INTRODUCTION
Helicobacter pylori (H. pylori) is the main cause of gastritis and peptic ulcer disease. There is now evidence that H. pylori may also play a role in various non-gastric diseases. H. pylori has also been shown to affect the vascular risks and complications in patients with diabetes mellitus [Reference Hamed1, Reference Yoshikawa2]. Prevalence of H. pylori infection is higher in patients with diabetes mellitus compared to healthy controls [Reference Hamed1]. There are other studies showing a positive correlation between H. pylori infection and cardiovascular disease risk [Reference Yoshikawa2]. H. pylori colonization is associated with reduced circulating leptin levels, and fundic ghrelin and leptin levels are directly related, which suggests that H. pylori has an impact on human health and disease by its involvement in the regulation of leptin and ghrelin expression [Reference Herder3].
Epidemiological studies that have investigated circulating chemokine levels suggest that these processes are reflected at the systemic level [Reference Kim4–Reference Panichi7]. Elevated levels of chemokines such as monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8) and IL-10 precede coronary events [Reference Kim4]. The circulating levels of MCP-1 and IL-8 may link obesity with obesity-related metabolic complications such as diabetes and atherosclerosis [Reference Wang5]. Other reports have elaborated on the involvement of chemokines in tumour growth, invasion and metastasis [Reference Benoy6, Reference Panichi7], indicating that increased serum IL-8 in breast cancer patients is correlated with early dissemination and survival [Reference Panichi7].
H. pylori plays an important role in the increased expression of various cytokines in gastric tissues, probably causing proliferation of gastric epithelial cells that enhances the development of gastric cancer [Reference Yamaoka8]. High expression of IL-8 has been demonstrated in gastric mucosa infected with H. pylori [Reference Kusugami9], and IL-8 has been shown to cause chemotaxis and activation of inflammatory cells in gastric mucosa infected with H. pylori [Reference Crabtree10]. Moreover, significantly increased levels of mucosal IL-8 have been detected when H. pylori is associated with active gastritis and gastric cancer [Reference Roper11]. Although many studies have reported IL-8 production in the gastric mucosa of patients infected with H. pylori, few data are available on circulating IL-8 levels.
In addition, previous studies have reported association of the IL-8 T-251A polymorphism within the IL-8 promoter with various diseases such as asthma, colorectal cancer and gastric diseases [Reference Hamajima12–Reference Ye14]. Individuals with both the IL-8 –251T/T and IL-10 –819T/T genotypes have a high probability of persistent H. pylori infection [Reference Hamajima12], and the IL-8 –251T allele is significantly associated with either gastritis or duodenal ulcer in subjects infected with H. pylori [Reference Hofner13]. The IL-8 –251 A allele is associated with higher mucosal IL-8 production, more severe inflammation, mucosal atrophy, and intestinal metaplasia than IL-8 –251T/T genotype in H. pylori-infected Koreans [Reference Ye14]. However, the association between the IL-8 gene polymorphism and circulating IL-8 levels has not been investigated.
Therefore, we examined the associations of plasma IL-8 levels with H. pylori infection and gastric atrophy in Japanese adults. We also evaluated the effects of H. pylori infection and the severity of atrophy of the gastric mucosa on plasma IL-8 levels, stratified by the IL-8 T-251A genotype.
METHODS
Subjects
Subjects were sampled from patients visiting the Daiko Medical Center of Nagoya University in 2004 who requested testing for H. pylori and subsequent eradication [Reference Ishida15]. We analysed the first 103 participants (36 men and 67 women) of those aged 20–69 years, who gave written informed consent, provided a 7-ml blood sample, and completed a questionnaire on their lifestyle, medical histories and medication. Of the 103 participants, we had no information on H. pylori infection and atrophy in one subject, atrophy status was not determined in two subjects, and plasma IL-8 was not measured in two others, which left 98 eligible for the present analysis. No participant was taking antibiotics or steroid drugs, and none were current smokers. Two participants reported a history of gastric ulcer, and three a history of gastritis. This study was approved by the ethics committee of the Nagoya University School of Medicine on 16 June 2004 (approval number 155).
Blood samples
Plasma and the buffy coat fraction were separated from blood samples in a test tube that contained EDTA-2Na, and were kept at −40°C until analysis.
Tests for H. pylori infection
H. pylori infection was evaluated using a [13C]urea breath test or serum anti-H. pylori antibody test. The [13C]urea breath test was conducted at the Daiko Medical Center with the UBiT® (Otsuka Pharmaceutical, Japan). Anti-H. pylori IgG antibody was measured with Detaminor H. pylori antibody kits (Scimedx, USA) by a single laboratory (SRL, Japan). Those with Δ13C >0·25% or an ELISA value ⩾2·3 were regarded as being infected.
