Invasive pneumococcal disease (IPD) is defined as an infection confirmed by isolation of Streptococcus pneumoniae (S. pneumoniae) from sterile sites (e.g. blood, cerebrospinal fluid) and can cause substantial morbidity and mortality. More than 90 S. pneumoniae serotypes have been reported and certain serotypes cause IPD. As compared with IPD caused by other serotypes, those attributed to S. pneumoniae serotype 12F (Sp12F) result in greater morbidity and mortality [Reference Deng1].
A 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in Japan in 2010 and a 13-valent PCV (PCV13) replaced the PCV7 in 2013. The interval from the start of PCV7 to the introduction of PCV13 was brief in Japan because PCV7 was introduced later than other countries. However, PCV13 is now widely used as a mandatory vaccine in the Japanese National Immunization Program (NIP) [Reference Saitoh and Okabe2]. Importantly, Sp12F is not covered by PCV7 or PCV13 but is covered by the 23-valent pneumococcal polysaccharide vaccine (PPSV23), which has been included in the NIP since October 2014 for adults aged 65 years or older.
After the introduction of PCV7 to the NIP, followed by that of PCV13, the IPD cases among children decreased down to about 40% [Reference Suga3]. However, the rate of IPD caused by non-PCV13 serotypes increased in both children [Reference Suga3] and adults [Reference Fukusumi4], because of serotype replacement, i.e., the increase in the prevalence of non-PCV serotypes replacing PCV serotypes and consequent decrease in PCV effectiveness [Reference Suga3, Reference Whitney5].
Tsuruoka city has a population of approximately 130 000 and is located in northern Japan, in Yamagata prefecture, near the Japan Sea. Tsuruoka Municipal Shonai Hospital is the only tertiary hospital in the area and all patients with severe infections are admitted to this hospital. In this report, we describe an outbreak of IPD cases caused by Sp12F during a 3-month period. Importantly, a paediatric case of IPD caused by Sp12F has been reported in Japan [Reference Nakano6] and elsewhere, there is no report of the outbreak spreading to both children and adults.
In this study, nine patients were admitted to the hospital under the diagnosis of a community-acquired IPD based on isolation of Sp12F from blood or cerebrospinal fluid from 1 March through 31 May 2016. Of note, no community outbreak caused by influenza, respiratory syncytial virus, or other respiratory viruses was observed during the period. All adult patients were evaluated for pneumonia and spleen presence by chest and abdominal computed tomography (CT), respectively. Spleen volume was calculated retrospectively by the following standard formula: spleen volume (cm3) = 30 + 0.58 (maximal width × height × thickness at hilum) [Reference Prassopoulos7].
IPD was defined as isolation of S. pneumoniae from blood or cerebrospinal fluid cultures. Occult bacteremia was defined as a positive blood culture without an obvious source of infection. Child contact was defined as living with children younger than 15 years or spending more than 8 hours per 4 weeks with children, as in previous studies [Reference Rodrigo8] among adult patients and visiting a day-care center daily among paediatric patients. The hypoplastic spleen was defined as a spleen volume less than 100 cm3 (normal range, 100–250 cm3) [Reference Prassopoulos7].
The patient clinical data were collected from the medical records and subsequent questionnaires distributed to patients and patients’ families. The questionnaires were sent to patients or their family members by the Shonai Public Health Department in October and November 2016. The clinical information collected included age, sex, past medical history, focus of IPD, Charlson Comorbidity Index (for adults), Pitt Bacteremia Score (for adults), outcome, pneumococcal vaccine history and history of child contact. The Charlson Comorbidity Index predicts mortality by comorbid conditions among adults and is useful in objectively evaluating patient background [Reference Quan9]. The Pitt Bacteremia Score predicts mortality in the intensive care unit among patients with bacteremia (cutoff, >4 points) [Reference Rhee10].
To investigate the source of infection, the locations of places where patients and their family members worked or visited were mapped by using the addresses of their workplace, school, group nursing home, friends’ homes and family home.
After the isolation of S. pneumoniae, serotyping was performed with pneumococcal typing antisera (Statens Serum Institut, Copenhagen, Denmark) at the National Institute of Infectious Diseases, Japan. Additionally, multilocus sequence typing (MLST) of each isolate was performed by a previously described method [Reference Enright and Spratt11]. Sequence types (STs) were determined by comparing sequences obtained from the current outbreak with those in the pneumococcal MLST database (https://pubmlst.org/spneumoniae/, accessed 4 June 2018).
