽4 mg/l for all strains) or daptomycin (CMI⩽1 mg/l for all strains).
In this study, interestingly, the SE, S. Ho and S. Ha species exhibited multiple resistances because at least 70% of the strains resistant to methicillin were also resistant to clindamycin, ofloxacin and rifampicin.
In contrast, the SL and SW strains were susceptible to most antibiotics; no resistant strain resistant to methicillin was detected and no more than 5% of the strains were resistant to fluoroquinolones or rifampicin.
All antibiotic susceptibilities are summarised in Table 1.
Antibiotic therapy
After the empirical treatment, the 179 patients received an oral combination of antibiotics.
Fifty-nine percent of the CNS strains were susceptible to ofloxacin and 80% to rifampicin.
For all patients diagnosed with a PJIs with CNS strain(s) susceptible to ofloxacin and rifampicin, they were treated by this recommended combination.
In case of SCN strain(s) resistant to rifampicin and susceptible to ofloxacin, the patient was treated by ofloxacin associated with clindamycin or SXT.
In case of SCN strain(s) resistant to ofloxacin but susceptible to rifampicin, the patient was treated by rifampicin associated with SXT or linezolid.
In case of SCN strain(s) resistant to both ofloxacin and rifampicin, the patient was treated by clindamycin associated with SXT or linezolid.
In case of multiresistant CNS strain, the patient was treated by linezolid monotherapy.
Follow-up
On the 179 patients included in the study, the evolution was favourable for 166 patients (93% of CNS PJIs). For 13 patients, a relapse or a new infection was reported regardless of surgical procedure (DAIR, one-stage or two-stage revision).
Discussion
To our knowledge, this is the first study comparing antibiotic susceptibilities in CNS PJIs.
CNS particularly S. epidermidis, may express multiple resistance factors that have a genetic flexibility and continuously generate novel variants [Reference Schoenfelder5]. The pathogenesis of CNS is linked to their ability to form a biofilm on device-related materials, particularly on specific components such as polysaccharide antigen [Reference Raad10, Reference Chokr11].
CNS, especially S. epidermidis, are known as the major cause of medical implant devices infections, especially with intravenous catheters [Reference Rogers, Fey and Rupp12]. CNS play a significant role in prosthetic joint (19–40%), vascular graft and surgical-site infections [Reference McCann, Gilmore and Gorman13].
With CNS, the most important challenge is assessing their clinical relevance.
In our retrospective study, 215 CNS PJIs were included. S. epidermidis was the most frequent species found, S. capitis, S. caprae and S. lugdunensis were emerging species. These results are in accordance with previous studies on CNS PJIs [Reference Pulido14].
Regarding antibiotic susceptibilities, 52.1% of our CNS strains were resistant to methicillin. This level is higher than in the studies of Titecat et al. (45%) [Reference Titecat15] and Tsukayama et al. (48%) [Reference Tsukayama, Estrada and Gustilo16] but much lower than the 85% of resistant strains reported by Hellmark et al. [Reference Hellmark17] or Sharma et al. 2008 [Reference Sharma18]. Resistance to glycopeptides was only observed for SE with 1.5% of the strains to vancomycin and 18.6% to teicoplanin, although this resistance is increasingly reported in other series [Reference Cremniter19]. These discordant results could be explained by a large proportion of SE in previous studies on CNS PJIs. No CNS strain was resistant to linezolid or daptomycin, as described in previous studies [Reference Hamad20]. Linezolid resistance was identified as the most frequent mechanism in CNS due to a mutation in 23S rDNA [Reference Decousser21] in rare studies.
S. epidermidis is recognised as a multi-resistant bacterium. Methicillin resistance was observed in 70% of the SE strains, which is comparable with other international studies. Methicillin resistance results are much higher than those described in S. aureus PJIs; previous studies showed less than 12–13% of MRSA PJI [Reference Dubost22, Reference Lora-Tamayo23]. Among these MRSA strains, additional resistance is exhibited, including resistance to quinolones, rifampicin, clindamycin or cotrimoxazole [Reference Decousser21, Reference Bogut24]. The SE strains are commonly resistant to various antimicrobial agents such as quinolones, rifampicin, cotrimoxazole and clindamycin [Reference Becker, Heilmann and Peters4, Reference Namvar25].
