Summations
-
∙ Microglial activation, disruption of blood–brain barrier (BBB), activation of toll-like receptor and antibody involvement seems to induce or aggravate epileptogenesis.
-
∙ In the present review, the hypothesis that indicates contribution of several inflammatory pathways to epileptogenesis is explored via investigating physiological aspects and promising agents which execute their effects through inflammatory mechanisms.
Considerations
-
∙ Evidence obtained from literatures suggests the involvement of inflammatory processes to epileptogenesis, and also, these processes include several agents that may have contribution to clinical use at present and in future, but further preclinical and clinical studies concerning their pharmacological effects are necessary.
Introduction
Epilepsy is a chronic neurological disease among the most common neurological disorders, affecting up to 1–2% of the population. It is characterised with pathological symptoms related to deterioration of the central nervous system (CNS) functions. Occurrence of at least two or more unprovoked seizures is comprised in the definement of epilepsy (Reference Radzik, Miziak, Dudka, Chrościńska-Krawczyk and Czuczwar1,Reference Mirza, Appleton and Burn2). These seizures are in property of confirmation by hypersynchronous discharges of cortical neurons. Seizure disorders can occur at any age during lifetime, but they are more likely to start in young children or geriatric patients. Prevalence of active epilepsy is in a range of 0.8–1%, whereas the lifetime likelihood of receiving a diagnosis of epilepsy is about 3% (Reference Steinborn, Zarowski, Winczewska-Wiktor, Wójcicka, Młodzikowska-Albrecht and Losy3).
Some conditions leading to pathological changes in brain like traumatic brain injuries, stroke, status epilepticus (SE) or infectious diseases of brain are accepted as identifiable causes of acquired epilepsies. Epileptogenesis is the phenomenon which is formed with neurobiological events after occurrence of these mentioned triggers or other causes like genetic or inflammatory factors. In course of time, epileptogenesis provides occurrence of spontaneous seizures and eventually epilepsy diagnosis (Reference Bovolenta, Zucchini and Paradiso4). In other words, epileptogenesis is a process determined by conversion of the healthy brain into the epileptic brain. In epileptic seizure development, in any groups of neurons, abnormal and excessive electrical discharges in brain are seen. Unbalanced neuronal excitability is the cause of these electrical discharges. Abrupt disturbance of the whole brain activity, accompanied by generalised seizures which are characterised with loss of consciousness as seen in tonic-clonic or absence seizures, is owing to unbalanced neuronal excitability and diffusion of excessive electrical discharges to neighbouring cells. In focal, in other words partial, epileptic seizures, which is the most common form of epilepsy, impulse is not transferred to other areas in brain rather than the epileptic focus which is consisted of spatial neuronal network. By extensive links contacting primary epileptic focus, secondary focus may develop among healthy nerve cells. Discrimination of focal epileptic seizure classification is executed via evaluating symptomatology and origins of seizures. Partial seizures without impairment of consciousness with simple symptomatology and possessing an origin in neocortex is a subclass of focal epileptic seizures, whereas partial seizures with impairment of consciousness with complex symptomatology and originating in old cortical structures of the temporal lobe (or for some cases in orbitofrontal cortex) is another type (Reference Radzik, Miziak, Dudka, Chrościńska-Krawczyk and Czuczwar1,Reference Mirza, Appleton and Burn2). When considering generalised seizures, there is an onset recorded simultaneously in both cerebral hemispheres (Reference Steinborn, Zarowski, Winczewska-Wiktor, Wójcicka, Młodzikowska-Albrecht and Losy3).
The aetiology of the most generalised epilepsies cannot be explicitly determined. Seizures are pronounced to be genetically determined by disturbances of receptors in CNS. For patients diagnosed with ‘idiopathic’ epilepsy, genetic predisposition has been shown to be present in 40%. Idiopathic epilepsy is the form of epilepsies of unknown cause and account for approximately one-third of all cases of epilepsy in adults and 23–35% in children.
As being a complex genetic disease, pathogenesis of epilepsy includes involvement of hundreds, perhaps thousands, of genes which are not still properly detected. This complexity of genetical approach is directing researchers to analyse processes underlying epilepsy in area of biological-pathway-based approach. Besides, epilepsy is not defined as a single disorder but rather a group of syndromes with a variety of underlying diseases, and also neurotransmitter distributions or other metabolic problems are supposed to involve in epileptogenesis (Reference Radzik, Miziak, Dudka, Chrościńska-Krawczyk and Czuczwar1–Reference Suleiman, Wright and Gill6).
For the causative roles of epileptogenesis, the immune system is suggested to play a role. For instance, direct connection of immune system disorders is asserted to function in Rasmussen’s encephalitis (chronic focal encephalitis, RE) through epileptic seizure mechanism (Reference Radzik, Miziak, Dudka, Chrościńska-Krawczyk and Czuczwar1).
Seizures caused by the presence of a pathogen (as in the case of meningitis), neurotropic pathogens, etc., autoimmune epilepsy syndromes (which are characterised by the presence of anti-neuronal autoantibodies that targets either ion channels, intracellular epitopes or neurotransmitter receptors), and also seizure disorders not presenting either of these mentioned features are pronounced as subtypes of ‘inflammation-related seizures’ (IRS). Because in these disorders, therapeutic response to immunomodulators, vascular changes derived from inflammatory processes or signs of inflammation observed in brain are seen (Reference Iffland, Carvalho-Tavares and Trigunaite7).
Most antiepileptic drugs (AEDs) which are in use for managing seizures simply suppress acute seizures and exert symptomatic effects, but they are not antiepileptogenic. Data obtained from literature indicates that AED administration have been shown to be unable to prevent either epileptogenesis or temporal lobe epilepsy (TLE) development (Reference Bovolenta, Zucchini and Paradiso4,Reference Löscher8,Reference Temkin9). A clear-cut relationship between inhibition of epileptogenesis and neuroprotection has been made, so preventive treatment with AEDs having significant neuroprotective effects can be considered as an opportunity to inhibit epileptogenesis (Reference Radzik, Miziak, Dudka, Chrościńska-Krawczyk and Czuczwar1,Reference Czuczwar10).
Lack of adequate data about pharmacological agents that have antiepileptogenic effects point to need of research on this field. Preventive antiepileptic treatment present promising prospects for future. To achieve this purpose, in this review, pathological mechanisms underlying epileptogenesis will be clarified. And also, via focussing on immune mechanisms in epileptogenesis, data from literature concerning involvement of inflammatory processes in epilepsy so far will be discussed.
Pathophysiological aspects of epileptogenesis
Various biological pathways are proved to be dysregulated in epileptogenesis (Fig. 1) (Reference Sadowski, Kotulska-Jóźwiak and Jóźwiak11). Considering the importance of defining the most critical disturbances which lead to epilepsy for generating novel therapeutic approaches with greater efficacy than currently available antiepileptics, understanding the aetiology of epilepsy possesses great importance.