Whole-genome sequencing and phylogenetic analysis were performed according to our previous study with modification to the removal of single-nucleotide variations (SNVs) on recombinogenic region [Reference Chang12]. To exclude SNVs on recombinogenic regions, SNV clusters (>2 single-nucleotide polymorphisms within 100 bp) were removed. The nucleotide sequence data obtained in this study were submitted to the DNA Data Bank of Japan Sequenced Read Archive under the accession numbers DRX114436-114444.
We identified nine cases of IPD caused by Sp12F during the period from 1 March through 31 May 2016 (Table 1), including two children (22%) and seven adults (78%). Among the nine patients, four (44%) had pneumonia and three (33%) had meningitis. Both children completed PCV13; however, no adults had received PCV13 or PPSV23, although three had the opportunity to receive PPSV23 as part of the NIP. Eight of the nine patients had child contacts. In addition, six of the seven adults (86%) had a smoking history.
Table 1. Summary of 9 cases of invasive pneumococcal disease caused by serotype 12F
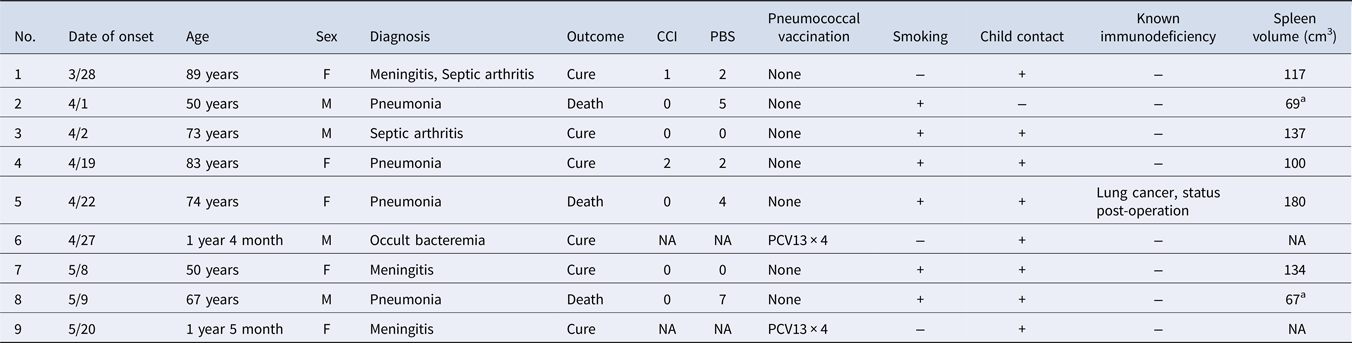
WGS, whole-genome sequencing; CCI, Charlson Comorbidity Index; PBS, Pitt Bacteremia Score; NA, not available; PCV13, 13-valent pneumococcal conjugate vaccine.
a Hypoplastic spleen (<100 cm3).
Every adult patient had a low Charlson Comorbidity Index score, which indicates that they did not have underlying diseases that increase sepsis risk. The three patients who died had a Pitt Bacteremia Score greater than 4 and were in critical condition on admission. These patients responded poorly to treatment and died on the day, or within a few days, of admission. Interestingly, although patients 2 and 8 did not have underlying diseases, abdominal CT revealed splenic hypoplasia (spleen volume <100 cm3). Analysis of questionnaire data on the locations where patients and their family members went, worked, or visited showed no obvious location where they congregated.
MLST showed that all Sp12F strains isolated from the nine patients belonged to a single sequence type: ST4846 (MLST allelic profile: aroE-gdh-gki-recP-spi-xpt-ddl: 12-32-111-1-13-48-6). The Sp12F isolates were susceptible to penicillin (minimal inhibitory concentration (MIC), 0.06 µg/ml) and cefotaxime (MIC, 0.06 µg/ml) but resistant to erythromycin (MIC, ⩾8 µg/ml) and clindamycin (MIC, ⩾8 µg/ml).
Among the 9 isolates of Sp12F, 8 formed a cluster in the SNV-based phylogenetic tree, which varied by 0 or 1 SNV. However, 1 isolate differed from isolates in that cluster, by 46 or 47 SNVs.
To our knowledge, this is the first report of local outbreak caused by Sp12F after the introduction of PCV13. We identified nine cases of IPD (including three adult deaths) that were attributable to the Sp12F strain and had occurred during a brief outbreak in Tsuruoka city, Japan. Because Sp12F is not covered by PCV13, strategies to prevent severe disease due to Sp12F need to be considered in the post-PCV13 era.