Testing for pepsinogen (PG)
PGI and PGII in plasma were measured using a chemiluminescent enzyme immunoassay (CLEIA) by SRL. Those with PGI ⩽70 ng/ml and a PGI/PGII ratio ⩽3 were classified as atrophy-positive, and those with PGI ⩽30 ng/ml and a PGI/PGII ratio ⩽2 were classified as having severe atrophy. Those who were classified as atrophy-positive but did not satisfy the criteria of severe atrophy were defined as having mild atrophy.
Measuring plasma IL-8 level
Plasma IL-8 level was measured in 100 μl thawed plasma using a Biochip Array Technology Analyzer Evidence Investigator, cytokines and growth factor array (Randox Laboratories, UK) at a single laboratory (Mitsubishi Kagaku Bio-clinical Laboratories, Japan). The principle of the array system has been described previously [Reference Fitzgerald16]. All assays were performed by staff that were blinded to the clinical and epidemiological data.
Genotyping of IL-8
DNA was extracted from the buffy coat fraction using a BioRobot EZ1 (Qiagen, Japan). The IL-8 gene was genotyped at the polymorphic site T-251A using the polymerase chain reaction by confronting two-pair primers (PCR–CTPP) [Reference Hamajima17]. The PCR amplification of IL-8 T-251A was conducted using the primers F1 (5′-CAT GAT AGC ATC TGT AAT TAA CTG) and R1 (5′-CAC AAT TTG GTG AAT TAT CAA A) for the T allele (a 169-bp fragment), and F2 (5′-GTT ATC TAG AAA TAA AAA AGC ATA CAA) and R2 (5′-CTC ATC TTT TCA TTA TGT CAG AG) for the A allele (a 228-bp fragment). The DNA amplified between F1 and R2 resulted in a 349-bp band common to both alleles.
Genomic DNA (30–100 ng) was used in a 25-μl reaction mixture that contained 0·2 mm dNTPs, 12·5 pmol of each primer, 0·5 U polymerase (AmpliTaq Gold; Applied Biosystems, USA), and 2·5 μl 10×PCR buffer including 15 mm MgCl2. The amplification conditions were an initial 10 min at 95°C, followed by 30 cycles of 1 min each at 95°C, 58°C and 72°C, and then 5 min at 72°C for final extension. The DNA products were visualized on 2% agarose gels with ethidium bromide staining.
Statistical analysis
Distribution of circulating IL-8 levels was skewed, and the geometric means of plasma IL-8 were calculated to compare groups. Differences in the demographic characteristics were analysed using the χ2 test, t test and one-way analysis of variance (ANOVA). The linear trend in the geometric means of IL-8 across atrophy status was assessed in subjects with H. pylori infection. The geometric mean levels for subjects with IL-8 –251 T/T and A allele carrier in respect to H. pylori infection and severity of atrophy were compared using the general linear model, with age, gender and comorbidities such as hyperuricaemia and bronchiectasis, which may affect plasma IL-8 as covariates. The frequency of the polymorphism was tested against Hardy–Weinberg equilibrium using the χ2 test.
All statistical analyses were performed using the Statistical Package for the Social Sciences version 14.0 for Windows (SPSS Inc., USA), and P<0·05 was considered statistically significant for all analyses.
RESULTS
Table 1 summarizes the characteristics of the 98 study subjects, of whom 71 were positive for H. pylori infection. Seven subjects (9·9%) with H. pylori infection were negative in the [13C]urea breath test and positive for serum anti-H. pylori antibody, while 64 (90·1%) with H. pylori infection were positive in both the [13C]urea breath test and for serum anti-H. pylori antibody. Of the 71 participants with H. pylori infection, 42 did not show gastric atrophy, while 29 had gastric atrophy, based on PG tests. Only one subject with gastric atrophy was negative for H. pylori infection.
Table 1. Subject characteristics by H. pylori infection and gastric atrophy
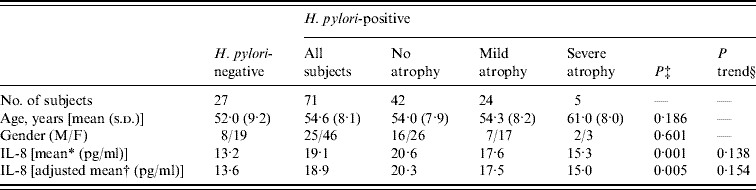
s.d., Standard deviation.
* Geometric mean.
† Geometric mean adjusted for age, gender and comorbidities.
