The term conjugated linoleic acid (CLA) describes a mixture of positional and geometric isomers of linoleic acid (C18 : 2n-6) which contain a conjugated double-bond system instead of the more common isolated double bonds (for a very recent review see Bhattacharya et al. Reference Bhattacharya, Banu, Rahman, Causey and Fernandes2006). Rumenic or cis-9, trans-11-octadecadienoic acid (cis9, trans11-C18 : 2) is the most common CLA isomer and is often regarded as the biologically most relevant one (Fritsche & Steinhart, Reference Fritsche and Steinhart1998). The various CLA are produced in the rumen of ruminant animals mainly by the bacteria Butyrivibrio fibrisolvens (Kepler et al. Reference Kepler, Hirons, McNeill and Tove1966; Kim et al. Reference Kim, Liu, Bond and Russell2000) through reactions of isomerization and biohydrogenation. These reactions lead as well to the formation of a wide variety of trans- and cis-monoenic fatty acids (especially C18 : 1 trans isomers). In addition, trans-vaccenic acid (trans11-C18 : 1, TVA) which originates from linoleic and linolenic acid plays an important role as precursor of rumenic acid. Very recent work has shown that the conversion of TVA in rumenic acid does occur as well in man (Mosley et al. Reference Mosley, McGuire, Williams and McGuire2006). CLA are currently receiving much attention in nutritional research, since there is experimental evidence suggesting that these fatty acids might have anti-carcinogenic, anti-atherosclerotic, anti-diabetic and immune-modulating effects, as well as a favourable influence on body fat composition, i.e. on the proportion of fat tissue to muscle mass (Belury, Reference Belury2002). Most of this experimental evidence derives from in vitro experiments or animal tests (Bhattacharya et al. Reference Bhattacharya, Banu, Rahman, Causey and Fernandes2006), which justifies the recent interest in clinical trials concerning the relevance of CLA for human health. The newly published reports concerning the effect of CLA supplementation on health-related outcomes have contradicting messages and in some cases an isomer-specific effect on the lipid profile could be shown (for a review see Tricon et al. Reference Tricon, Burdge, Williams, Calder and Yaqoob2005). A double-blind study revealed that the consumption of dairy products naturally enriched in cis9, trans11-C18 : 2 increases the level of this fatty acid in plasma and cellular lipids (Burdge et al. Reference Burdge, Tricon and Morgan2005). However, this change did not appear to have a significant effect on the whole blood lipid profile, including several CVD risk parameters (Tricon et al. Reference Tricon, Burdge and Jones2006).
It is known that the lipid composition of cow's milk is strongly influenced by the stable conditions and feeding management, with milk from cows held in organic farms (Germany, Italy) containing significantly more CLA than that from their conventionally held counterparts (Jahreis et al. Reference Jahreis, Fritsche and Steinhart1997; Bergamo et al. Reference Bergamo, Fedele, Iannibelli and Marzillo2003). Note that farms certified as ‘organic’ are those in which the use of synthetic inputs, such as synthetic fertilizers and pesticides, preventive veterinary drugs, genetically modified seeds and breeds, most preservatives, additives and irradiation are excluded (htpp://www.ifoam.org/sub/faq.html). Since the major source of CLA in man is the diet, we have hypothesized that the amount of CLA in the milk of breastfeeding women could be augmented by increasing the amount of organic dairy nutrients within their diet. The sources of CLA for man comprise not only dairy products but also ruminant meat (Ritzenthaler et al. Reference Ritzenthaler, McGuire, Falen, Shultz, Dasgupta and McGuire2001); therefore, emphasis was put on these two groups of nutrients. In a small pilot study in Switzerland, we have previously found that the milk from breastfeeding women who obtained more than 50 % of the energy content of their diet from organic products had about 30 % higher CLA content at 4 and 40 days post-partum, compared to controls consuming the same mixture (dairy products and meat) of conventional products (Rist et al. Reference Rist, Zweidler, von Mandach and Freyer2003). The aim of the KOALA Birth Cohort Study, which is being performed in the Netherlands, is to identify factors that influence the clinical expression of atopic disease with a main focus on lifestyle including, among other parameters, dietary habits, breastfeeding and breast milk composition (Kummeling et al. Reference Kummeling, Thijs and Penders2005). Accordingly, this cohort study comprised persons with alternative lifestyles, including organic food choice, which offered an opportunity to study the effect of organic food intake on the lipid composition of breast milk and, in particular, on the corresponding CLA content.
