1. Introduction
Following establishment of the Ordovician System in England and Wales by Lapworth (Reference Lapworth1879), the Anglo-Welsh Ordovician succession and its constituent series (Tremadoc, Arenig, Llanvirn, Caradoc, Ashgill; Fortey et al. Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000) served as a standard reference for Ordovician correlation for more than a century (e.g. Williams et al. Reference Williams, Strachan, Basset, Dean, Ingham, Wright and Whittington1972, fig. 2; Fortey et al. Reference Fortey, Bassett, Harper, Hughes, Ingham, Molyneux, Owen, Owens, Rushton, Sheldon, Barnes and Williams1991). Nevertheless, as a type area for the system, the Anglo-Welsh region has its limitations. Structural complexity is common and much of the succession, particularly in the Welsh Basin and NW England, has been subjected to low-grade metamorphism. In addition, there is a lack of long continuous sections and a predominance of clastic sedimentary rocks that hampers the recovery of some stratigraphically useful fossil groups, notably conodonts (Fortey et al. Reference Fortey, Bassett, Harper, Hughes, Ingham, Molyneux, Owen, Owens, Rushton, Sheldon, Barnes and Williams1991). Despite efforts to redefine the Anglo-Welsh series in accordance with modern stratigraphic practice and more appropriate stratotypes (Fortey et al. Reference Fortey, Harper, Ingham, Owen and Rushton1995, Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000), the global Ordovician series (Lower, Middle and Upper) and stages (Tremadocian, Floian, Dapingian, Darriwilian, Sandbian, Katian and Hirnantian; Bergström et al. Reference Bergström, Chen, Gutiérrez-Marco and Dronov2009) are now all defined outside the British Isles (Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012). Nevertheless, as the historic type region for the system, England and Wales remain a key reference area for Ordovician stratigraphy.
Graptolites and conodonts are the quintessential tools of Ordovician biostratigraphy, although trilobites and other shelly fossils have also been used (e.g. Fortey & Owens, Reference Fortey and Owens1987). Chitinozoans have the potential for global Ordovician biostratigraphic correlation, and chitinozoan biozonations have been developed for Baltica (Nõlvak, Reference Nõlvak1999; Nõlvak et al. Reference Nõlvak, Hints and Männik2006), different parts of Gondwana (Paris, Reference Paris1990; Paris et al. Reference Paris, Elaouad-Debbaj, Jaglin, Massa, Oulebsir, Cooper, Droser and Finney1995, Reference Paris, Le Hérissé, Monod, Kozlu, Ghienne, Dean, Vecoli and Günay2007; Grahn, Reference Grahn2006; Quintavalle & Playford, Reference Quintavalle and Playford2006; Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010; de la Puente & Rubinstein, Reference de la Puente and Rubinstein2013; Nowak et al. Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016), South China (X Wang et al. Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005, Reference Wang, Stouge, Chen, Li, Wang, Finney, Zeng, Zhou, Chen and Erdtmann2009; Chen et al. Reference Chen, Paris, Wang and Zhang2009; W Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013; Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017) and Laurentia (Achab, Reference Achab1989, Vandenbroucke et al. Reference Vandenbroucke, Verniers and Clarkson2003). However, a comparable biozonation covering the entire Ordovician succession for Avalonia, including England and Wales, has yet to be established. Data are available from Belgium (J Vanmeirhaeghe, unpub. PhD thesis, Univ. Ghent, 2006; Vanmeirhaeghe, Reference Vanmeirhaeghe2007) and the upper Middle and Upper Ordovician of England and Wales (Jenkins, Reference Jenkins1967; Vandenbroucke, Reference Vandenbroucke2008 a, b; Vandenbroucke et al. Reference Vandenbroucke, Rickards and Verniers2005, Reference Vandenbroucke, Williams, Zalasiewicz, Davies and Waters2008, Reference Vandenbroucke, Ancilletta, Fortey and Verniers2009 a; Challands et al. Reference Challands, Vandenbroucke, Armstrong and Davies2014), but the Lower Ordovician and the lower part of the Middle Ordovician in England and Wales have remained largely unstudied until now.
