Introduction
Khakiweed has been known by various scientific names since it was first documented in 1753 as Achyranthes repens L. (Mears Reference Mears1977). Khakiweed is a broadleaf weed species found in the southeastern United States in turfgrass, pasture, and pecan orchards (McCarty et al. Reference McCarty, Everest, Hall, Murphy and Yelverton2008). It has also been identified in South America, Africa, Australia, and the Mediterranean region (Llewellyn et al. Reference Llewellyn, Ronning, Clarke, Mayfield, Walker and Ouzman2016; Mears Reference Mears1977). The physiological appearance of khakiweed is variable, with stem color ranging from green to purple and leaf color ranging from deep to bright green (Sholedice and Renz Reference Sholedice and Renz2006). Leaves are pubescent or glabrous, obovate, and oppositely arranged, with small, white flowers that grow in clusters along the stem (Filippa and Espinar Reference Filippa and Espinar1993; Sholedice and Renz Reference Sholedice and Renz2006). Clusters mature into seed burrs that turn golden in color and injure people and animals, but limited research is available examining the extent of this damage. Following heavy rainfall, seeds germinate in the spring or summer and grow into a dense, prostrate mat (Nolte et al. Reference Nolte, Graf and Trammel2018). Though not yet ubiquitously present in Georgia, khakiweed can spread rapidly by multiple means of reproduction, including seed and shoot or root fragments. Furthermore, khakiweed can be difficult to control because of its woody taproot capable of carbohydrate storage (Sholedice and Renz Reference Sholedice and Renz2006).
Much of the available literature investigates khakiweed management in turfgrass environments. Increased khakiweed occurrence has been linked to long-term reductions in desirable grass (Umeda and Towers Reference Umeda and Towers2004). When managed under mowing heights commonly used in turf, plant diameters do not diminish, making mowing an unsuitable control method (Hephner et al. Reference Hephner, Cooper, Beck and Henry2013). Additionally, mowers can discharge khakiweed stems with nodes that are capable of rooting and regenerating into new plants (Parsons and Cuthbertson Reference Parsons and Cuthbertson2001). Instead, managers must depend on herbicides for sufficient control.
Acetolactate synthase (ALS) inhibitors like metsulfuron-methyl and nicosulfuron belong to the sulfonylurea chemical family. Whereas metsulfuron-methyl (half-life = 30 d) has variable residual activity, depending on soil conditions and application rate, nicosulfuron (half-life = 21 d) provides limited residual activity (Anonymous 1993; Bedmar et al. Reference Bedmar, Perdigon and Monterubbianesi2006; Carey and Kells Reference Carey and Kells1995; Lum et al. Reference Lum, Chikoye and Adesiyan2005; Shaner Reference Shaner2014; Walker et al. Reference Walker, Cotterill and Welch1989). Sequential applications of metsulfuron-methyl to khakiweed populations in turf provided 97% control at 12 wk after initial treatment (WAT) (Hephner et al. Reference Hephner, Cooper, Beck and Henry2012). When carfentrazone or sulfentrazone was applied in combination with metsulfuron-methyl, control was achieved sooner, but long-term (8 WAT) efficacy was not improved compared to metsulfuron-methyl alone, which provided >70% control at all tested rates (Brosnan et al. Reference Brosnan, Breeden, Henry and Walls2012).