We found 30.2% S. epidermidis strains resistant to rifampicin, which is comparable with results of previous studies [Reference Hellmark17], where strains showed between 30% and 39% resistance [Reference Hamad20].
We found 55% of SE strains resistant to quinolones. The mechanism of this resistance is usually a mutation in the grlA, gyrA or ParC genes [Reference Courvalin26]. Previous studies have shown varying results across countries. For example, Molina-Manso et al. reported 37.5% resistance to quinolones in SE PJI [Reference Molina-Manso27] and in 2015, Hamad et al. found that 81% of the strains were resistant to ciprofloxacin [Reference Hamad20]. Resistance to tetracyclines was observed in 41.8% of our strains. This rate is higher than some reported studies on SE, where 18–31% resistant strains were found [Reference Hamad20]. The mechanism of resistance to cyclines is located on the tet or otr genes [Reference Courvalin26]. However, doxycycline or minocycline was not tested in our study, whereas the latter can be efficient on CNS strains resistant to tetracycline and doxycycline [Reference Hamad20]. This resistance due to DHPS or DHFR mutations is often associated with methicillin and quinolones resistance, which could reduce its use and the choice of orally active antibiotics for the treatment of SE or S. Ho PJI.
S. capitis has been shown to cause pneumonia, urinary tract infections, ocular infections, bloodstream infections and endocarditis [Reference Pal and Ayyagari28, Reference Oren and Merzbach29]. However, very few bone and joint infections have been described [Reference Arciola30]. A previous study showed some isolates resistant to oxacillin, erythromycin and clindamycin but susceptible to glycopeptides in bloodstream bacteraemia [Reference Wang31].
S. caprae has been described as a causative agent in bone and joint infections [Reference Seng32]. In orthopaedic device infections, some autolysin and fibrinogen-binding proteins responsible for biofilm production and cell adhesion have been reported [Reference Allignet33]. A previous study by Seng et al. found a higher rate of susceptible strains with more than 90% susceptibility to methicillin, clindamycin, ofloxacin, cotrimoxazole and rifampicin [Reference Seng32].
Several studies on SL bone and joint infections have reported osteomyelitis, septic arthritis and PJI [Reference Herchline and Ayers34]. In our study, 62% of the strains were resistant to penicillin G but none to methicillin. These results are different from some previous studies, especially for penicillin G resistance [Reference Herchline and Ayers34]. Beta-lactamase production has increased during the past years, but the presence of the mecA gene remains rare for this species. As in previous studies, we found a high rate of susceptibility (90–100%) to quinolones, rifampicin and clindamycin [Reference Sampathkumar, Osmon and Cockerill35, Reference Shah36]. No strain was resistant to linezolid or SXT.
The main antibiotic regimen for treating PJIs includes glycopeptides, preferably with vancomycin because of a high rate of resistance to teicoplanin among SE. The intravenous antimicrobial therapy is followed by a long oral course (6 weeks–3 months). According to recommendations for oral antibiotics, whenever possible, a combination of fluoroquinolone and rifampicin is provided [Reference Osmon6, Reference Dupont and Dutronc9]. However, to treat multi-resistant CNS strains such as SE, S. Ho and S. Ha, other second-line antibiotics including linezolid, SXT, cyclines and clindamycin must be used.
Conclusion
We can conclude that the main CNS involved in PJIs are S. epidermidis. These bacteria are also the most resistant CNS strains to methicillin and multiple antibiotics used in PJI.
PJIs to S. capitis and S. lugdunensis are emerging and S. capitis strains are often resistant to methicillin and quinolones.
Vancomycin, linezolid or daptomycin may be an efficient treatment on the multi-resistant PJIs. Oral linezolid (combined with rifampicin whenever possible) can be also proposed as empirical treatment or in case of multi-resistant strains [Reference Bassetti37]. Tedizolid, which seems to have less potential to cause myelosuppression and neuropathy than linezolid, could also be an interesting option [Reference Flanagan38]. For these CNS PJI, a few data are available regarding combination therapy and duration of treatment. Other studies could be conducted to allow the best antibiotic therapies for these complex infections.