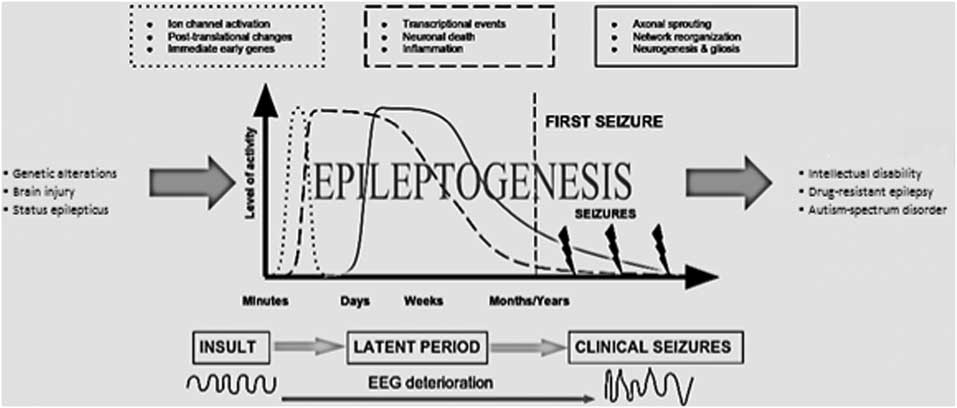
Fig. 1 Relationship between epileptogenesis and blood–brain barrier.
In this section, inflammatory aspects of epileptogenesis will be focused via considering several concepts like role of immune system, BBB and antibody involvement in epileptogenesis.
BBB has a unique role in immune privilege of CNS (Reference Bechmann, Galea and Perry12). Ionic homoeostasis is maintained by BBB and thus, BBB has the regulator role for neuronal excitability (Reference De Vries, Kooij, Frenkel, Georgopoulos, Monsonego and Janigro13–Reference Marchi, Granata, Ghosh and Janigro15). It was reported that BBB act as the cross-road for several pathophysiologic process like altered neuroglial physiology, inflammatory reactions in brain, changes in brain milieu and haemodynamic changes leading to energy mismatch. Drugs that provide BBB repair are reviewed by Marchi et al. (Reference Marchi, Granata, Ghosh and Janigro15). In that paper, add-on cerebrovascular drug is suggested as an efficient therapeutic strategy for reducing seizure burden.
Currently, inflammation of CNS via BBB leakage has been implicated in the progression of epilepsy, and BBB leakage has been shown to participate not only in progression of epileptogenesis but also in the induction of seizures (Reference Seiffert, Dreier and Ivens16–Reference Marchi, Angelov and Masaryk19). Michalak et al. (Reference Michalak, Lebrun and Di Miceli20) showed that BBB disruption, as observed in seizure generation regions in brain, is defined by large deposits of extravasated immunoglobulin G (IgG). In seizure disorders, presence of IgGs in brain is a fact that provides acceptance of autoimmune involvement in aetiology. Even in the absence of autoimmune diagnosis, lithium/pilocarpine-induced seizures cause presence of IgGs in mice brain. The importance or results of IgG extravasation into the CNS is still a matter of concern.
In addition, long-term effects of inflammatory mediators have been shown to play a role in alterations in permeability properties of BBB. Seizure threshold shown to be decreased via ionic imbalance inducement in the extracellular milieu (Reference Bauer, Vezzani and Bien21). On the other hand, altered BBB permeability in epileptic seizures suggested to cause accumulation of peripheral hormones like erythropoietin which may show potent neuroprotectant effect (Reference Acharya, Hattiangady and Shetty22). In case of some IRS that are caused by response to vascular changes accompanied by an continuing inflammatory process suggested to be related to BBB disruption (Reference Iffland, Carvalho-Tavares and Trigunaite7).
Neurotrophic factors
Neurotrophic factors may serve as keepers of BBB integrity
Vasoactive neuropeptides such as pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide have key roles in blood vessels in CNS just as maintaining functional integrity of BBB (Reference Staines, Brenu and Marshall-Gradisnik23). In addition, brain-derived neurotrophic factor (BDNF) has been shown to reduce BBB breakdown, tissue injury and edema formation in brain significantly when administered via intracerebroventricular route (Reference Sharma and Johanson24). Also, in vivo studies have shown the role of fibroblast growth factor-2 (FGF-2) in regulation of BBB permeability (Reference Reuss, Dono and Unsicker25). One of the possible pathways forming epileptogenesis which is induced by SE is suggested to be the activation of TrkB, a BDNF receptor. Transgenic overexpression of TrkB or BDNF is shown to conclude in an increasement of seizure severity or susceptibility. In vivo models of TLE is suggested to be characterised with increased BDNF expression and enhanced TrkB activation (Reference McNamara and Scharfman26). In another study, BBB leakage is shown to peak around 4 days after pilocarpine-induced SE (Reference Ndode-Ekane, Hayward, Gröhn and Pitkänen27), whereas after injection of a vector, short-term (1 week) increase in FGF-2 expression and a bit longer lasting (at least 11 days) increase in BDNF expression is shown to occur (Reference Paradiso, Marconi and Zucchini28). On the other hand, Larmet et al. (Reference Larmet, Reibel, Carnahan, Nawa, Marescaux and Depaulis29) showed possible antiepileptogenic effects of BDNF via indicating its inhibitory effects on development of hippocampal kindling and duration of electrographic seizure by chronic intrahippocampal infusion. Also, exposure to BDNF was found to suppress TrkB receptor responsiveness, reduce TrkB messenger RNA levels in vitro, and also in hippocampus, decrease TrkB receptor levels by 80% in vivo (Reference Frank, Ventimiglia, Anderson, Lindsay and Rudge30,Reference Knusel, Gao and Okazaki31). Gu et al. (Reference Gu, Huang, He, Joshi, Jang and McNamara32) specified TrkB-activated signalling pathway as a responsible pathway for TLE and showed that phospholipase Cγ1 is the dominant signalling effector by which excessive TrkB activation promotes epilepsy. Proceeding from this, therapeutic approaches targeting receptor tyrosine signalling seems to be a novel strategy for treatment of epilepsy.