In summary, we addressed the question whether an organic diet of the mothers can result in increased levels of CLA and TVA in human milk. Accordingly, the relative amounts of these fatty acids were measured in the milk from 312 breastfeeding women following diets with different content of organic dairy and/or meat products. While about 50 % of the study participants consumed conventional food, the remaining women included organic dairy and meat products in their diet. The present results, showing that an organic diet can lead to increased levels of CLA and TVA, are discussed in view of the possible health-favourable properties of these fatty acids.
Subjects and methods
Subjects and collection of breast milk
Breast milk samples were donated by breastfeeding participants to the KOALA study, a prospective birth cohort study described in detail elsewhere (Kummeling et al. Reference Kummeling, Thijs and Penders2005). Briefly, we recruited participants with varying lifestyles (conventional and alternative). Pregnant women with a conventional lifestyle (n 2343) were recruited from an ongoing prospective cohort study on Pregnancy-related Pelvic Girdle pain in the Netherlands (Bastiaanssen et al. Reference Bastiaanssen, de Bie, Bastiaenen, Heuts, Kroese, Essed and van den Brandt2005). During the same recruitment period (December 2002 to August 2003), pregnant women with an alternative lifestyle, which included the use of organic food (n 491), were recruited through several channels, such as organic food shops, anthroposophic clinicians and midwives, Rudolf Steiner schools and relevant magazines. Finally, 312 (146 from the conventional and 166 from the alternative recruitment group) were enrolled, each donating one sample of breast milk, 1 month post-partum. The study was approved by the Medical Ethical Committee of Maastricht University/Academic Hospital Maastricht, Maastricht, The Netherlands.
Breast milk sampling and extraction of total milk lipids
Mothers received a sterile 50 ml tube (Cellstar PP-test tubes; Greiner Bio-One, Kremsmuenster, Austria) and were instructed to collect the milk sample in the morning, before breastfeeding their child, from the contra-lateral breast (since the last feeding) and to keep the tube in the refrigerator (at approximately 4°C) until it was collected by one of the researchers. If the mother was not able to collect the milk sample by herself (with or without a pumping regimen), an electric breast pump (Medela, Baar, Switzerland) was used with the help of one of the researchers, within the same day. Collection and processing of the breast milk samples occurred on the same day. During transport the milk samples were stored in a cooler (Coleman Company, Inc., Breda, The Netherlands) on packed ice (at approximately 4°C). Fractions for fatty acids analysis were preserved by mixing approximately 2 ml milk with 2 μl butylated hydroxytoluene–methanol (1 : 1, v/v). The samples were stored at − 80 °C in plastic storage vials (Sarstedt, Nümbrecht, Germany) at the European Biobank in Maastricht (the Netherlands), until analysis. Lipids were extracted from the 0·2 ml milk samples with 3 ml chloroform–methanol (2 : 1, v/v containing 0·001 % butylated hydroxytoluene) after adding water to improve phase separation and 200 μl of the internal standard (containing approximately 200 μg heptadecenoic acid methyl ester in n-hexane, cis10-C17 : 1). The lower organic phase was transferred into a Pyrex glass tube and extraction was repeated twice. The combined organic phases were evaporated to dryness under a nitrogen stream at 40°C.
Fatty acid analysis
The lipid extracts were transmethylated with 5 % potassium methylate solution in methanol for 30 min at 60°C. After cooling to room temperature, 3 ml 0·5 m-methanolic sulphuric acid in methanol were added. Thereafter, the extracts were vortexed and heated at 60°C for 15 min. After cooling, 3 ml saturated sodium chloride solution in water and 2 ml n-hexane phase were added. The newly formed fatty acid methyl esters were then extracted into the n-hexane phase by vortexing. The upper n-hexane phase was transferred after centrifugation into a 4 ml glass vial; the extraction was repeated once. The combined n-hexane phases were evaporated to dryness under a stream of nitrogen and solved in 500 μl n-hexane. Fatty acid methyl esters were analysed by GC–free induction decay (GC–FID) and Ag+–HPLC essentially as previously described (Müller et al. Reference Müller, Ringseis, Dusterloh, Gahler, Eder and Steinhart2005). For the GC–FID analysis, an Agilent 6890 GC (Agilent Technologies, Waldbroon, Germany) equipped with a split/splitless injector at 230°C, a flame ionization detector at 260°C, an autosampler and a CP SIL 88 column (100 m, 0·25 mm, 0·2 μm film thickness; Varian, Darmstadt, Germany) was used. Hydrogen was used as carrier, at a constant flow rate of 1 ml/min. The temperature of the GC oven was set to 70°C for 3 min, increased at 8°C/min up to 180°C, held for 2 min, increased at 4°C/min up to 210°C, held for 4 min, increased at 2°C/min to a