Indaziflam, a cellulose synthesis inhibitor belonging to the alkylazine chemical family, is a highly effective herbicide that has both PRE and POST activity on broadleaf and grass weeds (Besancon and Bouchelle Reference Besancon and Bouchelle2023; Brosnan et al. Reference Brosnan, McCullough and Breeden2011; Dyer et al. Reference Dyer, Henry, McCullough, Belcher and Basinger2024), with registrations in turf, forages, and perennial crops (Grey et al. Reference Grey, Rucker, Webster and Luo2016; Grey et al. Reference Grey, Rucker, Wells and Luo2018; Hurdle et al. Reference Hurdle, Grey, McCullough, Shilling and Belcher2019; McCullough et al. Reference McCullough, Johnston, Reed and Yu2015). Because indaziflam has a half-life >150 d, it provides residual weed control months after initial application (Ghirardello et al. Reference Ghirardello, Araujo, da Silva, Silva, de Campos and Filho2022; Kaapro and Hall Reference Kaapro and Hall2012). However, control varies by species. Limited information exists examining indaziflam for khakiweed control, but indaziflam is used for control of other members of the Amaranthaceae family in a variety of cropping systems (Aulakh Reference Aulakh2020; Ekeleme et al. Reference Ekeleme, Dixon, Aster, Hauser, Chikoye, Olorunmaiye, Olojede, Korie and Weller2020; Grey et al. Reference Grey, Turpin, Wells and Webster2014; Smith et al. Reference Smith, Jennings, Monks, Jordan, Reberg and Schwarz2022). When indaziflam was applied at 20 or 41 g ai ha−1, redroot pigweed (Amaranthus retroflexus L.) control during the summer was >88% in Christmas tree [Abies balsamea (L.) Mill. var. phanerolepis Fernald] production (Aulakh Reference Aulakh2020). At 6 to 7 WAT, indaziflam gave 93% control of a variety of broadleaf weed species, including Palmer amaranth (Amaranthus palmeri S. Watson) and redroot pigweed in conventional-tillage sweet potato [Ipomoea batatas (L.) Lam.] systems (Smith et al. Reference Smith, Jennings, Monks, Jordan, Reberg and Schwarz2022).
Pendimethalin is a microtubule assembly inhibitor absorbed by plant roots to give PRE control of small-seeded broadleafs and grass weeds (Boydston et al. Reference Boydston, Collins and Fransen2010; Taylor-Lovell et al. Reference Taylor-Lovell, Wax and Bollero2002; Zain et al. Reference Zain, Dafaallah and Zaroug2020). Pendimethalin has a half-life of 50 to 100 d in soil, providing residual weed control during this period (Jha et al. Reference Jha, Kumar, Garcia and Reichard2015; Schleicher et al. Reference Schleicher, Shea, Stougaard and Tupy1995; Zimdahl et al. Reference Zimdahl, Catizone and Butcher1984). Pendimethalin alone may not be effective in controlling Amaranthaceae species over time, but mixtures with imazethapyr increased redroot pigweed control to >90% up to 8 WAT (Kahramanoglu Reference Kahramanoglu2014; Soltani et al. Reference Soltani, Shropshire and Sikkema2022).
Synthetic auxin herbicides that mimic natural auxins are more active and persist longer in plants than natural indole-3-acetic acid (Shaner Reference Shaner2014). Although 2,4-D effectively controls some broadleaf weed species, it provided only 23% control of khakiweed at 40 d after treatment (DAT) when a single application was used (835 g ai ha−1) (Kopec et al. Reference Kopec, Gilbert, Pessarakli and Moreno2004; Robinson et al. Reference Robinson, Simpson and Johnson2012). Florpyrauxifen-benzyl and aminopyralid are auxin mimics belonging to the pyridine-carboxylate chemical family. Florpyrauxifen-benzyl controls grass and broadleaf species in rice (Oryza sativa L.), pasture, and aquatic environments (Howell et al. Reference Howell, Hofstra, Heilman and Richardson2021; Miller and Norsworthy Reference Miller and Norsworthy2018). Florpyrauxifen-benzyl has no residual activity (Wright et al. Reference Wright, Norsworthy, Roberts, Scott, Hardke and Gbur2020). Aminopyralid is used for weed control in pasture and rangeland (Enloe et al. Reference Enloe, Lym, Wilson, Westra, Nissen, Beck, Moechnig, Peterson, Masters and Halstyedt2007). Although 2,4-D and florpyrauxifen-benzyl provide little to no residual weed control, aminopyralid at 0.12 kg ha−1 reduced tropical soda apple (Solanum viarum Dunal) populations by 97% 335 DAT (Ferrell et al. Reference Ferrell, Mullahey, Langeland and Kline2006).
Examination of chemical control options for khakiweed in pecan orchards and pasture areas is limited. The efficacies of several herbicides available for use in these crops were examined to possibly expand control options. The objective of the PRE study was to measure the efficacy of soil-applied herbicides for khakiweed control. The POST study was conducted to measure the effect of foliar-applied herbicides on khakiweed growth.