The aims of this paper are (i) to describe chitinozoan assemblages from the upper Tremadoc, Arenig and lowermost Llanvirn series in Wales, including the historical type area of the Arenig Series in North Wales and complementary sections in South Wales; (ii) to establish a biostratigraphical framework; and (iii) to assess biostratigraphic ages of sampled successions based on species ranges in Gondwana, Baltica and Laurentia, and therefore to correlate the British regional series with the equivalent global Tremadocian, Floian, Dapingian and lower Darriwilian stages. The new data are also expected to contribute towards tracking biogeographical affinities with Gondwana, Baltica, South China and Laurentia, and to help constrain the stratigraphy of a time interval that is becoming increasingly pivotal in understanding the evolution of Ordovician climate (e.g. Trotter et al. Reference Trotter, Williams, Barnes, Lécuyer and Nicoll2008; Vandenbroucke et al. Reference Vandenbroucke, Armstrong, Williams, Zalasiewicz and Sabbe2009 b; Turner et al. Reference Turner, Armstrong and Holt2011; Dabard et al. Reference Dabard, Loi, Paris, Ghienne, Pistis and Vidal2015; Amberg et al. Reference Amberg, Collart, Salenbien, Egger, Munnecke, Nielsen, Monnet, Hammer and Vandenbroucke2016, Pohl et al. Reference Pohl, Donnadieu, Le Hir, Ladant, Dumas, Alvarez-Solas and Vandenbroucke2016 a, b; Rasmussen et al. Reference Rasmussen, Ullmann, Jakobsen, Lindskog, Hansen, Hansen, Eriksson, Dronov, Frei, Korte, Nielsen and Harper2016; Elrick, Reference Elrick2022).
2. Geological setting
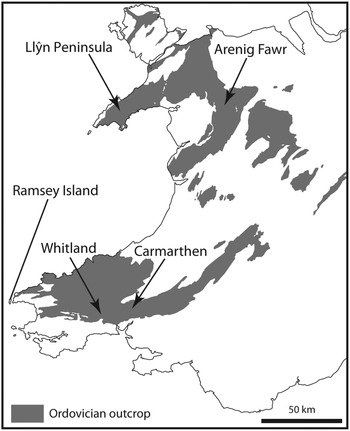
The term ‘Arenig’ was first used by Sedgwick (Reference Sedgwick1852) for rocks that crop out on Arenig Fawr Mountain in North Wales, where the Ordovician System extends around the Cambrian Harlech Dome from the Llⓨn Peninsula in the west to the Bala area in the east and the Arenig Mountains in central Wales (Fig. 1). The Arenig Fawr section is stratigraphically incomplete (Zalasiewicz, Reference Zalasiewicz1984), however, and more complete sections are to be found in South Wales, where lower and upper Arenig successions are well developed in the Carmarthen area (Fortey & Owens, Reference Fortey and Owens1978) and around Whitland (Fortey & Owens, Reference Fortey and Owens1987) respectively. The Ordovician System in South Wales extends over 160 km from SW Wales to east central Wales (Fig. 1), following the Tywi lineament (Fortey et al. Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000). The Arenig Series is at the centre of this Ordovician tract and extends from Ramsey Island, off the westernmost point of the Pembrokeshire coast, towards Llandeilo, east of Carmarthen.

Fig. 1. Ordovician outcrop in Wales indicating sampled areas at Arenig Fawr in North Wales and the Carmarthen and Whitland areas in South Wales.
The Arenig Series in South Wales has a relatively rich and diverse macrofauna comprising trilobites, brachiopods, other shelly fossils and graptolites (Fortey & Owens, Reference Fortey and Owens1978, Reference Fortey and Owens1987; Cope, Reference Cope1996, Reference Cope2005; Cocks & Popov, Reference Cocks and Popov2019) and has also yielded acritarchs and chitinozoans (Molyneux, Reference Molyneux1987). Fortey and Owens (Reference Fortey and Owens1987) proposed a subdivision of the Arenig Series in South Wales into the Moridunian, Whitlandian and Fennian stages, although the base of the series itself was not defined there (Fortey et al. Reference Fortey, Harper, Ingham, Owen and Rushton1995). Wider correlations have been hindered, however, by the provincialism of many of the macrofossil species present (Cocks & Fortey, Reference Cocks and Fortey1982), the generally poorly preserved and sparse acritarchs with low species richness, and the preliminary nature of the published chitinozoan record. Graptolites are poorly represented in the Moridunian and Whitlandian stages, but become more numerous, diverse and stratigraphically useful in the Fennian Stage. The lithostratigraphy and biostratigraphy of the areas sampled are summarized below.