final temperature of 240°C and held for 25 min. The data were analysed using the HP Chemstation software (Rev. A08.03); the percentage method which excludes the internal standard was used, to allow a better comparison of the fatty acids among the various samples. Conjugated fatty acid isomers were separated using Ag+–HPLC– diode-array detection. The system consisted of an isocratic Merck-Hitachi L-6000 A HPLC pump equipped with a Waters 717 autosampler (Waters, Eschborn, Germany) and a Waters 996 diode-array detector operated at wavelength between 210·4 and 395·4 nm. Three Chromspher 5 lipid columns (250 mm × 4·6 mm, 5 μm) were used in series with a 50 mm × 4·6 mm pre-column of the same column material (Varian). Propionitrile at 0·02 % in n-hexane was used as eluent at a flow rate of 1 ml/min (approximately 80 bar). Millennium32 software (Version 3.20; Waters) was used for data analysis. The following CLA isomers were considered in the analysis: trans12, trans14-, trans11, trans13-, trans10, trans12-, trans9, trans11-, trans8, trans10-, trans7, trans9-, cis11, trans13-, trans10, cis12-, cis11, trans13-, trans11, cis13-, cis9, trans11-, trans8, cis10-, cis11, cis13-, cis10, cis12-, cis9, cis11- and cis8, cis10-C18 : 2.
FFQ
The FFQ (Kummeling et al. Reference Kummeling, Thijs and Penders2005) was included in a self-administered questionnaire in week 34 of the pregnancy. The questionnaire was based on an existing validated one (Grootenhuis et al. Reference Grootenhuis, Westenbrink, Sie, de Neeling, Kok and Bouter1995), which was extended and modified to meet the specific aims of the present study. To make the questionnaire suitable for subjects with a vegetarian, anthroposophic, macrobiotic or other alternative dietary lifestyle, specific foods often used by these groups were included as well. The FFQ consisted of approximately 160 food items, for which the frequency of consumption and portion size were to be estimated. Furthermore, we have asked for information concerning the origin of the various food groups, for each of the three following food categories: dairy products, meat and certain other food items. The study participants had to specify whether the aliments had originated from conventional, organic or biodynamic – a special form of organic agriculture in which emphasis is put on activating the life of the soil by using natural preparations from plant and animal origin – production. The patients who consumed organic (including biodynamic) food were asked whether these constituted < 50 %, 50–90 % or >90 % of the food, within the corresponding food group. Since biodynamic foods are expensive, difficult to find and often used as an adjunct to organic foods, we only asked whether subjects used ‘any’ foods of biodynamic origin, again distinguishing between dairy products, meat and other food groups.
Subjects were classified into four groups distinguished in terms of the origin of the meat and dairy products: (1) conventional (if < 50 % of both the meat and dairy they used was of organic origin, or they ate no meat and < 50 % of the dairy they used was of organic origin, or they ate no dairy and < 50 % of the meat they used was of organic origin); (2) 50–90 % organic (if >50 % of both the meat and dairy they used was of organic origin but < 90 % of one of the two was of organic origin, or they ate no meat and 50–90 % of the dairy they used was of organic origin, or they ate no dairy and 50–90 % of the meat they used was of organic origin); (3) >90 % organic (if >90 % of both the meat and dairy they used was of organic origin, or they ate no meat and >90 % of the dairy they used was of organic origin, or they ate no dairy and >90 % of the meat they used was of organic origin); (4) other (including any combination of < 50 % meat of organic origin and >50 % dairy of organic origin or vice versa, and missing and inconsistent data). For the purpose of the present study, only those food items which are relevant dietary sources of CLA were documented: milk and milk products, including cheese and butter (nineteen food items), meat and meat products (nineteen food items). Fat intake from these food groups was calculated using the most recent Dutch Food Composition Table (Anonymous, 2001). Calculation of fat intake from meat was limited to that of ruminant cattle such as beef and veal (omitting minced and processed meat because it is often a mixture of beef and pork), lamb and mutton; throughout this paper, we refer to these nutrients as ‘meat’ only, for simplicity reasons. Since we expected that the fatty acid composition of the breast milk could be influenced by the use of dietary supplements during pregnancy and lactation, the questionnaire administered during the pregnancy and a questionnaire administered at the moment of breast milk sampling included detailed questions on the use of supplements with borage oil or primrose oil (both containing γ-linolenic acid, C18 : 3n-6) and fish oil (containing eicosapentaenoic acid, C20 : 5n-3; docosapentaenoic acid, C22 : 5n-3; docosahexaenoic acid, C22 : 6n-3).