Materials and Methods
Greenhouse experiments were conducted in 2022 and 2023 at the University of Georgia Tifton Campus in Tifton, GA (31.28°N, 83.31°W). In the PRE study, the bottom 2.5 cm of 10.2 × 10.2 × 12.7-cm pots (Greenhouse Megastore, Danville, IL, USA) were filled with potting media (Sta-Green, Mooresville, NC, USA) to improve soil and water retention, while the remaining 10.2 cm was filled with Tifton loamy sand (87%, 7%, and 6% sand, silt, and clay, respectively; fine-loamy, kaolinitic, thermic Plinthic Kandiudult) previously sterilized in an autoclave to reduce weed competition. The Tifton soil had pH 6.3 and 0.8% organic matter. Potting media was not used as the sole soil source because high organic matter content (50% to 60%) could sorb the herbicides and decrease herbicidal activity (López-Piñeiro et al. Reference López-Piñeiro, Peña, Albarrán, Becerra and Sánchez-Llerena2013; Rojas et al. Reference Rojas, Morillo, Usero, Delgado-Moreno and Gan2023). For the POST-only experiment, these same-sized pots were filled with potting media alone to reduce soil activity of the herbicides and emphasize only the POST effects of application (López-Piñeiro et al. Reference López-Piñeiro, Peña, Albarrán, Becerra and Sánchez-Llerena2013; Rojas et al. Reference Rojas, Morillo, Usero, Delgado-Moreno and Gan2023).
Plants were initially collected from Terrell County, GA (31.69°N, 81.52°W), on April 21, 2022, to create an established population in the greenhouse. Cuttings were taken from the established population and used for all experiments. Similar to the method used by Proctor et al. (Reference Proctor, Gaussoin and Reicher2011), the bottom 2.5 cm of khakiweed plant cuttings were treated with root growth media (TakeRoot®, Garden Safe, Bridgeton, MO, USA) and planted in the pots filled as described earlier for the PRE and POST studies. Cuttings grew for 3 wk in the greenhouse at an average day temperature of 32 C and average night temperature of 25 C to allow plants to develop a substantial root system. Plants were subjected to natural light and watered via misting sprayer at 115 ml d−1 pot−1. Two weeks after planting, each pot was fertilized with 10-10-10 (Gro Tec, Madison, GA, USA) at 50 kg ha−1. At this stage, plants were between 10 and 16 cm in length, and all plants had three or more nodes. Five plants were randomly assigned to each treatment to create a randomized complete block design, taking care to evenly distribute plant sizes.
All herbicide treatments were mixed in the laboratory via serial dilution, where the most concentrated spray was mixed first and used as a stock solution. For each rate, a 300-ml aliquot was mixed in 500-ml Pyrex® graduated cylinders. Four rates for each herbicide were then generated by mixing and included 0.25X, 0.5X, 1X, and 2X levels of the label recommended rate for general broadleaf weed control (Table 1). All herbicides were applied at 140 L ha−1 based on the known soil surface area (104 cm2 pot−1). For the PRE and POST studies, there were five replicates per run, with three runs in time. Five nontreated plants were included as a control in both studies. Spray dates for each run were July 10, 2022, October 3, 2022, and April 21, 2023. Day length was 14, 12, and 13 hr for run 1, 2, and 3, respectively. Preemergence treatments were pipetted onto the soil to avoid contact with plant tissue, whereas POST treatments were applied via a spray chamber (University of Georgia Fabrication Lab, Athens, GA, USA) at 24 PSI using a TeeJet® XR 11002 VS nozzle (TeeJet® Technologies, Glendale Heights, IL, USA). All PRE and POST herbicide treatments are detailed in Table 1. Nonionic surfactant (Southern Ag, Hendersonville, NC, USA) was included at 0.25% v/v for metsulfuron-methyl, metsulfuron-methyl + nicosulfuron, indaziflam, and 2,4-D amine. Methylated seed soil (Southern Ag) was added to the 2,4-D amine + florpyrauxifen-benzyl and aminopyralid + florpyrauxifen-benzyl treatments at 1% v/v. After application of the PRE and POST treatments, watering was delayed by 12 hr to ensure that herbicides were not leached or washed off. Pots were watered as described earlier.