Antibody involvement in epileptogenesis
Via activation of several inflammatory pathways and also by elevation of inflammatory mediators in neurons; astrocytes and cells among microglia/macrophage lineage suggested to be activated in epileptic tissues. In vivo studies provide evidence which proves participation of elevated inflammatory mediators in epileptogenic areas. Also, proconvulsant effects are suggested to be mediated by specific proinflammatory pathways in forebrain, so these pathways have potency for evaluating new therapeutic approaches to epileptogenesis treatment (Reference Vezzani and Granata33–Reference Vezzani, Balosso, Maroso, Zardoni, Noé and Ravizza35). Activation of the interleukin-1beta (IL-1β)–Interleukin-1 receptor type I (IL-1R1) (IL-1β–IL-1R1) system, which is observed in TLE patients, suggested to come before onset of epilepsy, and may contribute to seizure generation. Occurrence of an ongoing inflammatory progress in experimental models of TLE provides evidence to this assertion (Reference Vezzani, Ravizza, Balosso and Aronica34). In serum of patients who have disorders like stiff person syndrome, cerebellar ataxia, epilepsy and limbic encephalitis, which are all characterised by dysfunction of the Gamma-Aminobutyric acid system (GABAergic system), elevated levels of antibodies against glutamic acid decarboxylase shown to exist (Reference Saiz, Blanco and Sabater36–Reference Ali, Rowley, Jayakrishnan, Teuber, Gershwin and Mackay38).
Interaction of microglia with epilepsy
Lately, glial cells are considered as not only being signs of neuropathology, but also having a protagonistic role. Protagonistic role of glial cells are suggested to occur via release of inflammatory cytokines, chemokines and also by altering neuronal function (Reference Sofroniew39). It should be noted that microglial–neuronal cross-talk is a principal factor for neuronal synaptic activity and microglial activation (Reference Barres40,Reference Walter and Neumann41). Considering various mechanisms which proves contribution of glial cells to epileptogenesis, it is assumed that inflammatory processes involving glial activation plays a significant role. This activation is characterised by release of inflammatory proteins, more likely cytokines and chemokines. Examples of such proteins which are mostly secreted by astrocytes and microglia cells and able to facilitate hyperexcitability processes are macrophage inflammatory proteins (MIP), interleukin 6 (IL-6), the C–C motif ligand 2 (CCL2) and IL-1β (Reference Vezzani and Granata33,Reference Vezzani, Ravizza, Balosso and Aronica34,Reference Foresti, Arisi, Katki, Montañez, Sanchez and Shapiro42–Reference Xu, Long, Tang, Zhang, Hut and Tang44).
In the area where the new born neurons are, microglial activation induced via inflammation that is generated with lipopolysaccharide has been proved to impair basal hippocampal neurogenesis in rats (Reference Ekdahl, Claasen, Bonde, Kokaia and Lindvall45). Role of hippocampal neurogenesis has been related to memory formation (Reference Shors, Miesegaes, Beylin, Zhao, Rydel and Gould46) and mood regulation (Reference Santarelli, Saxe and Gross47). On the other hand, after brain damage, microglial activation may have beneficial effects like release of neurotrophic factors (Reference Schwartz48). So any therapeutic approach developed must be designed without having any changing effect on these useful features.
Fractalkine, an inflammatory chemokine secreted by neurons and astrocytes, shown to act on CX3CR1 receptors. These receptors are present mostly on microglial cells, and regulates its several activities (Reference Ali, Chugh and Ekdahl49). The fractalkine–CX3CR1 signalling pathway has been suggested to play a role in the pathogenesis of epilepsy in a study conducted by Xu et al. (Reference Xu, Zeng and Han50) which suggests CX3CL1 to be a possible biomarker of brain inflammation in epileptic patients. Ali et al. (Reference Ali, Chugh and Ekdahl49) described a role of this pathway for the acute pathological changes in the brain following an epileptic insult. Also they showed that blocking this pathway by the anti-CX3CR1 antibody diminishes electrical SE-induced microglial activation and neurodegeneration. In the cerebrospinal fluid of epileptic patients, increased levels of fractalkine has also been reported (Reference Xu, Zeng and Han50). The fractalkine–CX3CR1 pathway has been also shown to regulate synaptic transport, via affecting both glutamatergic and GABAergic transmission (Reference Ali, Chugh and Ekdahl49). Modulation of the fractalkine–CX3CR1 pathway by administrating recombinant fractalkine (in cortical brain tissues from TLE patients) shown to modulate the decrease on GABA current (Reference Roseti, Fucile and Lauro51).
Alteration of glial inflammatory processes via various ways like blocking IL-1β, which has been shown to indicate significant anticonvulsant effects (Reference Vezzani, Balosso, Maroso, Zardoni, Noé and Ravizza35) can also be assumed as another potential therapeutic strategy. The P2×7 receptor, which has been focussed recently in terms of its effects on preclinical models of SE, is a member of P2 class of ionotropic and metabotropic purinoceptors. This class is expressed by neurons and glia, and some subtypes are shown to upregulate after SE. The P2×7R mediates inflammatory responses in the brain after injury, microglial activation and the release of the proepileptogenic inflammatory cytokine interleukin 1β. This release is accepted as an important process in epilepsy (Reference Henshall, Clark, Adelson, Chen, Watkins and Simon52–Reference Johnson, Behmoaras and Bottolo55). Also, prolonged seizures have been shown to activate these receptors. Report of a study conducted by Henshall and Engel (Reference Henshall and Engel56) showed that SE produced by intraamygdala microinjection of kainic acid (KA) causes an increase in P2×7R expression in the hippocampus and neocortex of mice. And antagonism of P2×7R shown to reduce seizure severity, microglial activation and interleukin 1β release. Mesuret et al. (Reference Mesuret, Engel and Hessel57) also showed that inhibition of P2×7 receptor causes interruption in the progression of seizures and reduction in hippocampal damage in immature rats.
Among glial cell types, astrocytes are shown to modulate synaptic transmission by release of glutamate, purines, d-serine and GABA (Reference Barres40). They also shown to regulate synaptic homoeostasis via expressing neurotransmitter transporters such as glutamate, GABA and glycine transporters (Reference Sofroniew and Vinters58). With having hippocampal sclerosis as a predominant pathology, in terms of TLE, underlying pathology includes gliosis in regions of neuronal loss and also, altered astrocytic transporters and receptors in hippocampus. This alteration is suggested to result in more suitable conditions to seizure initiation and development (Reference Seifert, Schilling and Steinhäuser59). Also, glutamate released by astrocytes is shown to cause paroxysmal depolarisation shifts in neurons. What is more, in acute models of epilepsy, neuronal synchronisation seems to be affected by stimulation of astrocytes (Reference Tian, Azmi and Takano60).
Adenosine that is released by astrocytes provides an inhibitory neuromodulator effect and suggested to indicate endogenous anticonvulsant properties. Considering the need to decrease hyperexcitability in epilepsy, regulating levels of substances released by astrocytes (like adenosine and adenosine kinase) seems to be an affectual therapeutic approach (Reference Boison61).