‡ P values between all subjects with H. pylori infection and those without H. pylori infection by t test.
§ P trend values for association between extent of gastric atrophy and plasma IL-8 in subjects with H. pylori infection.
Plasma IL-8 levels in the subjects with H. pylori infection were significantly higher than in those without H. pylori infection (geometric mean: 13·2 and 19·1 pg/ml, respectively; P=0·001). Adjustments for age, gender and comorbidities did not change this result (P=0·005). Of the 71 participants with H. pylori infection, individuals who were positive for the [13C]urea breath test showed higher IL-8 levels than those who were [13C]urea breath test negative/serum anti-H. pylori antibody-positive (geometric mean: 19·5 and 16·1 pg/ml, respectively; P=0·536).
The four groups, H. pylori(−), H. pylori(+)/atrophy(−), H. pylori(+)/mild atrophy(+), and H. pylori(+)/severe atrophy(+), had statistically different IL-8 levels (P=0·005, by ANOVA), whereas no differences were observed according to age or gender. The H. pylori(+)/atrophy(−) group had the highest mean IL-8 level of all the groups. There was a statistically significant difference in the mean IL-8 level between H. pylori(−) and H. pylori(+)/atrophy(−) groups (P=0·001). Development of atrophy was negatively associated with the geometric means of IL-8 in the 71 participants with H. pylori infection, although not significantly so. There was no statistically significant difference of mean IL-8 level between H. pylori(−) and H. pylori(+)/severe atrophy(+) groups (P=0·61).
The distribution of the IL-8 T-251A genotype was not significantly different from that expected by Hardy–Weinberg equilibrium (P=0·13). IL-8 levels in A allele carriers of the IL-8 T-251A genotypes were slightly higher than those in subjects with T/T genotype, but the difference was not statistically significant (geometric mean: 17·5 and 17·0 pg/ml, respectively).
For both the T/T genotype and A allele carriers, plasma IL-8 levels were elevated in individuals with H. pylori(+)/atrophy(−), and decreased with the development of atrophy. Development of atrophy was negatively associated with the geometric means of IL-8 levels in those with the T/T genotype in H. pylori-positive individuals (P trend=0·088).
In each ANCOVA, IL-8 levels in those with the T/T genotype were associated with H. pylori infection and atrophy status (P=0·016) for three groups, while the differences for those carrying the A allele were not significant (P=0·326) (Fig. 1). There was a statistically significant difference in the mean IL-8 level in those with the T/T genotype between H. pylori(−) and H. pylori(+)/atrophy(−) groups (P=0·021). The differences in the IL-8 concentration between those with the T/T and A allele carriers in each group in relation to H. pylori infection and atrophy status were not statistically significant.
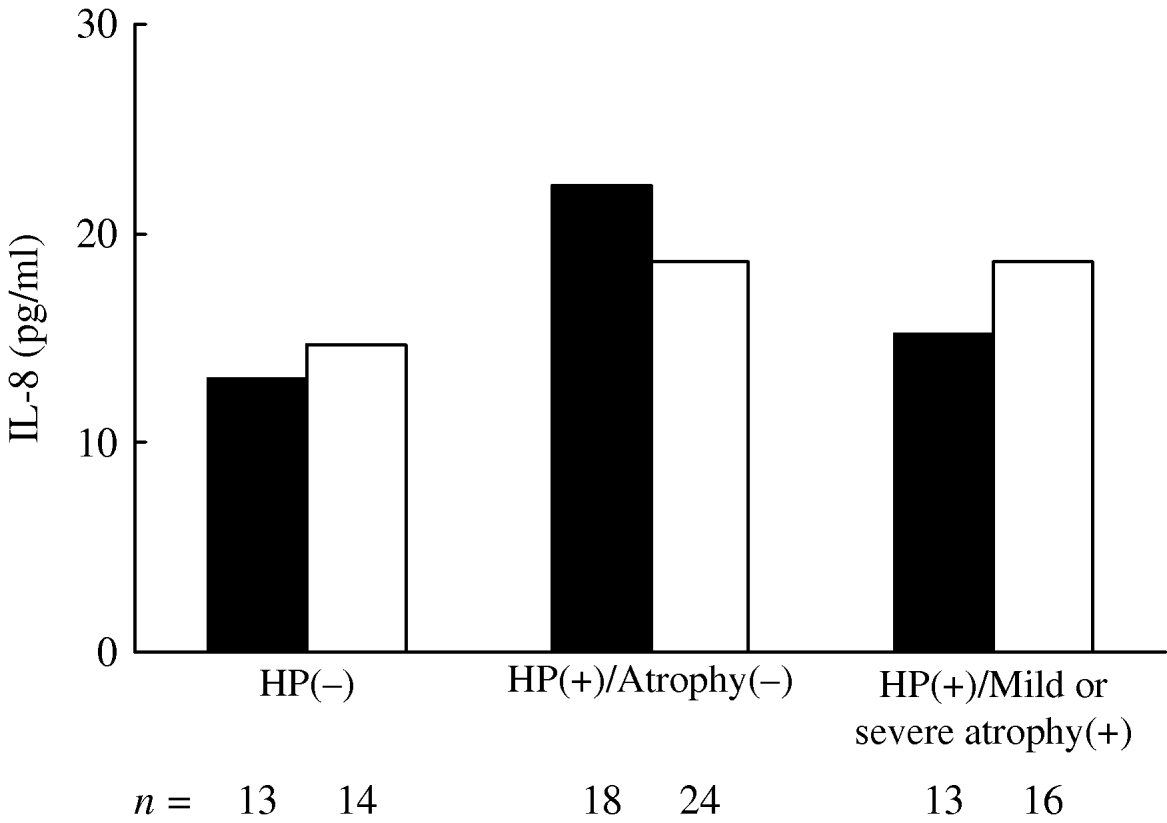
Fig. 1. Geometric mean of plasma IL-8 levels in subjects with IL-8–251T/T (▪) and A allele carriers (□) in respect of H. pylori infection and severity of gastric atrophy. IL-8 levels were adjusted for age, gender and comorbidities. In each analysis of covariance, IL-8 levels in those with the T/T genotype were associated with H. pylori infection and atrophy status (P=0·016) for three groups, while the differences for those carrying the A allele were not significant (P=0·326). There was a statistically significant difference in the mean IL-8 level in those with the T/T genotype between H. pylori(−) and H. pylori(+)/atrophy(−) groups (P=0·021).