Table 1. PRE and POST herbicides evaluated in the study, listed with modes of action, Herbicide Resistance Action Committee group numbers, and rates examined. a,b,c
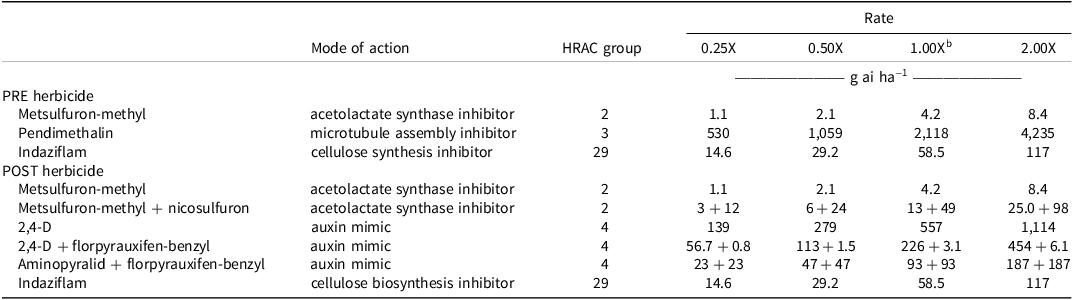
a For metsulfuron-methyl, pendimethalin, indaziflam, and 2,4-D, pecan crop application rates were used. Metsulfuron-methyl + nicosulfuron, 2,4-D + florpyrauxifen-benzyl, and aminopyralid + florpyrauxifen-benzyl were evaluated at the pasture rate.
b Abbreviation: HRAC, Herbicide Resistance Action Committee.
c Label recommended rate for general broadleaf control.
Khakiweed was visually assessed for herbicide symptomology at 3, 7, 14, and 21 DAT for all PRE and POST treatments. Epinasty, chlorosis, and necrosis observations were recorded as percentages, where 0% indicated no visual injury and 100% indicated injury on all plant tissue (Frans et al. Reference Frans, Talbert and Crowley1986). Aboveground biomass was collected by clipping all tissue above the soil line at the end of this first evaluation period 21 DAT. Biomass harvest was done to stimulate khakiweed regrowth from the roots. Fresh biomass was recorded in grams before plant tissue was placed in a dryer at 50 C for 3 d.
After the initial biomass collection, pots were returned to the greenhouse and regrown for 3 wk. During this regrowth period, plants were maintained in the same greenhouse conditions previously described. At the end of week 3, above- and belowground biomass (roots) was collected. Aboveground biomass was collected as previously mentioned, and belowground biomass was collected by gently rinsing the soil away from the root tissue. Excess water was removed by blotting with a paper towel. Fresh masses were recorded, then tissue was placed in a dryer for 3 d at 50 C. Percent control values were calculated for the dry biomass taken from the first aboveground biomass harvest, the second aboveground biomass harvest, and the root harvest.
The PRE and POST studies were analyzed separately. JMP® Pro 17 (JMP, Cary, NC, USA) was used to detect data outliers using distribution plots and to confirm data normality via a goodness-of-fit test. ANOVA was conducted in SAS OnDemand (SAS Institute, Cary, NC, USA) to determine the significance (α = 0.05) of rate on mass percent control where replication and run were treated as random effects. Nonlinear regression was performed on the mass percent control values where applicable. The Gompertz three-parameter regression (Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016; Schwartz-Lazaro et al. Reference Schwartz-Lazaro, Norsworthy, Scott and Barner2017) on the mass response data produced maximum control and GR50 values with 95% CIs:
Maximum control at all rates was estimated by parameter a, the upper asymptote of the curve. An increase in percent khakiweed control induced by an increase in herbicide application rate is reported as parameter b, the curve slope. The application rate needed to reduce mass by 50% (GR50) is reported as parameter c, the curve inflection point.