2.a. Carmarthen area
2.a.1. Stratigraphy
Fortey and Owens (Reference Fortey and Owens1978) provided a modern account of the stratigraphy of the Carmarthen area where the lower Arenig Moridunian Stage is best exposed (Figs 2, 3). The ‘Login beds’, consisting of siltstone, shale and sandstone, comprise the lowest exposed unit in the succession and were dated by acritarchs as latest Tremadoc to earliest Arenig (Molyneux & Dorning, Reference Molyneux and Dorning1989; Molyneux et al. Reference Molyneux, Raevskaya and Servais2007).
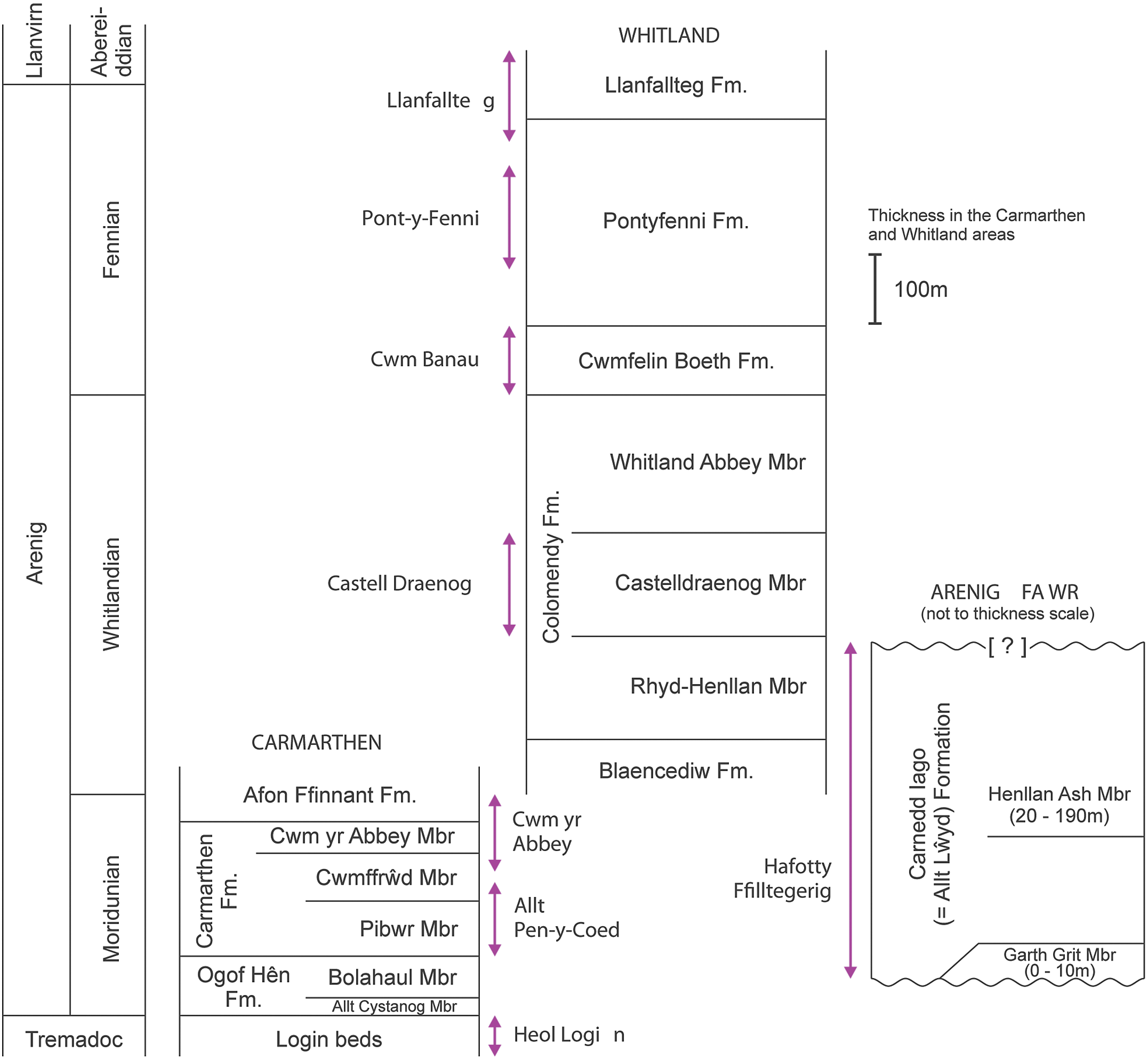
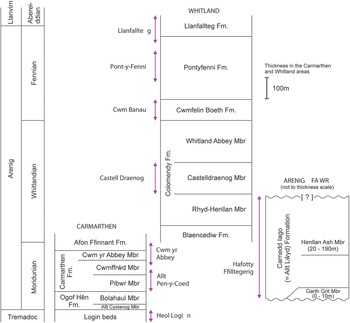
Fig. 2. Generalized vertical sections for the Arenig successions in the Carmarthen, Whitland and Arenig Fawr areas. Arrowed lines indicate the stratigraphical extent of sampled sections.