DISCUSSION
This is believed to be the first study to examine the effects of H. pylori infection, gastric atrophy and IL-8 T-251A polymorphism on circulating IL-8 levels. We found plasma IL-8 levels were significantly associated with H. pylori infection. Higher plasma IL-8 levels were particularly observed in H. pylori(+)/atrophy(−) participants. The differences in plasma IL-8 levels were clearer in those with the IL-8 –251T/T genotype than those carrying the A allele.
There is a paradigm according to which a host–microbial interaction occurs in some cases that may promote pathological conditions, whereas in other cases it may protect against pathology. Current studies showed that eradication of H. pylori reduced the risk of gastric cancer [Reference Fukase18]. Meanwhile, it has now become clear that it is inversely associated with the development of oesophageal diseases, and the more interactive CagA-positive strains are associated with the strongest inverse effects [Reference Peek and Blaser19]. Inverse associations of H. pylori and childhood asthma, allergic rhinitis and atopy have also been found [Reference Blaser, Chen and Reibman20]. Our findings revealed the possibility that H. pylori has an impact on human health by its involvement in the regulation of IL-8 expression.
Previous reports have described conflicting results concerning the association between H. pylori infection and circulating IL-8 levels. We found a positive association, which supports the results of Cichoz-Lach et al. [Reference Cichoz-Lach21] and Mehmet et al. [Reference Mehmet22], but not those of Russo et al. [Reference Russo23], Bayraktaroğlu et al. [Reference Bayraktaroğlu24], and Di Bonaventura et al. [Reference Di Bonaventura25]. Bayraktaroğlu et al. [Reference Bayraktaroğlu24] examined circulating cytokine levels in 42 patients with dyspeptic symptoms and found that 30 were infected with H. pylori, while 12 were not. They observed elevated circulatory levels of IL-8 in those who were H. pylori-positive, but the difference was not statistically significant (P=0·079). In part, the sample sizes might account for the discrepancy in the results. Infections with specific H. pylori strains that possess the CagA pathogenicity island induce significantly higher levels of chemokines than CagA-negative strains [Reference Innocenti26]. The distribution of H. pylori strains possessing CagA might also have affected the results.
Antigens released by H. pylori can stimulate endothelial cells, macrophages and epithelial cells to make large amounts of chemokines, such as IL-8 and growth-regulated oncogene-alpha, which produces a chemotactic gradient for the migration of neutrophils into the gastric mucosa [Reference Sieveking, Mitchell and Day27–Reference de Jonge29]. The stomach has a large surface area, with locally produced cytokines which might spill over into the bloodstream continuously, and H. pylori infection and the severity of gastric atrophy in those infected may alter the expression levels of IL-8.