Results and Discussion
PRE Herbicides
Preemergence herbicides resulted in <50% initial control of the established plants. However, all herbicides and rates inhibited regrowth >85% compared to the control, with no difference between herbicides (P = 0.09) or rates (P = 0.16) (data not shown). A similar effect was observed in a study measuring the effect of PRE indaziflam on Palmer amaranth control. Data were combined across rates with 43% to 86% control of newly emerged and mature weeds 6 wk after the initial application, depending on timing with respect to weed size (Smith et al. Reference Smith, Jennings, Monks, Jordan, Reberg and Schwarz2022). Efficacy of pendimethalin and metsulfuron-methyl alone and combined with POST treatments in preventing Amaranthaceae species like Palmer amaranth and redroot pigweed is well documented, and control achieved in this study often exceeded reported values (Beiermann et al. Reference Beiermann, Creech, Knezevic, Jhala, Harveson and Lawrence2022; Crow et al. Reference Crow, Steckel, Hayes and Mueller2015; Moyer Reference Moyer1995; Whitaker et al. Reference Whitaker, York, Jordan and Culpepper2010). Preemergence herbicides are a highly effective way to control khakiweed regrowth from roots.
POST Herbicides
Although most response trends were evident within mode of action groups, some were observed across all herbicides. Khakiweed plants were most sensitive to herbicide application during the initial growth period. However, control plateaued at 35% to 71% (Table 2) as indicated in Equation 1. For all POST herbicides, root biomass GR50 (parameter c of Equation 1) was greater than initial and regrowth-period aboveground biomass GR50 values, indicating that khakiweed roots are more tolerant of herbicide application than aboveground biomass (Table 2). For all herbicide treatments except those containing florpyrauxifen-benzyl, control after biomass removal was greater than the initial control (Table 2).
Table 2. Khakiweed biomass response to POST herbicide application modeled with the Gompertz three-parameter equation. a,b
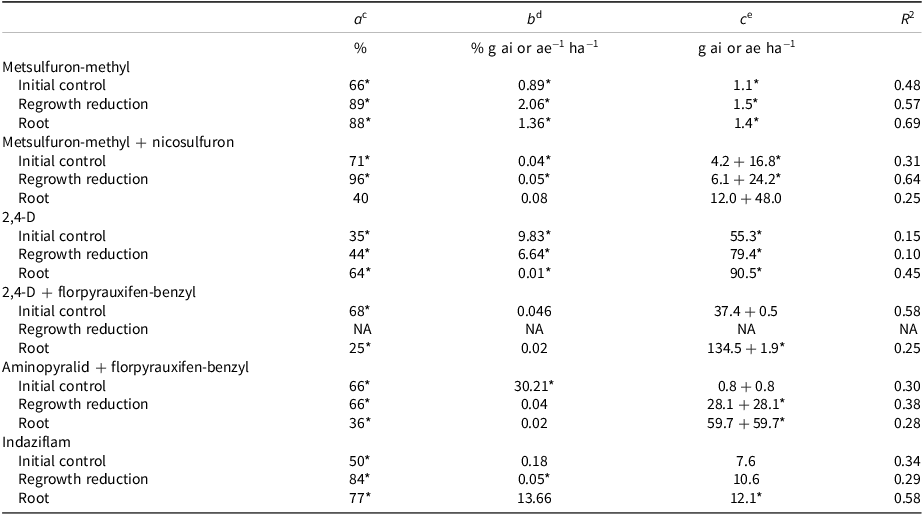
a
The Gompertz three-parameter model
![]() $y = a \times {e^{ - {e^{ - b\left( {x - c} \right)}}}}$
was used to generate parameters a, b, and c, where x = herbicide rate.
$y = a \times {e^{ - {e^{ - b\left( {x - c} \right)}}}}$
was used to generate parameters a, b, and c, where x = herbicide rate.
b Abbreviations: NA, not applicable; *, P < 0.05, where values were generated via ANOVA using least squared means comparisons and α = 0.05.
c Maximum control across rates.
d Slope.
e GR50: herbicide rise required to decrease plant biomass by 50%.