Several inflammatory processes, which have been linked to epileptogenesis, can be triggered via an injury-induced fluctuation in adenosine levels. Recent findings also demonstrate that increased levels of adenosine induce hypomethylation of hippocampal DNA (Reference Williams-Karnesky, Sandau and Lusardi62) and this reducement could induce the transcription of epileptogenesis genes. Consequently, these data suggests a novel mechanism by which adenosine may trigger epileptogenesis (Reference Boison, Sandau, Ruskin, Kawamura and Masino63). Also, adenosine 2A receptor (A2AR) activation shown to exert bidirectional effect (detrimental or protective) in acute neurological injuries. A2AR subtype affects differently depending on where it is localised: neuron, microglia, bone marrow-derived cells (BMDC) or astrocyte. For instance A2AR on microglia mediate inhibition of glutamate uptake transporter GLT-1 and stimulate glutamate outflow (Reference Nishizaki, Nagai and Nomura64). Li et al. (Reference Li, Oskouian and Day65) reported that, inflammatory BMDCs are the primary targets of A2AR agonist-mediated protection in spinal cord injury, whereas protection via global A2AR deletion or pharmacological blockade is mediated by A2AR present on cells other than BMDCs. Consequently, there is a need of developing cell-type-specific adenosine receptor agents to selectively target the receptors (Reference Dai and Zhou66).
Early microglial activation is a pathological finding of Cstb2/2 mouse, an animal model for progressive myoclonus epilepsy of Unverricht–Lundborg type (EPM1). EPM1 is an inherited neurodegenerative disorder caused by mutations in the cystatin B gene (CSTB) and characterised with myoclonus, seizures and ataxia. CSTB deficiency has shown to be linked to neuroinflammation with early activation and dysfunction of microglia in a study that used Cstb2/2 mouse (Reference Okuneva, Korber and Li67).
After SE albumin extravasation shown to be prominent in astrocytes and neurons, in case of TLE patients, also in hippocampus. Existence of albumin and tracers in microglia, astrocytes and neurons of the rat supports these data (Reference Van Vliet, Da Costa Araújo, Redeker, Van Schaik, Aronica and Gorter18). Ivens et al. (Reference Ivens, Kaufer and Flores17) demonstrated association between direct brain exposure to serum albumin and albumin uptake into astrocytes. This association was also suggested to be mediated by transforming growth factor β receptors (TGF-βRs). Albumin uptake to astrocytes shown to result in down-regulation of inward-rectifying potassium channels, which generates diminished extracellular potassium buffering. This process indicated to result in neuronal hyperexcitability and, after all, epileptiform activity. Blocking TGF-βR is suggested to be a novel therapeutic target for epilepsy. Data shows the fact that TGF-β signalling increases after brain injuries, but its role in neuronal survival is less consistent and depend on the model and outcome measure (Reference Friedman, Bar-Klein, Serlin, Parmet, Heinemann and Kaufer68). For example, when TGF-β1 were introduced to cultured rat’s cortical neurons and glia cells in glutamate neurotoxicity, it is shown to reduce l-glutamate-induced neuronal injury. In case of permanent middle cerebral artery occlusion, pretreatment with TGF β-1 slightly reduced the area of ischaemia (Reference Prehn, Backhauss and Krieglstein69).
Neurodegeneration which is caused by brain inflammation includes immune cell activation, and microglial activation which involves producement of several proinflammatory factors (Reference Pocock and Liddle70–Reference Gebicke-Haerter72). In neurological diseases such as ischaemia and epilepsy, and also in neurodegenerative diseases, activated microglia shown to release glutamate and this is suggested to be concluded with excito-neurotoxicity, so as neurodegeneration. Thus, blockage of microglial glutamate release may be a favourable therapeutic approach for epilepsy treatment (Reference Takeuchi, Jin and Suzuki73). Activated microglia demonstrated to produce glutamate by glutaminase, and hemichannels of gap junctions are shown to be the release location (Reference Takeuchi, Jin and Wang74). In experimental models, various brain insults are shown to be the reason of rapid and lasting glial activation, and associated with this, inflammatory processes. The occurring chronic inflammation may contribute to epileptogenesis (Reference Vezzani, Balosso, Maroso, Zardoni, Noé and Ravizza35).
In a transient ischaemic brain injury model, administration of a gap junction blocker and an anti-inflammatory drug; carbenoxolone and also a glutaminase inhibitor and an antitumor drug; 6-diazo-5-oxo-l-norleucine is shown to suppress microglial excessive release of glutamate both in vivo and in vitro. Microglial excessive release of glutamate has been shown to cause neuronal death in vitro. In the same study, in case of transient global ischaemia, neuroprotective effect of these agents in the hippocampal CA1 region of gerbils was also shown. These agents are promising for use in neurodegeneration in terms of microglial activation, because on the contrary to the N-methyl-d-aspartate (NMDA) receptor blockers which show adverse effects in a dose-dependent manner, these agents provides specificity to microglial glutamate producement pathways. In terms of ischaemia, neuronal damage includes involvement of neuronal and astrocytic gap junctions, so blockade of gap junction may exert neuroprotection additively (Reference Takeuchi, Jin and Suzuki73).
Adult neurogenesis is regulated by microglial activation in several aspects. Arrangement of abnormal hippocampal neurogenesis which is induced by seizure occurrence is shown to be altered by glial activity, mostly associated with inflammation (Reference Ekdahl, Claasen, Bonde, Kokaia and Lindvall45). Regulation of this alteration seems to be a therapeutic approach for epileptogenesis. For instance, Jung et al. (Reference Jung, Chu and Lee75) showed ameliorative effect of a cyclooxygenase-2 inhibitor on altered hippocampal neurogenesis and microglial activation after seizure occurrence, along with a decrease in spontaneous recurrent seizures. After pilocarpine-induced seizure, Yang et al. (Reference Yang, Liu and Chen76) reported that selective microglial inhibition via minocycline treatment results in formation of hilar basal dendrites and inhibition on aberrant migration of newborn neurons. After KA-induced seizures, minocycline shown to be neuroprotective in the hippocampus (Reference Heo, Cho and Cho77). Further, melatonin has been shown to indicate inhibitory effect on microglial activation, leading attenuation of hippocampal neurodegeneration which is induced by KA (Reference Chung and Han78).
Contribution of cytotoxic T cells to epileptogenesis
CD8-positive T lymphocyte elevation is a characteristic feature for a variety of CNS inflammatory diseases (Reference Bernal, Graus, Pifarre, Saiz, Benyahia and Ribalta79–Reference Petito, Torres-Munoz, Zielger and Mccarthy81). As mentioned before, a variety of diseases can be a reason of epileptic seizures. One of them is brain inflammation or encephalitis. Studies including analysis of brain immune cell composition of patients with RE supports this finding. In brain parenchyma, composition of T lymphocyte fraction is shown to include mainly CD8-positive cells. Besides, CD4-positive cells proved to accumulate mostly in the perivascular space of blood vessels, not tend to migrate into brain parenchyma. In addition, in human brain disease, cytotoxic T-cell mechanism has been suggested to contribute to loss of neurons (Reference Bien, Bauer and Deckwerth82).