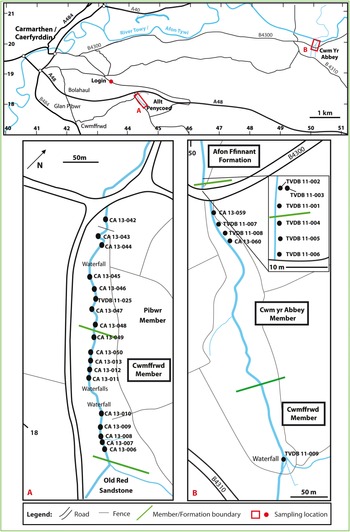
Fig. 3. Sample localities in the Carmarthen area, South Wales.
The Login beds are overlain by the Moridunian Ogof Hên Formation, although the contact between the two units is not exposed (Fortey & Owens, Reference Fortey and Owens1978). The Ogof Hên Formation includes conglomerate, sandstone and siltstone of the Allt Cystanog Member overlain by micaceous mudstone and shale of the Bolahaul Member. The overlying Carmarthen Formation comprises, in upwards succession, black mudstone of the Pibwr Member, turbidite beds and shale of the Cwmffrⓦd Member, and grey mudstone of the Cwm yr Abbey Member. The Carmarthen Formation passes up into the Afon Ffinnant Formation, consisting of turbidite deposits. The base of the middle Arenig Whitlandian Stage is placed 40 m above the base of the Afon Ffinnant Formation (Fortey & Owens, Reference Fortey and Owens1987, p. 87). Traynor (Reference Traynor1988) assigned the Ogof Hên Formation to his facies 3, comprising fluviodeltaic deposits. The mudstones of the Pibwr and Cwm yr Abbey members are placed in facies 6 (deep water mudstone), and the Cwmffrⓦd Member and Afon Ffinnant Formation are placed in facies 5, representing deep water sediment-gravity flows in which turbidites are interbedded with facies 6 mudstone (Traynor, Reference Traynor1988).
2.a.2. Fauna and microflora
The acritarch assemblage from the Login beds (Molyneux & Dorning, Reference Molyneux and Dorning1989) is similar to the messaoudensis–trifidum acritarch assemblage from the English Lake District and has been recorded as an occurrence of that assemblage in South Wales (Molyneux et al. Reference Molyneux, Raevskaya and Servais2007). Molyneux and Dorning (Reference Molyneux and Dorning1989) concluded that the Login assemblage was from the uppermost Tremadoc or lowest Arenig series. Fortey et al. (Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000, fig. 7), however, considered the acritarchs to indicate the upper Tremadoc Migneintian Stage and suggested correlation with the Araneograptus murrayi graptolite Biozone.