Metsulfuron-methyl alone and in combination with nicosulfuron was examined for POST control of khakiweed biomass. Overall, these herbicides yielded the greatest level of control for all mass measurements (Table 2). Metsulfuron-methyl alone and in combination gave <71% control of khakiweed during the initial growth period (Table 2). In contrast, a study examining control of ALS-resistant and -susceptible Palmer amaranth observed that POST applications of all 16 ALS herbicides at all rates (0.5X, 1X, 2X, and 4X the label recommended rate) controlled (>80%) the susceptible biotype (Gaeddert et al. Reference Gaeddert, Peterson and Horak1997). Regrowth was reduced 89% and 96% by metsulfuron-methyl and metsulfuron-methyl + nicosulfuron, respectively, following an aboveground biomass harvest (Table 2). When metsulfuron-methyl was applied at 21 and 42 g ai ha−1 for khakiweed control on weekly mowed bermudagrass [Cynodon dactylon (L.) Pers.] plots, 79% and 87% control was achieved 8 WAT, respectively (Hephner et al. Reference Hephner, Cooper, Beck and Henry2012). The results of this study and previous literature indicate that ALS herbicide application in combination with mowing may be a successful control mechanism for khakiweed. Regrowth GR50 values were similar to those of the 0.5X application rates (2.1 and 3 + 12 g ai ha−1 for metsulfuron-methyl and metsulfuron-methyl + nicosulfuron, respectively) (Table 2). The response of 12 aquatic weed species to variable rates of metsulfuron-methyl demonstrated that plants with a small exposed leaf area and rapid growth rate, like khakiweed, were the most sensitive to this herbicide, with the common aquatic bioassay species common duckweed (Lemna minor L.) having a GR50 of 0.18 μg L−1 (Cedergreen et al. Reference Cedergreen, Streibig and Spliid2004). Though aboveground control values were similar for these herbicide treatments, metsulfuron-methyl reduced root mass by 88% at the maximum rate (8.4 g ai ha−1), while metsulfuron-methyl + nicosulfuron had a variable effect on root mass (Table 2). Nicosulfuron can have antagonism with other sulfonylurea herbicides (Mekki and Leroux Reference Mekki and Leroux1994; Rabaey and Harvey Reference Rabaey and Harvey1997). A study examining the effects of nicosulfuron + rimsulfuron indicated synergism for smooth crabgrass [Digitaria ischaemum (Schreb.) Schreb. ex Muhl.] but an antagonistic effect on soybean [Glycine max (L.) Merr.] (Mekki and Leroux Reference Mekki and Leroux1994). Because antagonism is species-dependent, it is possible that the metsulfuron-methyl + nicosulfuron applications caused decreased root control in khakiweed.
Although indaziflam was effective at all rates as a PRE treatment for regrowth inhibition, variable plant response to POST application limits its use for khakiweed control. The maximum control provided by indaziflam during the initial and regrowth period was 50% and 84%, respectively (Table 2). Initial mass reduction values were lower than those reported by a simulated drift study examining the effect of a similar range of indaziflam rates on row crop biomass (Jeffries et al. Reference Jeffries, Mahoney and Gannon2014). Additionally, indaziflam at 35 g ai ha−1 or more provided >97% control of smooth crabgrass, indicating that khakiweed was less sensitive than crabgrass to POST indaziflam application (Brosnan et al. Reference Brosnan, McCullough and Breeden2011). However, GR50 values were unable to be generated for aboveground control due to highly variable plant response to indaziflam (Table 2). Final root mass was well correlated to application rate, where the maximum application rate (117 g ai ha−1) caused a 77% reduction compared to the nontreated control plants (Table 2). The indaziflam GR50 for root application was 12 g ai ha−1 (Table 2). This value is significantly larger than the barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], broadleaf signalgrass [Brachiaria platyphylla (Munro ex C. Wright) Nash], doveweed [Murdannia nudiflora (L.) Brenan], large crabgrass [Digitaria sanguinalis (L.) Scop.], and purple nutsedge (Cyperus rotundus L.) root GR50 values reported (Ramanathan et al. Reference Ramanathan, Gannon and Maxwell2023).