Assuming density of infiltrating T lymphocytes is a criterion for inflammation, disease duration and also neuronal loss are correlated contrarily with this feature. As disease progresses, amount of T lymphocytes decreases, whereas neuronal loss increases. But it should be noted that the level of T lymphocytes still remains above the levels that is present in normal individuals (Reference Bien, Urbach and Deckert83), and these findings support the idea that neuronal loss may cause from a cytotoxic T-cell response against neurons.
Presence of T-cell inflammation in the CNS is shown in paraneoplastic encephalitis (PE). PE with anti-Yo, anti-Hu or anti-Ma antibodies have been suggested to involve with an elevated amount of CD8-positive T cells in the infiltrates (Reference Graus, Ribalta, Campo, Monforte, Urbano and Rozman84–Reference Voltz, Gultekin, Posner, Rosenfeld and Dalmau88). There are also studies reporting that these T cells contain cytotoxic granules and they are close to neurons (Reference Bauer and Bien5). This approach may suggest a role of these cells in neuronal cell death.
Inflammatory approaches to epilepsy in terms of toll-like receptors
Epilepsy suggested to be a part of disorders associated with the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signalling (PI3K–Akt–mTOR) pathway irregularity (Reference Mirzaa, Dodge and Glass89–Reference LEE, Smith and Paciorkowski91). The mammalian target of rapamycin (mTOR) pathway, which is involved in the control of major multiple cellular events like the ones that occur in highly epileptogenic conditions, may be a potential target for the suppression of epileptogenesis (Reference Sadowski, Kotulska-Jóźwiak and Jóźwiak11).
In a study which rapamycin-resistant mutants of yeast were isolated and corresponding mutations were discovered, the affected genes were named as TOR1 and TOR2 (Reference Hetiman, Movva and Hall92). Several years later, mTOR was identified and defined as the rapamycin target in mammals (Reference Sabatini, Erdjument-Bromage, Lui, Tempst and Snyder93). Nowadays, like cardiovascular diseases, metabolic disorders, many types of cancer and neurological disorders; so many human diseases have been linked to mTOR pathway dysregulation. Mostly, these linkages includes hyperactivity of mTOR pathway (Reference Huffman, Mothe-Satney and Lawrence94–Reference Ravikumar, Vacher and Berger96). Therefore, mTOR pathway inhibition may suggest an attractive therapeutic choice, which might have regulative effect in epileptogenesis.
The mTOR pathway has a regulative role in functions of neurons like cell proliferation, survival, growth, metabolism and plasticity. Also, mTOR signalling pathway plays a crucial role in antigen-presenting cell and T-cell regulation (Reference Thomson, Turnquist and Raimondi97). Discovery of mTOR is highly related to finding of rapamycin, its inhibitor (Reference Vezina, Kudelski and Sehgal98). Rapamycin and its analogs seem to share same mechanism of action. Everolimus, deforolimus, ridaforolimus and temsirolimus, are small-molecule kinase inhibitors. This group has a mode of action that essentially includes binding to FKBP12 proteins. The rapamycin–FKBP12 complex has an inhibitory effect on the mTOR pathway via direct binding to mTOR complex 1 (mTORC1), which is one of the two complexes that mTOR forms. The mechanism of immunosuppressive effect of rapamycin is generally linked to inhibition of T-cell proliferation which is induced by growth factor (Reference Sadowski, Kotulska-Jóźwiak and Jóźwiak11).
mTOR pathways appears to play a significant role in arrangement of neuroinflammatory processes. In addition, scientific studies conducted in animal models of Tuberous Sclerosis (TSC) and included administration of mTOR inhibitors shown to prevent progress of epilepsy and reduce underlying brain abnormalities. In animal model of absence epilepsy, rapamycin shown to decrease and dampen release of Lipopolysaccharide (LPS)-induced neuroinflammatory cytokines (Reference Russo, Andreozzi and Iuliano99). In addition, reduction of microglia-mediated neuroinflammation has been suggested to involve mTOR pathway (Reference Xie, Sun and Wang100). First study that provides clear evidence about antiepileptogenic activity of rapamycin introduced suppressive effect on seizures and extender effect on survival. And also early treatment with rapamycin has been shown to have protective effects against the development of epilepsy and premature death of in Tsc1 GFAP CKO mice. Tsc1 GFAP CKO mice are characterised with conditional inactivation of the Tsc1 gene primarily in glia, development of abnormal glial proliferation, progressive epilepsy and premature death. Rapamycin shown to inhibit the astrogliosis, abnormal activation of the mTOR pathway and neuronal disorganisation. Interestingly, when rapamycin treatment was ended, neurological and histological abnormalities suggested to reappear within few weeks (Reference Zeng, Xu, Gutmann and Wong101). Rapamycin treatment has been shown to reverse early increases in glutamatergic neurotransmission and seizure susceptibility when applied immediately before and after seizures, and also to reduce later life epilepsy and autistic-like behaviour (Reference Talos, Sun and Zhou102). Postnatal treatment with rapamycin has shown to both prevent affected animals from seizures and premature mortality and reverse cellular type abnormalities. Single dose of rapamycin was given to pregnant dams for investigation of effects on prenatal mice. A foetal brain model of TSC characterised with Tsc1 cc Nes-cre + mouse was used. In that model, recombination and loss of Tsc1 in neural progenitor cells cause brain enlargement, hyperactivation of mTOR and neonatal death via pup–maternal interaction reducement are characteristic features. Data obtained showed that rapamycin treatment rescued the lethality for mutant mice (Reference Anderl, Freeland, Kwiatkowski and Goto103). Suggestion of mTOR inhibitors as antiepileptic therapy has been arised (Reference Wong104). Within cultured hippocampal neurons, the PI3K–Akt–mTOR signalling pathway shown to promote the growth and branching of dendrites. According to this finding, mTOR is indicated to play a central role in the control of dendrite growth and branching during development by regulating global, and also local protein translation (Reference Jaworski, Spangler, Seeburg, Hoogenraad and Sheng105). By adjusting synaptic plasticity, mTOR pathway can be suggested to influence some mechanisms involved in epileptogenesis.
One possible way of activation for mTORC1 is suggested to occur after seizure at developmental stage, at the same time comprising a critical period of synaptogenesis. This contributes, in later life, to formation of epileptic networks and autistic-like behaviour. In vivo model of neonatal hypoxia-ischaemia was used to investigate mTOR activation in hippocampus and neocortex. Data obtained from that study showed that rapamycin treatment immediately before and after seizures causes significant suppression of mTOR activation not only in hippocampus but also in neocortex. Also, rapamycin treatment suggested to decrease autistic-like social deficits. In that study, inducement of mTOR pathway after seizures was identified again via showing this increasement within dendritic portion of glutamatergic neurons (Reference Talos, Sun and Zhou102). In another study which includes KA-induced SE model, mTOR signalling has been shown to be activated in several brain areas such as cortex and hippocampus. Interestingly, long-term treatment with rapamycin seemed to sensitise animals to KA. These data were suggested to associate with a change in threshold for epileptic discharges generated via KA and size reduction of hippocampus (Reference Macias, Blazejczyk and Kazmierska106).