H. pylori has been well documented to upregulate the Th1 cytokines. Goll et al. [Reference Goll30] have reported that the cytokine profile of the H. pylori-infected gastric mucosa shows a mixed Th1–Th2 profile. Both the Th1 and Th2 mediator genes are upregulated in the gastric mucosa of H. pylori-positive subjects. Our finding of decreased IL-8 levels in the plasma of H. pylori-infected subjects with atrophy, compared to those without, is consistent with switching of the immune response from an innate to an adaptive one mediated by Th1 cells [Reference Robinson, Argent and Atherton31]. H. pylori gradually decreases during the development of glandular atrophy [Reference Zhang32], which also supported our findings.
Our results did not clearly show the modification of effects of H. pylori infection on plasma IL-8 levels by the IL-8 T-251A polymorphism, which were significantly elevated in individuals with H. pylori(+)/atrophy(−) for the T/T genotype. The association between the IL-8 gene polymorphism and disease in H. pylori-infected patients has not been well documented. Shirai et al. [Reference Shirai33] have revealed that gastric carcinoma with a high frequency of microsatellite instability occurs in H. pylori-infected patients, and is associated with the IL-8 –251T/T genotype (low-expression genotype). The IL-8 –251T/T genotype is also significantly associated with an increased risk of non-cardia gastric carcinoma in H. pylori-positive individuals [Reference Lee34]. The low inflammatory cytokine expression genotype may lead to carcinogenesis via the mutator pathway, presumably due to a higher rate of H. pylori colonization [Reference Shirai33]. Meanwhile, there are contradictory reports. Ye et al. [Reference Ye14] found a positive association between the IL-8 –251 A allele and the degree of atrophy and intestinal metaplasia. The IL-8 –251 A allele carriers showed a higher risk of gastric adenocarcinoma in that study. We could not sufficiently analyse the associations of IL-8 with severity of gastric atrophy and the IL-8 T-251A polymorphism in H. pylori-positive subjects because of the small sample size. Further study in a larger sample size is needed to examine the modifying effect of IL-8 gene polymorphism on circulating IL-8 levels and disease progression over time in H. pylori-infected patients.
Our study found that IL-8 levels were elevated in H. pylori(+)/atrophy(−) individuals. Circulating IL-8 was significantly higher in patients with coronary artery disease with H. pylori infection than in control subjects [Reference Kowalski35]. H. pylori infection may be a trigger factor in the pathophysiology of ischaemic heart disease, through induction of an inflammatory cascade concentrated on the gastric mucosa [Reference Di Bonaventura25]. Similarly, the associations between H. pylori infection, vascular diseases and diabetes mellitus could be mediated by increasing cytokine levels [Reference Moyaert36]. Follow-up data for H. pylori(+)/atrophy(−) individuals may provide a clue concerning the potential role of circulating IL-8 as a risk marker for gastric diseases and other systemic illnesses, such as cardiovascular diseases.
The current study has some limitations. Circulating IL-8 levels can be affected by factors other than H. pylori infection. Most of our subjects were apparently healthy, and the analysis included adjustment for potential confounding factors. Information on the use of antibiotics or steroid drugs and smoking status was all self-reported, which may have led to misclassification. Study subjects were outpatients who requested testing for H. pylori and subsequent eradication, although only 5% were found to suffer from gastric disease. Since the subjects did not know their PG level, IL-8 level, and IL-8 genotype, the selection bias seemed to be limited. The cross-sectional study design and small sample size were also limitations of this study.
Another limitation involves the diagnosis of gastric atrophy. This was done entirely on the basis of serum PG levels and not through histological assessment. However, the PG method is well established as a surrogate marker of gastric atrophy [Reference Miki37–Reference Oksanen39]. The validation criterion is PGI ⩽70 ng/ml and PGI/PGII ratio of ⩽3. A relatively small proportion of our participants had severe gastric atrophy (5·1%), which prevented analysis stratified by the level of atrophy. Although cagA antibody was not tested in the plasma of our subjects, nearly 100% of H. pylori strains in Japan possess CagA [Reference Ito40].
In conclusion, we found that plasma IL-8 levels were significantly higher in those with H. pylori infection, and inversely correlated with the level of gastric atrophy in H. pylori-infected Japanese individuals. Our findings did not indicate that the effects of H. pylori infection on plasma IL-8 levels were modified by the IL-8 T-251A polymorphism.
ACKNOWLEDGEMENTS
The authors are grateful to Ms. Yoko Mitsuda for her technical assistance. This study was supported in part by a Grant-in-Aid for Scientific Research on Special Priority Areas of Cancer from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
DECLARATION OF INTEREST
None.




