INTRODUCTION
Objective
The aim of this study was to assess, in a more definitive manner than prior research, the effect of epinephrine in OHCA and its safety and efficacy.
METHODS
Design
Randomized, double-blind trial
Setting
Five UK National Health Service ambulance services
Subjects
Adults (≥ 16 years of age) with OHCA in whom initial cardiopulmonary resuscitation (CPR) and defibrillation were unsuccessful. Exclusion criteria included suspected pregnancy, cardiac arrest from anaphylaxis or asthma, and epinephrine before the arrival of trial-trained paramedics. Traumatic arrests were excluded at one site.
Intervention
IV or intraosseous epinephrine, 1 mg, or 0.9% normal saline placebo every 3–5 minutes.
Outcomes
Primary outcomes included rate of survival at 30 days. Secondary outcomes included rates of survival until hospital admission, at-hospital discharge and 3-months, lengths of hospital and intensive care unit (ICU) stay, and neurologic outcome at hospital discharge.
RESULTS
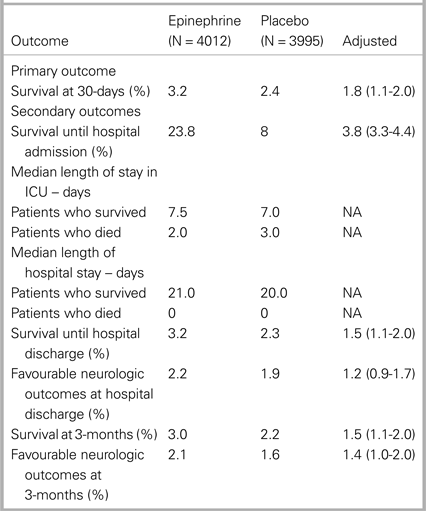
Results of the study are shown in Table 1.
Table 1. Primary and secondary outcomes (from Table 2 in Perkins et al., 2017, “A randomized trial of epinephrine in out-of-hospital cardiac arrest”)
APPRAISAL
Strengths
• Large, multicentre, double-blind randomized controlled trial (RCT), expanding upon previously observational research
• Outcomes clearly defined and clinically relevant
• Primary outcome in accordance with International Liaison Committee on Resuscitation (ILCOR) guidelines
• Well-defined population
• Similar baseline characteristics between groups
• CPR data included when available
Limitations
• Emergency department and hospital care not defined by the study protocol, which could distort the accuracy or generalizability of the results
• Overall rate of survival following cardiac arrest significantly lower than anticipated
• Median time to administration of study agent > 21 minutes, which could distort the accuracy or generalizability of the results
• No discussion on shockable versus non-shockable rhythms included (However, a subgroup analysis reported in the supplementary material found no significant differences.)
• CPR quality during resuscitation efforts known to contribute heavily to outcomes and not assessed
• Not necessarily generalizable to other epinephrine dosing strategies
CONTEXT
Multiple cohort studies with conflicting results on the efficacy and safety of epinephrine in OHCA have been published. In 2011, Jacobs et al. published the only other RCT on this topic; however, it was terminated early with incomplete enrolment.Reference Jacobs, Finn and Jelinek2 Current Advanced Cardiac Life Support guidelines recommend the routine administration of 1-mg (standard dose) epinephrine every 3–5 minutes in OHCA, despite a lack of strong evidence to support this practice.Reference Nolan, Hazinski and Aickin3 Trials focused on different epinephrine doses and frequencies, infusions, or other vasopressor agents would be helpful.
BOTTOM LINE
The results of this study provide persuasive evidence to reconsider current epinephrine guidelines in OHCA. Although epinephrine was associated with increased 30-day survival, it did not increase the probability of survival with good neurologic outcome owing to an increased rate of severe neurologic disability in the treatment group. The number needed to treat in this trial to obtain one additional survivor was 112. The slight increased survival coupled with worsened neurologic outcome in the treatment group does not support the routine use of epinephrine in OHCA; however, the possibility of benefit in subgroups remains, and further research is required. Clinicians should continue to use epinephrine in OHCA until such time that society and national guidelines are revised.



INTRODUCTION
Background
There is clinical uncertainty regarding the safety and efficacy of epinephrine administration in out-of-hospital cardiac arrest (OHCA).Reference Loomba, Nijhawan, Aggarwal and Arora1
Objective
The aim of this study was to assess, in a more definitive manner than prior research, the effect of epinephrine in OHCA and its safety and efficacy.
METHODS
Design
Randomized, double-blind trial
Setting
Five UK National Health Service ambulance services
Subjects
Adults (≥ 16 years of age) with OHCA in whom initial cardiopulmonary resuscitation (CPR) and defibrillation were unsuccessful. Exclusion criteria included suspected pregnancy, cardiac arrest from anaphylaxis or asthma, and epinephrine before the arrival of trial-trained paramedics. Traumatic arrests were excluded at one site.
Intervention
IV or intraosseous epinephrine, 1 mg, or 0.9% normal saline placebo every 3–5 minutes.
Outcomes
Primary outcomes included rate of survival at 30 days. Secondary outcomes included rates of survival until hospital admission, at-hospital discharge and 3-months, lengths of hospital and intensive care unit (ICU) stay, and neurologic outcome at hospital discharge.
RESULTS
Results of the study are shown in Table 1.
Table 1. Primary and secondary outcomes (from Table 2 in Perkins et al., 2017, “A randomized trial of epinephrine in out-of-hospital cardiac arrest”)
APPRAISAL
Strengths
• Large, multicentre, double-blind randomized controlled trial (RCT), expanding upon previously observational research
• Outcomes clearly defined and clinically relevant
• Primary outcome in accordance with International Liaison Committee on Resuscitation (ILCOR) guidelines
• Well-defined population
• Similar baseline characteristics between groups
• CPR data included when available
Limitations
• Emergency department and hospital care not defined by the study protocol, which could distort the accuracy or generalizability of the results
• Overall rate of survival following cardiac arrest significantly lower than anticipated
• Median time to administration of study agent > 21 minutes, which could distort the accuracy or generalizability of the results
• No discussion on shockable versus non-shockable rhythms included (However, a subgroup analysis reported in the supplementary material found no significant differences.)
• CPR quality during resuscitation efforts known to contribute heavily to outcomes and not assessed
• Not necessarily generalizable to other epinephrine dosing strategies
CONTEXT
Multiple cohort studies with conflicting results on the efficacy and safety of epinephrine in OHCA have been published. In 2011, Jacobs et al. published the only other RCT on this topic; however, it was terminated early with incomplete enrolment.Reference Jacobs, Finn and Jelinek2 Current Advanced Cardiac Life Support guidelines recommend the routine administration of 1-mg (standard dose) epinephrine every 3–5 minutes in OHCA, despite a lack of strong evidence to support this practice.Reference Nolan, Hazinski and Aickin3 Trials focused on different epinephrine doses and frequencies, infusions, or other vasopressor agents would be helpful.
BOTTOM LINE
The results of this study provide persuasive evidence to reconsider current epinephrine guidelines in OHCA. Although epinephrine was associated with increased 30-day survival, it did not increase the probability of survival with good neurologic outcome owing to an increased rate of severe neurologic disability in the treatment group. The number needed to treat in this trial to obtain one additional survivor was 112. The slight increased survival coupled with worsened neurologic outcome in the treatment group does not support the routine use of epinephrine in OHCA; however, the possibility of benefit in subgroups remains, and further research is required. Clinicians should continue to use epinephrine in OHCA until such time that society and national guidelines are revised.
Competing interests
None declared.