S. pneumoniae commonly causes asymptomatic nasopharyngeal colonisation in children and elderly adults. Colonised organisms can be transmitted from person to person. In general, colonised organisms spread in closed communities such as homes, group nursing homes and hospitals. After the introduction of PCVs for children, the numbers of IPD cases in elderly adults decreased because of a decline in pneumococcal carriage among children [Reference Whitney5]. Therefore, nasopharyngeal colonisation among children may affect IPD rates in children and adults.
Although Sp12F may be more likely to cause meningitis and can be more invasive than other serotypes, no previous study has reported a brief outbreak of Sp12F affecting both adults and children. Additionally, most past outbreaks were caused by ST218 [Reference Deng1], the present outbreak was caused by ST4846, which was not previously reported. Whole-genome sequencing analyses showed that 8 isolates, which varied by 0 and 1 SNV, were genetically identical strains. However, 1 isolate (case 9) was different from that cluster by 46 and 47 SNVs. This case was reported at the end of the study period, so a different strain of Sp12F may have circulated in the community and caused IPD during the same period, or the strain may have acquired additional genetic changes later during the period.
In Japan, the incidence of IPD decreased in children after the introduction of PCV7; however, IPD caused by non-PCV13 serotypes have been increasing because of serotype replacement [Reference Suga3]. Thus, the total number of IPD has not changed since the introduction of PCV13 (unpublished data). When we calculate the IPD rates based on the population (approximately 130 000) living in Tsuruoka city, the IPD rate during the present outbreak was 27.7/100 000 population; in contrast, the rate was 5.3/100 000 population during 2005–2015 from 76 hospitalised patients (unpublished data). This significant increase in the IPD rate needs for caution and continuous monitoring of IPD with the determination of pneumococcal serotype is warranted in the post-PCV13 era.
IPD caused by non-PCV13 serotypes increased after the introduction of PCV13 and the increase in IPD caused by Sp12F has been reported in different countries, including the latest and largest report from Israel [Reference Rokney13]. Of note, there is no outbreak of Sp12F reported in Asia. In Japan, IPD were infrequently caused by Sp12F [Reference Nakano6]; however, the frequency of such infections has been increasing since the introduction of PCV13. In addition, the current local outbreak of Sp12F is important, because it spread rapidly in a small area causing high morbidity and mortality. In the near future, Sp12F is expected to be the most important serotype related to IPD. Continuous IPD surveillance, including serotype determination, is therefore warranted.
Because the effectiveness of PPSV23 is not promised in children younger than 2 years, immunization by PCV13 is necessary to protect children from IPD. The incidence of IPD caused by non-PCV13 serotypes has been increasing; thus, we need additional strategies to protect children from IPD, including new PCVs that cover non-PCV13 serotypes and non-conjugated vaccines with novel mechanisms. In addition, PPCV23 administration in adults may affect IPD incidence in children.
Overwhelming post-splenectomy infection (OPSI) is well recognised and shows how splenic function is strongly related to overwhelming infection of encapsulated organisms like S. pneumoniae. However, it is unclear if hypoplastic spleens have a low splenic function and methods for evaluating spleen function have not been established. Because hypoplastic spleen is a fortuitous imaging or autopsy finding, its prevalence is unknown. In the current study, two of the nine patients had hypoplastic spleens, a fulminant clinical course, like that of OPSI and died on the day of admission. Only a few previous cases of fulminant pneumococcal infection with hypoplastic spleen have been reported [Reference Emori, Takeuchi and Soneda14]. The hypoplastic spleen may be associated with the low splenic function and affected patients may have a higher risk of fulminant infection by encapsulated organisms. Thus, patients with an incidental finding of hypoplastic spleen on CT or ultrasonography might benefit from receiving PCV13 or PPSV23.
This study has a few limitations. First, only a small number of IPD cases caused by Sp12F were identified during this brief local outbreak. Second, no samples were collected from family members to confirm the intrafamilial spread of S. pneumoniae. Such analysis could be helpful in understanding routes of S. pneumoniae transmission in a family.
In conclusion, An IPD outbreak caused by genetically identical Sp12F resulted in high morbidity and mortality after the post-PCV era, in Tsuruoka city, Japan. Sp12F could become an important serotype associated with endemic severe IPD. Continuous monitoring of IPD, along with the determination of pneumococcal serotype, is thus mandatory to improve understanding of IPD. In addition, new IPD control strategies may be needed if this type of fatal outbreak continues to occur.
Acknowledgements
We acknowledge David Kipler for editing the manuscript. We are also grateful to the patients, their parents or guardians, family members and the physicians who participated in this study. This study was supported in part by a grant from the Ministry of Health, Labour and Welfare of Japan (grant number H29 Shinko-Shitei-001).
Conflict of interests
The authors declare that they have no conflicts of interest.