Statistical methods
Duplicate values of fatty acids – expressed as weight percentage (wt%) of total fatty acids in breast milk fat – were averaged for each subject, and the resulting mean values were used for further calculations. Mean wt% of rumenic acid and other CLA (total CLA minus rumenic acid) were computed for groups of subjects classified by organic origin of dairy and meat, using Student's t test to assess differences between the groups (not assuming equality of variances); a difference between two groups was considered to be statistically significant if P ≤ 0·05. A linear regression analysis used rumenic acid level and TVA (wt%) as the dependent variables, while the independent variables were the categories of organic or biodynamic origin of dairy and meat and the fat intake from ruminant meat and dairy (g/d). Possible interactions between origin and fat intake were tested by adding interaction terms to the linear regression models. Since we expected CLA levels in fresh dairy products to be higher in summer months, we also included the season in which the breast milk was sampled in the multivariate analysis, to correct for a possible confounding effect (dichotomized into two periods: December 2002 to May 2003; June 2003 to September 2003). Other covariables in the regression analyses were: recruitment group (conventional/alternative), maternal age, education, and the use of oil supplements during pregnancy or lactation (yes/no). All statistical analyses were done in SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Of the 312 participants, thirty-three (10·5 %) used 50–90 % meat and dairy of organic origin, while thirty-seven (11·8 %) used more than 90 % of these aliments (Table 1). The subjects differed substantially in terms of recruitment group, and slightly in terms of the month during which breast milk sampling took place, education level and age of the mother, as well as in the use of oil supplements (Table 1). Therefore, we have included these characteristics as covariables in the multivariate linear regression analysis. Only six (3 %) subjects in the conventional group used any meat and dairy of biodynamic origin, whereas, as expected, this percentage was higher in the groups of organic users, increasing up to 30 % use of biodynamic meat and 76 % use of biodynamic dairy in the >90 % organic group (Table 1); the ‘other’ group had an intermediary position. As shown in Table 1, the total fat intake from the main dietary fat sources included in the FFQ was comparable between the groups, but the percentage contributed by dairy fat was almost twice as high in the groups with 50–90 % (70 %) and >90 % (75 %) meat and dairy of organic origin compared to the conventional group (39 %).
Table 1 Relevant characteristics of the study participants†
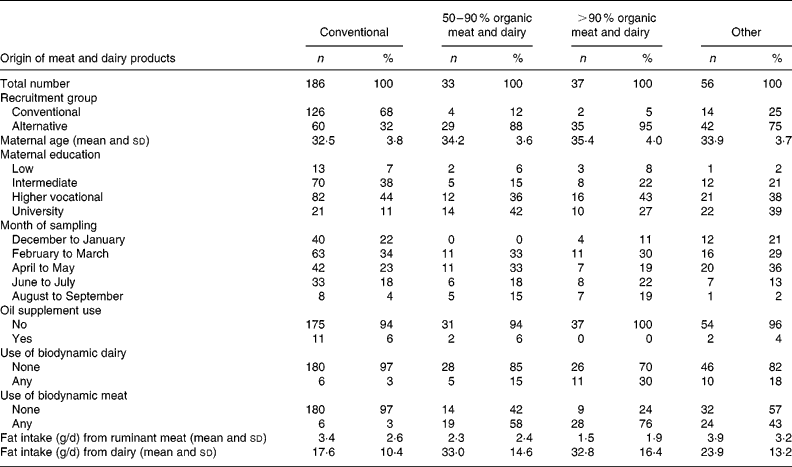
† The participants (n 312) were distributed by the various groups (conventional, 50–90 % organic, >90 % organic and other) according to the origin of the dairy and meat products included in the corresponding diet. The characteristics of the participants attributed to each of the four groups, in terms of number, age, maternal education, month of breast milk sampling, use of oil supplement and use of biodynamic dairy and meat products are depicted.