Above the Login beds, much of the Arenig succession in the Carmarthen area has yielded shelly faunas, rare graptolites and acritarchs (Fortey & Owens, Reference Fortey and Owens1978, Reference Fortey and Owens1987; Molyneux, Reference Molyneux1987; Cope, Reference Cope1996, Reference Cope2005; Cocks & Popov, Reference Cocks and Popov2019; Ebbestad & Cope, Reference Ebbestad and Cope2021). No shelly fossils or graptolites have been recorded from the Allt Cystanog Member, but shelly faunas from the rest of the succession are varied. Echinoderm fragments, brachiopods and gastropods occur in the Bolahaul Member, bivalves in the Bolahaul, Pibwr and Cwmffrⓦd members, orthoconic nautiloids in the Cwm yr Abbey Member, and trilobites throughout. Fortey and Owens (Reference Fortey and Owens1987) established a succession of seven trilobite biozones in the Arenig succession of South Wales, of which the lower three occur in the Carmarthen area: the Merlinia selwynii Biozone in the upper Ogof Hên and lower Carmarthen formations (Bolahaul and Pibwr members), the Merlinia rhyakos Biozone in the upper part of the Carmarthen Formation (Cwmffrⓦd and Cwm yr Abbey members), and the Furcalithus radix Biozone in the lower Afon Ffinnant Formation.
Graptolites recorded from the Carmarthen area include Phyllograptus cf. densus and Pseudophyllograptus aff. angustifolius from the Pibwr Member, dendroid graptolites from the the Cwm yr Abbey Member, including Callograptus cf. tenuis, Callograptus salteri and Palaeodictyota sp., and Azygograptus eivionicus and A. hicksii from the Whitlandian part of the Afon Ffinnant Formation (Fortey & Owens, Reference Fortey and Owens1978, Reference Fortey and Owens1987).
Molyneux (Reference Molyneux1987) described four acritarch assemblages from the Moridunian Stage in the Carmarthen area, informally designated Assemblage I to Assemblage IV in upwards succession. All comprise relatively sparse and poorly preserved specimens in assemblages of generally low diversity, however, and do not currently provide enough evidence to assist correlation.
2.b. Whitland area
2.b.1. Stratigraphy
The Whitlandian and Fennian stages are better exposed in the Whitland area (Fig. 2), where the succession was described by Fortey and Owens (Reference Fortey and Owens1987). The Whitlandian Blaencediw Formation at the base of the succession consists of turbidite deposits, shale and siltstone, and is correlated with the upper part of the Afon Ffinnant Formation in the Carmarthen area (Fig. 2). The overlying Colomendy Formation, also Whitlandian, is divided into the sandy shale of the Rhyd-Henllan Member, the grey shale of the Castelldraenog Member and the black shale of the Whitland Abbey Member. The base of the Fennian Stage is placed at the base of the Cwmfelin Boeth Formation, which consists of turbidite beds and black shale. The overlying Pontyfenni Formation comprises black to dark grey shale and mudstone, and the Llanfallteg Formation at the top of the Arenig succession, passing up into the Llanvirn Series, comprises light grey mudstone and shale. The Colomendy, Pontyfenni and Llanfallteg formations were classed as facies 6 deep water mudstone units by Traynor (Reference Traynor1988), interbedded with two coarse facies 5 deep water turbidite units represented by the Blaencediw and Cwmfelin Boeth formations.
2.b.2. Fauna and microflora
Trilobites have been collected from all lithostratigraphical units in the Whitland area except the Castelldraenog Member of the Colomendy Formation, graptolites from all except the Rhyd-Henllan Member, and brachiopods, chordates and ostracods are known from some levels (Fortey & Owens, Reference Fortey and Owens1987; Cocks & Popov, Reference Cocks and Popov2019). The Rhyd-Henllan and Whitland Abbey members contain the typical Whitlandian trilobite Bohemopyge scutarix. The latter also contains the graptolite Expansograptus simulans (recorded as Didymograptus simulans), which is present throughout the Whitlandian Stage (Fortey et al. Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000; Cooper et al. Reference Cooper, Fortey, Hughes, Molyneux, Moore, Rushton and Stone2004; Zalasiewicz et al. Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009). Fossils are numerous in the upper part of the Cwmfelin Boeth Formation and include brachiopods and the trilobite Asaphellus. Fortey and Owens (Reference Fortey and Owens1987) considered them likely to be derived from a relatively shallow source.