In absence seizures, acute treatment effects of rapamycin was suggested to be associated with anti-inflammatory effects of this molecule which is mediated via microglia inhibition and cytokines (Reference Russo, Citraro and Donato107). Persistent mTOR activation characterised with change in progenitor cell proliferation through epileptogenesis have been suggested to play a role in development of the absence seizures in rats (Reference Russo, Follesa and Citraro108). Rapamycin has been shown to totally inhibit spontaneous seizure development in an in vivo study in which electrical stimulation of the angular bundle was used to induce SE (Reference Van Vliet, Forte and Holtman109). Data obtained from this study indicated inhibitory effect on sprouting and neuroprotective effect of this drug. Cell loss and sprouting was also suggested to be significantly less in rapamycin-treated rats compared with vehicle-treated rats (Reference Heng, Haney and Buckmaster110). In a model of pilocarpine-induced SE in rats, rapamycin has shown to decrease mTOR activation that induced by SE and attenuate microgliosis mostly in CA1 area. In that study, rapamycin suggested to show a significant difference compared with control group in tests evaluating spatial learning and memory deficit parameters (Reference Brewster, Lugo and Patil111).
In terms of childhood epilepsy, TSC is a major genetic cause. Brain hamartomas may be a marker for prenatal TSC diagnosis. In TSC, preliminary seizures are generally subtle and focal, but epilepsy usually progress to more severe forms like infantile spasms (Reference Chu-Shore, Major, Camposano, Muzykewicz and Thiele112). Proceeding from these facts, TSC can be suggested to be a preferred model to investigate role of mTOR signalling pathways in epilepsy.
Improvement of median survival was observed in a mouse model of TSC, which comprise ablation of Tsc1 gene in most neurons during cortical development and rapamycin treatment (Reference Meikle, Pollizzi and Egnor113). In that study, myelination, neurofilament abnormalities and cell enlargement suggested to improve via rapamycin treatment, however no significant change was observed in dysplastic neuronal features and changes in dendritic spine density and length shown to be in a moderate range.
In clinical studies, seizure reduction and potential disease-modifying effect of mTOR inhibitors for TSC patients have been shown. Autophagy is suggested to increase after neonatal hypoxia-ischaemia in neuronal cells. mTOR pathway has been shown to inhibit autophagy. Regarding this, autophagy and apoptosis seem to be related to mTOR pathway (Reference Carloni, Buonocore and Balduini114). Proceeding from these data, in such circumstances mTOR inhibitors may provide neuroprotective effect.
There are some similarities between focal cortical dysplasia (FCD) and TSC-associated lesions, for both so similar pathological pathways are suspected. Upstream modulators of the mTOR pathway are suggested to be affected in both conditions which leads to mTOR activation. In a study conducted via evaluating blockage of paroxysmal activity induced by 4-aminopyridine by rapamycin treatment in resected epileptogenic cortex tissue samples from TSC, FCD and other non-FCD lesions has shown that this drug blocks mentioned activity in both TSC and FCD samples (Reference Cepeda, Andrè and Yamazaki115). mTOR activation was determined in microglia, immature cell types and dysmorphic neurons in a sequence of epileptic surgical pathologies (Reference Liu, Reeves and Michalak116). That study introduces a proof that mTOR dysregulation is a possible mechanism involved in various acquired forms of epilepsy, especially when a dysmorphic cytopathology coexist.
Apart from these studies, mTOR inhibition has been shown to cause significant reduction in seizure development in spite of presence of microglial activation, which leads to consideration of different mechanisms of this molecule about seizure development other than its effects on control of inflammatory processes. Rapamycin has been suggested to interrupt seizure development via mTOR inhibition and also, possibly, via affecting BBB leakage (Reference Van Vliet, Forte and Holtman109).
In post-SE rat model of TLE, rapamycin has been suggested to provide possible antiepileptic and antiepileptogenic effects (Reference Zeng, Rensing and Wong117). Findings obtained from study conducted by Buckmaster et al. (Reference Buckmaster, Ingram and Wen118) suggested that mossy fibre sprouting development was suppressed with continual treatment by inhibition of the mTOR signalling pathway. That study also revealed absence of reversal of already established axon reorganisation. Mossy fibre sprouting reduction, which accepted as protective mechanism of mTOR pathway inhibitors in SE can be presented as potential mechanism of antiseizure effect. On the other hand, there is data concerning absence of antiseizure effect of rapamycin although suppression of mossy fibre sprouting was determined. Study conducted by Buckmaster and Lew (Reference Buckmaster and Lew119) showed that there may not be a relation between reduction of seizures with mossy fibre sprouting, because rapamycin treatment was shown to cause no significant reduction of seizure frequency, whereas it provided a reduction in mossy fibre sprouting. But data from this study is consistent with the hypotheses that ectopic granule cells and hilar neuron loss may contribute to TLE, because they showed that rapamycin does not significantly affect hilar neuron loss, or generation of ectopic granule cells. Also, in amygdala stimulation TLE model, post-treatment with rapamycin shown to introduce no significant difference in terms of areas that are occupied by mossy fibres (Reference Sliwa, Plucinska, Bednarczyk and Lukasiuk120). So, whether exactly mTOR inhibition processes play a causative role in the potential antiepileptogenic effect of rapamycin (RAP) will be subject of future studies.
Effect of everolimus on seizures caused from TSC has been investigated and as a result, significant reducement of seizures was observed (Reference Krueger121). In a case study, rapamycin treatment is reported to be effective in drug-resistant epilepsy (Reference Muncy, Butler and Koenig122). Afterwards, clinical studies conducted among patients with subependymal giant cell astrocytomas, some related to TSC presenting seizures have suggested that everolimus treatment could reduce seizure activity, and even lead to cessation of seizures (Reference Krueger, Care and Holland123,Reference Perek-Polnik, Jozwiak, Jurkiewicz, Perek and Kotulska124).
Relation between seizure occurrence and mutation of genes related to epileptic pathways still remains to be clarified. One of these genes is PTEN, which inhibits PI3K–Akt–mTOR pathway (Reference Mester and Eng125). Mutation in PTEN has been related to seizure development (Reference Marchese, Conti and Valvo126). In addition, seizures in NS-Pten conditional knockout mice has been shown to be suppressed by rapamycin (Reference Sunnen, Brewster and Lugo127).