The levels of rumenic acid were higher in the groups of organic meat and dairy users, with an increasing trend going from the ‘other’ group to the 50–90 % organic meat and dairy group to the >90 % organic meat and dairy group (Table 2). The difference between the levels of rumenic acid in these groups and that in the conventional group was always statistically significant. No such trend was found for other CLA, and the relative amount of all the other CLA (wt%) was slightly lower in the 50–90 % group (Table 2). The mean level of TVA was about twice the level of rumenic acid in breast milk and correlated with rumenic acid (r 0·51, P < 0·001). Like rumenic acid, TVA content showed an increasing trend over the organic groups relative to the conventional group (Table 2), reaching statistical significance in the >90 % organic meat and dairy group. However, this was not the case for the differences among the other groups, which was probably due to the relatively high standard deviation values. The most abundant fatty acids present in milk are depicted in Table 3, to better understand the context of the mentioned changes in CLA and TVA levels. The increases in the levels of these fatty acids seem to be associated with relative decreases in the levels of trans-C18 : 1 and of C20 : 4 fatty acids.
Table 2 Rumenic acid, other conjugated linoleic acids, trans-vaccenic acid and other relevant fatty acid classes in breast milk (as weight percentage (wt%) of total milk fat) by origin of meat and dairy (n 312)† (Mean values and standard deviations)
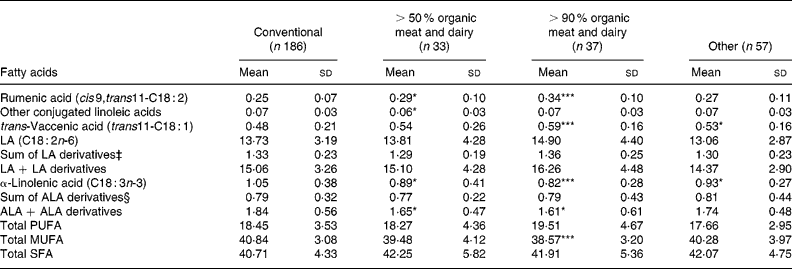
ALA, α-linolenic acid; LA, linoleic acid
Mean values were significantly different from those of the conventional group (Student's t test): *P < 0·05; **P < 0·01; ***P < 0·001.
† For details of procedures, see pp. 736–737.
‡ Sum of LA derivatives includes: C18 : 3n-6, C20 : 3n-6, C20 : 4n-6, C22 : 4n-6 and C22 : 5n-6.
§ Sum of ALA derivatives includes : C18 : 4n-3, C20 : 4n-3, C22 : 4n-3, C22 : 5n-3 and C22 : 6n-3.
Table 3 Most abundant fatty acids in breast milk (as weight percentage (wt%) of total milk fat) by origin of meat and dairy (n 312)† (Mean values and standard deviations)
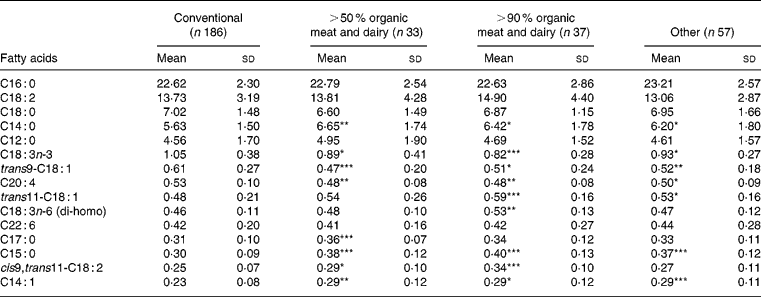
Mean values were significantly different from those of the conventional group (Student's t test): *P < 0·05; **P < 0·01; ***P < 0·001.
† Only fatty acids present in amounts higher than 0·2 % of total milk fat are shown. For details of procedures, see pp. 736–737.
After adjusting for covariables (alternative v. conventional recruitment group; maternal age; maternal education; use of supplements; winter v. summer months), rumenic acid remained statistically significantly higher in the >90 % organic group (v. conventional) (Table 4, model A). Fat intake from ruminant meat and from dairy contributed equally to the rumenic acid level in breast milk, but only the result for dairy fat was statistically significant: 0·021 increment of rumenic acid level (as wt% of total milk fat) per 10 g/d increment of daily dairy fat intake, P < 0·001 (Table 4, model B). When we additionally adjusted for fat intake from dairy and ruminant meat, rumenic acid remained significantly higher in the >90 % organic group compared to the conventional group (Table 4, model C). In addition to the organic origin, dairy fat intake still contributed to the rumenic acid level in breast milk (Table 4, model C). In linear regression analysis of breast milk TVA, results were very similar to those of rumenic acid: TVA (wt% of total milk fat) was statistically significantly higher in the >90 % organic group (v. conventional, regression coefficient 0·097, se 0·040, P = 0·015). Moreover, TVA was dependent on dairy fat intake in a statistically significant way: 0·023 increment of TVA (as wt% of total milk fat) per 10 g/d increment of daily dairy fat intake (se 0·009, P = 0·009), linear regression adjusting for the covariables mentioned earlier. It is worth mentioning that rumenic acid in breast milk peaked in the early summer months in the conventional and 50–90 % organic meat and dairy users, and somewhat later in the >90 % organic group (data not shown).