Auxin mimic performance was dependent on rate and plant size. Plants smaller than 11 cm were controlled by auxin herbicide application at the 1X rate or greater (data not shown). Auxin herbicides are known to be less effective on larger plants (Faji et al. Reference Faji, Kebede, Fevissa, Mohammed, Mengistu and Terefe2022; Peterson et al. Reference Peterson, McMaster, Riechers, Skelton and Stahlman2016). During the initial growth period, larger plants demonstrated chlorosis (>75%) and epinasty (>80%) symptomology, but recovery was evident within 2 wk in most cases (data not shown). Auxin mimics provided poor long-term control of above- and belowground biomass (<68%) (Table 2). 2,4-D at the maximum application rate (1,114 g ai ha−1) reduced plant mass by only 44% compared to the nontreated control (Table 2), which agreed with previous literature (Kopec et al. Reference Kopec, Gilbert, Pessarakli and Moreno2004; Mears Reference Mears1966). This indicates that 2,4-D alone is not effective for consistent golf course, private lawn, and agricultural khakiweed control (Kopec et al. Reference Kopec, Gilbert, Pessarakli and Moreno2004; Mears Reference Mears1966). For plants subjected to 2,4-D or aminopyralid + florpyrauxifen-benzyl at the 1X rates (557 and 93 + 93 g ai ha−1, respectively), plant mass was greater compared to the 0.5X and 2X rates (Table 2). This decrease in efficacy may be because auxin herbicides can stimulate plant growth at low application levels without inducing significant plant injury (Agusti et al. Reference Agusti, Zaragoza, Iglesias, Almela, Primo-Millo and Talόn2001; Song Reference Song2014). Additionally, there is response variability between dicot species for auxin mimic herbicides. A study examining the effect of auxin mimic herbicide application rate on tall fescue (Festuca arundinacea Schreb.) injury reported that 70 + 140 g ha−1 of dicamba + 2,4-D caused equivalent or greater injury than the 280 + 1,120 g ha−1 rate (Moyer and Kelley Reference Moyer and Kelley1995).
Of the auxin mimics, aminopyralid + florpyrauxifen-benzyl provided the greatest control (66%) of khakiweed during the regrowth phase, but control was not comparable to that achieved by POST ALS or cellulose biosynthesis inhibitor herbicide application (Table 2). 2,4-D is symplastically translocated to roots, inhibiting biomass accumulation (Cords Reference Cords1966; Shaner Reference Shaner2014; Wyrill and Burnside Reference Wyrill and Burnside1976). When 2,4-D was applied alone at the maximum rate (1,114 g ai ha−1), 64% control was achieved (Table 2). However, a mixture of 2,4-D + florpyrauxifen-benzyl resulted in the lowest level of root control of all auxin mimic herbicides examined (Table 2). This could be due to the reduced amount of 2,4-D in the combination treatments.
Of the three modes of action examined in this study, the ALS inhibitors gave the highest level of aboveground POST control. Metsulfuron-methyl alone provided the greatest reduction in root mass of all herbicides examined (Table 2). Indaziflam gave 84% and 77% control of regrowth and root mass, respectively, but was less effective than the ALS inhibitors for initial control (Table 2). The auxin mimics gave poor long-term control of khakiweed, with aminopyralid + florpyrauxifen-benzyl giving only 66% control of regrowth at the highest application rate (Table 2).
Practical Implications
Khakiweed, though not yet prolific in the southeastern United States, has limited control options. Single-application chemical control alone is not effective for long-term khakiweed control. When PRE herbicide or POST ALS herbicide application is paired with biomass removal, >85% long-term control can be achieved. This points to mowing as an essential component of control in pecan and pasture areas. However, mowing should be done only after effective herbicide application to reduce the risk of spreading viable plant parts for establishment into new khakiweed plants. Because khakiweed germination occurs in spring or summer, pasture mowing should be timed to decrease interference with the crop. Whereas this study utilized young plants, khakiweed plants in the field may grow several feet in diameter. Further research is needed to evaluate the effect of herbicides on mature khakiweed plants. ALS-inhibitor herbicides were highly effective in this study, but overreliance on a single mode of action increases the risk of resistance within the species. Resistance to ALS herbicides is abundant in family Amaranthaceae. Therefore further research is needed to evaluate novel chemical and cultural control strategies.
Acknowledgments
Thanks to Sidney Cromer, Hagen Walker, Morgan Funk, and Ebson Silva at the University of Georgia for providing technical support.
Funding
Funding for this research was provided by the University of Georgia College of Agricultural Sciences, the Georgia Pecan Commission, and BASF Agriculture.
Competing interests
The authors declare no conflicts of interest.