TSC suggested to result from mutations of protein coding genes, TSC1 and TSC2 (Reference Northrup, Koenig, Pearson and Au128). Tuberin and hamartin, these genes’ protein products, shown to take a part in regulation of mTOR pathway. TSC function loss appears to result in mTOR hyperactivity (Reference Kwiatkowski129). Also as mentioned before TSC is associated with epilepsy, and as a proof for this proposition, presence of epilepsy among 90% of patients with TSC can be indicated (Reference Kotulska, Jurkiewicz, Donmańska-Pakiela, Grajkowska, Mandera and Borkowska130).
Vigabatrin, which has been shown to inhibit mTOR (Reference Zhang, Mcdaniel, Rensing and Wong131), has become a therapeutic option to infantile spasms and partial seizures related to TSC (Reference Curatolo, Jozwiak and Nabbout132). Also, in terms of mTOR modulation, second generation of dual mTORC1/mTORC2 inhibitors which are known as ATP-competitive mTOR kinase inhibitors are promoting agents. These agents block the feedback activation of PI3K/AKT signalling pathway via showing an inhibitory effect on kinase-dependent functions of mTORC1 and mTORC2 (Reference Zaytseva, Valentino, Gulhati and Evers133).
All mentioned mechanisms may have potential to contribute to neuromodulatory and antiepileptogenic effects of mTOR inhibitors, and also preliminary results about these candidates are promising to investigate molecular aspects of epileptogenesis.
Role of proinflammatory cytokines in epileptogenesis
Recently, epileptic seizure aetiology has been related to potential involvement of cytokines. In experimental models and clinical studies, activation of proinflammatory cytokine production and inflammatory interactions in brain after occurrence of seizures have been investigated. Histopathological analysis obtained from epilepsy patients draw attention as data obtained from these analyses gives proof about the role of inflammation in epileptogenesis. Within these data, neuronal loss, cortical morphological malformations and reactive gliosis, which in turn proves existence of a chronic inflammation, have been shown (Reference Steinborn, Zarowski, Winczewska-Wiktor, Wójcicka, Młodzikowska-Albrecht and Losy3).
The complement system consists of proteins that can cause several processes leading to microglial activation or secretion of proinflammatory cytokines. In a study conducted by Aronica et al. (Reference Aronica, Boer and Van Vliet134), authors showed the dynamics of the complement cascade during epileptogenesis in a rat model of TLE and concluded on the finding that the persistence of complement activation could contribute to a sustained inflammatory response and could destabilise neuronal networks involved.
In terms of neuronal plasticity and synaptic transmission, involvement of various immune modulators have been shown (Reference Yirmiya and Goshen135). In CNS, the components that are able to produce and secrete cytokines are glia cells, neurons and T lymphocytes. Tumour necrosis factor alpha (TNF-α) and IL-6 can also pass BBB by active and passive transport (Reference Stokłosa136). Proinflammatory cytokines like IL-1β, IL-6 and TNF-α have been suggested to induce an increase in excitatory synaptic transmission and a reducement in inhibitory synaptic transmission in the spinal cord (Reference Kawasaki, Zhang, Cheng and Ji137). Enhancement of seizure activity by intra-cerebral application of IL-1β has been shown in experimental models (Reference Vezzani, Conti and De Luigi138). TNF-α has been shown to induce microglial glutamate release by up-regulating glutaminase and gap junctions (Reference Takeuchi, Jin and Wang74). In treatment of autoimmune diseases, TNF-α neutralising antibodies are suggested to be an effective choice (Reference Illei and Lipsky139).
In terms of receptors for inflammatory molecules, including receptors of proinflammatory cytokines (IL-1β, IL-6 and TNF-α) and TLRs, overexpression of these receptors suggested to occur via glial cells and neurons (Reference Bauer, Vezzani and Bien21). As well as this suggestion, all cell types among brain are proposed to be capable of expressing cytokines and their receptors. IL-1β, TNF-α and IL-6 are suggested to be expressed primarily in activated microglia and astrocytes, whereas cytokine receptor expression seems to be up-regulated in astrocytes, microglia and neurons (Reference Vezzani, French, Bartfai and Baram54). TNF-α, IL-6 and IL-1β are proinflammatory cytokines that are able to induce seizures via modulation of glutamatergic transmission (Reference Mlodzikowska-Albrecht, Steinborn and Zarowski140). These cytokines also have capability to affect neuronal viability, glial activation and proliferation, participate in BBB permeability enhancement, but they also can inhibit neurogenesis (Reference Steinborn, Zarowski, Winczewska-Wiktor, Wójcicka, Młodzikowska-Albrecht and Losy3). Studies conducted via rat models indicate that intensity of IL-1β, IL-6, TNF-α synthesis regulates the extent of hippocampal neuronal injury (Reference Zhang, Zhang, Fauser and Schluesener141).
Proinflammatory cytokines like IL-1β, IL-2, IL-6 sustain at very low concentration levels in healthy brain and they are shown to increase after generalised seizures. Just after tonic-clonic seizures, in vivo studies indicated the elevation of production and secretion of proinflammatory cytokines like IL-1β, IL-6, TNF-α in hippocampus. Evidence obtained from in vivo studies led to conclusions which shows that levels of cytokines mentioned are elevated in CSF and blood serum in patients with epilepsy (Reference Vezzani and Granata33,Reference Vezzani, Balosso and Ravizza142). IL-1β has an inhibitory effect on glutamate reuptake and enhancer activity on glutamate release by astrocytes (Reference Jacque and Tchelingerian143). In addition to IL-1β, IL-6 and TNF-α have a stimulator effect on glutamatergic neurotransmission, too (Reference Evseev, Vetrile and Karpova144). In animal models, tonic-clonic seizures suggested to increase blood serum levels of IL-6 significantly (Reference Vezzani145). In a clinical aspect, study conducted by Ichiyama et al. (Reference Ichiyama, Nishikawa, Yoshitomi, Hayashi and Furukawa146) showed that after prolonged febrile seizures, cytokines IL-1β, IL-6 and TNF-α shown to be increased in CSF of children. In terms of IL-2, modulatory effect on dopaminergic neuron activity and indirect effect on glutamatergic, cholinergic, serotonergic and noradrenergic neurotransmission of this cytokine have been shown (Reference Jacque and Tchelingerian143). In addition, proconvulsant effect of IL-2 has been observed in mice (Reference De Sarro, Rotiroti, Audino, Gratteri and Nistico147). Conversely, data obtained from a study concerning serum and CSF concentration of IL-2 in patients with epilepsy do not exactly confirm the mentioned suggestions, with no decrease in IL-2 concentration at seizure-free period (Reference Sinha, Patil, Jayalekshmy and Satishchandra148).