Table 4 Rumenic acid in breast milk as a function of the use of either meat and dairy of organic origin (model A), or dietary fat intake from ruminant meat and dairy products (model B) or both (model C)†
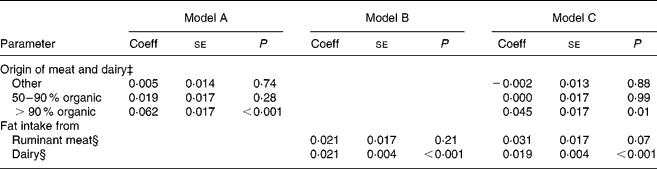
Coeff, linear regression coefficient; se, standard error of the regression coefficient.
† The difference in rumenic acid (as weight percentage (wt%) of total milk fat) was assumed to vary linearly with one of the two or with both parameters; data from the 312 participants were incorporated in the linear regression. Linear regression models controlled for the following covariables: alternative v. conventional group; maternal age; maternal education level; use of supplements; winter v. summer months.
‡ Coefficients denote the difference in rumenic acid level (wt%) in each group compared to the conventional group.
§ Coefficients denote increase in rumenic acid level (wt%) with 10 g/d increase of fat intake.
Discussion
CLA and TVA are often formed by isomerization and biohydrogenation of dietary linoleic and linolenic acid by microorganisms (mainly Butyrovibrio fibrisolvens) living in the rumen of ruminant animals. These reactions lead to the formation of various positional and geometric isomers, which differ substantially in nutritional value (Banni et al. Reference Banni, Angioni, Casu, Melis, Carta, Corongiu, Thompson and Ip1999). TVA is the major trans-fatty acid in ruminant milk fat and an intermediate in the bioconversion of linoleic acid (C18 : 2n-6) to rumenic acid (cis9, trans11-C18 : 2). In man, it can be converted by Δ9 desaturation to rumenic acid (Turpeinen et al. Reference Turpeinen, Mutanen, Aro, Salminen, Basu, Palmquist and Griinari2002), being probably responsible for one-quarter of the human CLA pool (Kuhnt et al. Reference Kuhnt, Kraft, Moeckel and Jahreis2006). This conversion has been shown to occur in lactating women (Mosley et al. Reference Mosley, McGuire, Williams and McGuire2006); in this precursor study, consumed TVA was converted in rumenic acid which was detectable in the human milk. Other trans-fatty acids, such as trans-10-octadecenoic acid (trans10-C18 : 1) cannot be desaturated. Bertschi et al. (Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Butikofer, Wechsler, Folkers and von Mandach2005) have recently described an about 50 % concomitant increase of both TVA and rumenic acid levels in human breast milk after consumption of alpine butter, in comparison with margarine. Since alpine butter had a high content of these fatty acids whereas the tested margarine had hardly any (Bertschi et al. Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Butikofer, Wechsler, Folkers and von Mandach2005), this observation suggests that the CLA and TVA present in human milk have a dietary origin. At least in the Netherlands the main sources of CLA and TVA are of dairy origin (Voorrips et al. Reference Voorrips, Brants, Kardinaal, Hiddink, van den Brandt and Goldbohm2002). In the present work, however, no determination of the content in these fatty acids in the dairy and meat products normally consumed by the mothers of the different groups was performed.
It should be mentioned that industrially produced trans-fatty acids, as often incorporated in commercial products, are likely to contribute to pathological situations, such as IHD (Stender & Dyerberg, Reference Stender and Dyerberg2004) and type 2 diabetes (Odegaard & Pereira, Reference Odegaard and Pereira2006). This possible contribution is leading several governments to limit the total amount of these fatty acids – mainly elaidic acid (trans9-C18 : 1), but to some extent also TVA (trans11-C18 : 1) – which can be included in oils and fats. For instance, in the case of the Danish government, this limit has been set to 2 % of the total fat content (Stender et al. Reference Stender and Dyerberg2004). The oscillations of the levels of TVA reported in the present study occur therefore within a range which is clearly different from the one of industrially produced trans-fatty acids, meaning that no unfavourable effects on human health are to be expected. Furthermore, and as often occurs in natural mixtures, the observed oscillations occur concomitantly to other alterations, namely to an increase in the CLA levels, which are likely to exert beneficial effects on health performance.