The Pontyfenni Formation yielded a rich fauna of graptolites, trilobites, chordates and ostracods (Fortey & Owens, Reference Fortey and Owens1987; Jefferies in Fortey & Owens, Reference Fortey and Owens1987). A diverse graptolite fauna includes Expansograptus? uniformis lepidus, Expansograptus hirundo and Undulograptus cumbrensis (=‘Glyptograptus’ dentatus of Fortey & Owens, Reference Fortey and Owens1987; Owens, Reference Owens, Rushton, Owen, Owens and Prigmore1999). Fortey and Owens (in Fortey et al. Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000) placed the Pontyfenni Formation of the Whitland area in the Isograptus gibberulus graptolite Biozone. Trilobites are nowhere common but comprise diverse cyclopygid and atheloptic assemblages with Pricyclopyge binodosa eurycephalathe, Placoparia cambriensis and Selenopeltis buchii macrophthalma, the last two species being typical of the Fennian Stage (Fortey & Owens, Reference Fortey and Owens1987).
Fortey et al. (Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000, fig. 7) depicted the Llanfallteg Formation as straddling the Arenig–Llanvirn boundary, corresponding to the upper Fennian Didymograptus hirundo graptolite Biozone (since replaced by the Aulograptus cucullus graptolite Biozone: Rushton in Cooper et al. Reference Cooper, Fortey, Hughes, Molyneux, Moore, Rushton and Stone2004; Zalasiewicz et al. Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009) and the lower Abereiddian (lowest Llanvirn) Didymograptus artus graptolite Biozone. Graptolites include Undulograptus cumbrensis (recorded in part as ‘Glyptograptus’ dentatus by Fortey & Owens, Reference Fortey and Owens1987; see Owens, Reference Owens, Rushton, Owen, Owens and Prigmore1999), found only in the uppermost Arenig section at Llanfallteg but spanning the Arenig–Llanvirn boundary elsewhere (Zalasiewicz et al. Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009, fig. 4), and Acrograptus acutidens and U. austrodentatus, which range across the Arenig–Llanvirn boundary at Llanfallteg. A diverse trilobite fauna is also present, with Dionide levigena, the eponymous species of the uppermost Arenig trilobite biozone, although it also ranges into the Llanvirn Series, and Ectillaenus perovalis, Barrandia homfrayi, Stapeleyella inconstans, Amphyx linleyensis and P. cambriensis, all of which have ranges that span the Arenig–Llanvirn boundary at Llanfallteg (Fortey & Owens, Reference Fortey and Owens1987).
Trilobite biozones established by Fortey and Owens (Reference Fortey and Owens1987) in the Whitland area comprise, in upwards succession, the Gymnostomix gibbsii Biozone, Stapeleyella abyfrons Biozone, Bergamia rushtoni Biozone and Dionide levigena Biozone. Gymnostomix gibbsii occurs in the Rhyd-Henllan and Whitland Abbey members of the Whitland area, meaning that the gibbsii Biozone coincides with most of the upper part of the Whitlandian Stage. The other three zones all occur in the Fennian Stage, the Stapeleyella abyfrons Biozone in the basal Pontyfenni Formation immediately overlying the Cwmfelin Boeth Formation, the Bergamia rushtoni Biozone through an estimated two-thirds of the Pontyfenni Formation, and the Dionide levigena Biozone in the upper Fennian part of the Llanfallteg Formation.
Molyneux (Reference Molyneux1987) described one Whitlandian acritarch assemblage, Assemblage V from the Whitland Abbey Member. The assemblage, like those from the Moridunian succession in the Carmarthen area, is of low diversity and comprises mainly small acanthomorph acritarchs (Micrhystridium spp.) that do not assist correlation. More diverse acritarch assemblages of Fennian age, with stratigraphically useful forms, were described from the Pontyfenni Formation (Molyneux, Reference Molyneux1987). Assemblage VI, from just above the base of the formation, is distinguished by the presence of Coryphidium bohemicum Vavrdová, ?Frankea hamata Burmann, Orthosphaeridium sp., Stellechinatum uncinatum (Downie) Molyneux, ?Striatotheca mutua Burmann, S. rarirrugulata (Cramer et al.) Eisenack et al. and species of Uncinisphaera. Assemblage VII, from higher in the formation at Pont-y-Fenni, includes Coryphidium bohemicum Vavrdová, Dasydorus cirritus? Playford & Martin, Orthosphaeridium ternatum (Burmann) Eisenack et al., Stellechinatum papulessum Molyneux, and species of Solisphaeridium, Stelliferidium and Uncinisphaera. Chitinozoans assigned to species of Belonechitina, Conochitina and Lagenochitina were recorded from the Pontyfenni Formation at Pont-y-Fenni (Molyneux, Reference Molyneux1987).