Synapsins are proteins that have been linked to pathogenesis of epilepsy in clinical studies. Clinical studies revealed that mutations in genes encoding synapsins are associated with epilepsy (Reference Cavalleri, Weale and Shianna149–Reference Lakhan, Kalita, Misra, Kumari and Mittal151) and their deletion has been shown to cause an excitatory/inhibitory imbalance and seizures. Increase in amounts of IL-6 and TNF-α in epileptogenic phase has been shown by Chugh et al. (Reference Chugh, Ali, Bakochi, Bahonjic, Etholm and Ekdahl152), who evaluated brain inflammation, synaptic protein expression and adult hippocampal neurogenesis in the epileptogenic and tonic-clonic phase among synapsin 2 knockout mice. Also a significant region-specific microglial activation was shown, which points to the fact that inflammation may serve as a precipitating factor for generating seizures.
In a model of partial SE, significant alteration of an adhesion molecule which is associated with inhibitory synapses, neuroligin-2, and also of a post-synaptic scaffolding protein gephyrin has been shown to occur in newly formed hippocampal neurons (Reference Jackson, Chugh and Nilsson153). Expression of adhesion molecules seems to be regulated by inflammatory molecules. For instance, N-cadherin – an adhesion molecule involved in the morphogenesis of synapses, which in turn plays a key role in cognitive function, and has an regulative role on molecular organisation of inhibitory and excitatory synaptic circuits – suggested to be modulated via TNF-α (Reference Kubota, Inoue and Hashimoto154,Reference Teocchı and D’Souza-Li155). Also, apart from being an essential component mediating IL-1 cytokine-related immune responses, IL-1 receptor accessory protein (IL-1RAcP) provides an interesting molecular link between immune systems and synapse formation in the brain. IL-1RAcP itself is able to arrange synapse formation. In neurons these isoforms can serve as a trans-synaptic cell adhesion molecule (Reference Yoshida, Shiroshima and Lee156).
Accompaniment of neuronal apoptosis has been shown in in vivo models of epilepsy (Reference Tuunanan, Lukasiuk, Halonen and Pitkanen157). In terms of apoptosis mediated by members of the TNF – nerve growth factor superfamily, interaction between the nervous and the immune system seems to be affected by these molecules. But also, involvement of these molecules in the induction of glial–neuronal cell death in case of neuroinflammatory diseases has been suggested.
TNF-related apoptosis-inducing ligand (TRAIL) seems to be not expressed in the human brain, apoptosis-mediating and apoptosis-blocking TRAIL receptors are both located on astrocytes, neurons and oligodendrocytes. In terms of therapeutic approaches to T-cell-mediated autoimmune diseases of the CNS, this death receptor–ligand system is a potential field to be investigated (Reference Dorr, Bechmann and Waiczies158). The expression of TRAIL was shown to increase significantly in both patients with TLE and the rat model of epilepsy. In the same study, CX3CL1-induced cell death has been suggested to have a role as a biomarker of brain inflammation in epileptic patients, and CX3CL1-induced cell death has been shown to be affected by the expression of TRAIL (Reference Xu, Zeng and Han50).
Apoptosis initiating extrinsic signalling pathway of transmembrane receptor-mediated interactions through death receptors, which are members of the TNF receptor gene superfamily, shown to be a part of epileptic pathogenesis. It has been shown that some death receptor apoptotic systems may be associated with the maintenance and progression of TLE-associated hippocampal sclerosis. But effects of these receptors on epilepsy are still scarcely comprehended. TNF and TNF receptor superfamily pathways are suggested to be an important pharmacological target in terms of anti-inflammatory therapy in TLE-associated hippocampal sclerosis patients (Reference Teocchı and D’Souza-Li155).
As being an effective AED in various seizure types, valproic acid (VPA), a GABA transaminase inhibitor, has been investigated for its modulating effects on immune system. The indicated immunomodulator effect of VPA has not been clearly disclosed yet (Reference Ichiyama, Okada, Lipton, Matsubara, Hayashi and Furukawa159). In a clinical study which involves comparison of blood serum levels of IL-1β, IL-2, IL-6 and TNF-α in patients with generalised seizures before and after VPA treatment, the concentration of IL-6 shown to be decreased significantly after 4–6 months of VPA therapy. Other proinflammatory cytokines shown to be not changed significantly in these patients. Results of that study suggest that the anti-inflammatory properties of VPA also provides a role in effective control of seizures (Reference Steinborn, Zarowski, Winczewska-Wiktor, Wójcicka, Młodzikowska-Albrecht and Losy3). TNF-α may play an important role in the progression of neurodegeneration but, regardless of these properties, through serious side effects like increased risk for infections and cancer, TNF-α neutralising therapy is suggested to be not an applicable choice to neurological diseases (Reference Dorr, Bechmann and Waiczies158).
As TNF-α plays an important role in surveillance of malignancy, there is a suggested risk about increase in tumour formation with anti-TNF-α agents. Considering therapy by anti-TNF-α agents, there are clinical trials that point to increased risk of cancer. But in the contrary, there are observational studies where an increased risk of cancer is not detected, even though excess risks of certain tumours are reported. Data about effects of anti-TNF therapy on infectious diseases are contradictory, but it is suggested to be the best to elaborate this therapy associating with a doubling of the increased risk of serious infection (Reference Ding and Deighton160).
Perspectives and conclusions
Linkage between brain inflammation and the epileptic process was emphasised commonly in literature via observations in clinical studies regarding drug-resistant human epilepsies and the experimental findings. Especially for seizures which are refractory to up-to-date anticonvulsant treatments, controlling of progression needs novel pharmacological strategies. In terms of epileptogenesis, as involving complicated pathomechanisms and multiple molecular signals, a better understanding remains challenging. Clarification of inflammatory processes in epileptic brain and also characterisation of contribution of several inflammatory processes and components may allow us to offer new pharmacological treatment options and possibility of using molecular alterations in brain inflammation as diagnostic biomarkers and potential therapeutic targets for epilepsy. These components may include FGF-2 and TrkB signalling pathways, bidirectional effects of BDNF, detrimental proinflammatory pathways (such as the IL-1β–IL-1R1 system), involvement of mTOR inhibition, harmful and helpful actions of microglia, release of glial inflammatory proteins (such as MIP, IL-6, CCL2 and IL-1β), adhesion molecules which are suggested to function in signalling pathways between neurons and microglia, and also linkage between these molecules and proinflammatory cytokines may provide facility to assign these approaches. For adjusting effectiveness and usage of these approaches, further studies are necessary.
Acknowledgements
Authors’ Contributions: F.A. and M.D. drafted the article and critically reviewed important intellectual content. F.A. substantially contributed to data acquisition. M.D. approved the final version for publication.
Financial Support
The authors report no financial affiliation or relationship relevant to the subject of this article.
Conflicts of Interest
The authors declare no conflicts of interest.