We found that the levels of rumenic acid as well as of TVA in breast milk were higher in mothers which included organic dairy and meat products in their diet, in comparison with mothers who had pursued a conventional diet. Furthermore, the extent of the increase in rumenic acid depended on the amount of organic products consumed during the study, with those mothers using almost exclusively (more than 90 %) organic dairy and meat products in their diet having a higher content of this fatty acid in their milk than mothers with a moderately (50–90 %) organic dairy and meat diet. These data corroborate the results of our previous pilot study, which showed that mothers who consumed more than 50 % organic dairy and meat products had higher levels of CLA (Rist et al. Reference Rist, Zweidler, von Mandach and Freyer2003). Interestingly, it has been shown in a variety of studies (Jahreis et al. Reference Jahreis, Fritsche and Steinhart1997; Bergamo et al. Reference Bergamo, Fedele, Iannibelli and Marzillo2003; Gedek, Reference Gedek1980; Dewhurst et al. Reference Dewhurst, Evans, Scollan, Moorby, Merry and Wilkins2003) that the levels of CLA in cow's milk from organic producers in Europe, including the Netherlands (Adriaansen-Tennekes et al. Reference Adriaansen-Tennekes, Bloksma, Huber, Baars, De Wit and Baars2005), are significantly higher than CLA levels in the milk from conventional producers. Therefore, and since the fat from human breast milk is likely to be of dietary origin (see earlier), we believe that the larger amounts of rumenic acid and TVA in breast milk from the organic groups were due to the corresponding intake of organic dairy and meat products with higher levels of rumenic acid and TVA. This interpretation of the present results is strengthened by the fact that the total fat intake was comparable among the various groups and that the CLA content of the food is very stable and not influenced by storage or processing (Luna et al. Reference Luna, de la Fuente and Juarez2005). The fat intake from meat was five to twenty times lower than that from dairy products; therefore, it is likely that dairy products were more strongly influencing the final fat composition of the milk than the meat products. Much to our surprise, the multivariate analysis found an additional effect of the organic origin on the diet, stronger than that to be expected from the total dairy fat intake.
The CLA levels found in the breast milk from the >90 % organic dairy and meat products consumers were as high as those reached after supplementation with 30 g/d alpine butter for 10 d (Bertschi et al. Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Butikofer, Wechsler, Folkers and von Mandach2005): 0·33 g/100 g milk fat (margarine control: 0·22 g/100 g milk fat). Breast milk levels of rumenic acid in the conventional group (0·25 g/100 g fat) were in the same range as the values previously reported: 0·21 g/100 g fat (Park et al. Reference Park, McGuire, Behr, McGuire, Evans and Shultz1999); 0·28 g/100 g total fatty acids (Ritzenthaler et al. Reference Ritzenthaler, McGuire, McGuire, Shultz, Koepp, Luedecke, Hanson, Dasgupta and Chew2005), and 0·19 and 0·18 g/100 g fat (Jensen et al. Reference Jensen, Lammi-Keefe, Hill, Kind and Henderson1998; Jensen & Lammi-Keefe, Reference Jensen and Lammi-Keefe2001). The higher levels, namely 0·4 g/100 g fat, recorded in American (McGuire et al. Reference McGuire, Park, Behre, Harrison, Shultz and McGuire1997; Innis & King, Reference Innis and King1999) and in German mothers (Jahreis et al. Reference Jahreis, Fritsche, Möckel, Schone, Möller and Steinhart1999) can be attributed to a diet which normally includes higher amounts of dairy products and/or meat. The fact that the relative amounts of CLA and TVA correspond to less than 1 % of the total fat should not be taken as indicative of a reduced physiological relevance of these fatty acids. Their mechanism of action is likely to include the production of biologically active compounds and processes of intracellular signalling (Khan & Vanden Heuvel, Reference Khan and Vanden Heuvel2003), and it is typical for molecules participating in such processes that they are present in very small amounts. In this context it is worth mentioning that, although the levels of n-3 fatty acids in maternal milk are as well rather low, they have been shown to influence the risk of non-atopic eczema and asthma in the infant (Oddy et al. Reference Oddy, Pal and Kusel2006; Wijga et al. Reference Wijga, van Houwelingen, Kerkhof, Tabak, de Jongste, Gerritsen, Boshuizen, Brunekreef and Smit2006). Concerning the magnitude of the differences in breast milk CLA levels that we found among the various groups, it might be argued that they are minor. Nevertheless, it should be noted that the level in breast milk reflects CLA intake by maternal diet and could therefore be a marker of placental supply in uterus and possibly of ongoing supply to the child from dietary sources of dairy products and meat shared by the family. Taken together, these factors are likely to represent a lifelong cumulative effect.
The health effects of CLA and TVA on human newborns are still unknown; nevertheless there is promising evidence stemming from animal models and from clinical studies involving human adults. Often, the positive effects of CLA on health parameters revealed themselves stronger in animal models than in clinical studies with man (Bhattacharya et al. Reference Bhattacharya, Banu, Rahman, Causey and Fernandes2006). One possible explanation for this discrepancy is that, while animal studies have concentrated on very young growing rats or mice, clinical studies have exclusively focused on adult man. This strengthens the need for long-term clinical studies starting with very young participants, as is possible within the frame of the KOALA study. An area in which the expectations concerning the CLA effects are relatively high concerns their immunomodulating properties (see review by O'Shea et al. Reference O'Shea, Bassaganya-Riera and Mohede2004). Indeed, in animal models, these fatty acids lead to a reduction of the harmful effects of intranasally administered influenza viruses, and to a reduction of the leukotriene and prostaglandin production which suggests a favourable effect in preventing inflammatory phenomena that are typical of an immediate immune response (O'Shea et al. Reference O'Shea, Bassaganya-Riera and Mohede2004). Moreover, CLA feeding was able to prevent wasting after endotoxin injections (Cook et al. Reference Cook, DeVoney, Drake, Pariza, Whigham and Yang1999). The examination of healthy human volunteers who had been vaccinated against hepatitis B revealed that supplementation with certain CLA isomers resulted in a statistically significant higher level of protective antibodies, indicative of a better immune-responsiveness to the vaccination (Albers et al. Reference Albers, van der Wielen, Brink, Hendriks, Dorovska-Taran and Mohede2003). CLA supplementation in young healthy men affected the immune function in terms of increased plasma IgA and IgM, and the anti-inflammatory cytokine IL-10, and decreased levels of IgE and the proinflammatory cytokines TNF-α and IL-1γ (Song et al. Reference Song, Grant, Rotondo, Mohede, Sattar, Heys and Wahle2005). Dietary studies have indicated a protective effect of butter relative to margarine against allergy and asthma (Bolte et al. Reference Bolte, Frye, Hoelscher, Meyer, Wjst and Heinrich2001; Dunder et al. Reference Dunder, Kuikka, Turtinen, Rasanen and Uhari2001; Woods et al. Reference Woods, Walters, Raven, Wolfe, Ireland, Thien and Abramson2003). Similarly, a 3-year prospective cohort study found a decreased risk of asthma in children who consumed full cream milk and butter daily, compared to those who did not (Wijga et al. Reference Wijga, Smit, Kerkhof, de Jongste, Gerritsen, Neijens, Boshuizen and Brunekreef2003). Since butter is normally rich in CLA, this might suggest a positive effect of CLA on the prevention of those diseases. Furthermore, it is known that children who grow up in families with an anthroposophic lifestyle have a reduced risk of atopic diseases compared to those in families with conventional lifestyles (Alm et al. Reference Alm, Swartz, Lilja, Scheynius and Pershagen1999; Alfven et al. Reference Alfven, Braun-Fahrlander and Brunekreef2006). An anthroposophic lifestyle comprises, besides a restrictive use of antibiotics and few vaccinations, a diet that usually contains raw milk and organic, or more specifically biodynamic, products. Given that an organic diet and organic dairy and/or meat products have a higher CLA content than their conventional counterparts (see earlier), this observation might suggest that CLA consumption could add to a protective effect against atopic diseases.
In conclusion, we show here that the levels of both rumenic acid and TVA in human breast milk were higher in the case of mothers following a diet that contained organic dairy and meat products, in comparison with mothers consuming a conventional diet. In view of the accumulating evidence pointing towards various positive effects of CLA on human health, in particular at a very young age, the present results are highly interesting. Further results of the KOALA Birth Cohort Study, in particular those concerning allergic sensitization and asthma in the children corresponding to the mothers that have participated in the present study, are awaited anxiously.
Acknowledgements
This study was financially supported by the Netherlands Organization for Health Research and Development (ZonMw, the Netherlands), Royal Friesland Foods (the Netherlands), Triodos Foundation (the Netherlands), UDEA organic retail (the Netherlands), Biologica organization for organic farming and food (the Netherlands), the Consumer Association for Bio-Dynamic Agriculture Zurich (Switzerland) and Weleda AG Arlesheim (Switzerland).