2.c. Arenig Fawr
2.c.1. Stratigraphy
The Arenig Series is represented in the Arenig Fawr area by a single formation, previously referred to as the Carnedd Iago Formation, with unconformities at the base and top (Fig.e 2; Fortey et al. Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000). The Carnedd Iago Formation is now considered to be equivalent to and has been superseded by the Allt Lⓦyd Formation throughout North Wales (Rushton & Howells, Reference Rushton and Howells1998), but the older terminology is retained here for better comparison with work reported in the literature.
The Carnedd Iago Formation was established by Lynas (Reference Lynas1973) and described by Zalasiewicz (Reference Zalasiewicz1984) as extending throughout the Arenig with three members: the Garth Grit Member, consisting of quartzo-feldspathic sandstone, the Llyfnant Member, consisting of laminated dark siltstone and pale sandstone, and the Henllan Ash Member, comprising variably bioturbated feldspathic sandstone and sandy mudstone. Fortey et al. (Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000) revised the stratigraphy of the Carnedd Iago Formation, restricting it to the lower Moridunian to middle Whitlandian stages and including only the Garth Grit and the Henllan Ash as separate members.
2.c.2. Fauna
The Llyfnant Member contains common Expansogratus aff. simulans, which suggests correlation with the E. simulans Biozone, the Isograptus victoriae victoriae Biozone, or possibly the Isograptus gibberulus Biozone of the English Lake District (Zalasiewicz, Reference Zalasiewicz1984; Zalasiewicz et al. Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009). Specimens of Corymbograptus aff. deflexus and Azygograptus cf. eivionicus have also been recorded. The ranges in England and Wales of the three nominal species, A. eivionicus, C. deflexus and E. simulans, overlap in the simulans Biozone, which is correlated with the lower Whitlandian Stage (Zalasiewicz et al. Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009).
Fortey and Owens (Reference Fortey and Owens1978, Reference Fortey and Owens1987) suggested correlation of the Henllan Ash Member with the Bolahaul Member or the Pibwr Member, or both, based on its abundant trilobite fauna (Whittington, Reference Whittington1966), and therefore with the middle Moridunian Stage. Graptolites from the uppermost Henllan Ash Member were re-examined by Zalasiewicz (Reference Zalasiewicz1984), who identified a fauna characterized by Expansograptus cf. praenuntius and Tetragraptus reclinatus. Fortey et al. (Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000) placed the Henllan Ash in the upper Moridunian Stage, but Zalasiewicz et al. (Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009) depicted E. cf. praenuntius as present in the simulans, victoriae and gibberulus graptolite biozones, the last with some doubt. This suggests a possible slightly younger, Whitlandian age than that indicated by Fortey et al. (Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000), albeit still Floian.
3. Sample localities
3.a. Carmarthen area
Samples were collected from the Login beds, Carmarthen Formation and Afon Ffinnant Formation in the Carmarthen area (Fig. 3). No samples were collected from the Ogof Hên Formation.
3.a.1. Heol Login
Ten samples from the Login beds, originally collected by SGM (Molyneux & Dorning, Reference Molyneux and Dorning1989) and curated in the British Geological Survey’s collections at Keyworth, Nottingham, UK (BGS sample registration numbers MPA 26829 to MPA 26838), were resampled. The samples are from a section along Heol Login (‘Login road’), about 2 km SE of Carmarthen (British National Grid References SN 4352 1873–SN 4364 1870; ‘Login’ in Fig. 3; Molyneux & Dorning, Reference Molyneux and Dorning1989, figs 1, 2).
3.a.2. Allt Pen-y-Coed
Allt Pen-y-Coed [SN 4425 1823–SN 4446 1803] (Fig. 3a) is a steep-sided, wooded stream section, oriented NW–SE, about 3 km SE of Carmarthen. It exposes the Pibwr and Cwmffrⓦd members of the Carmarthen Formation. The succession dips steeply to the south or SE so that the older beds are to the north. At the southern end of the section, the Cwmffrⓦd Member is unconformably overlain by upper Silurian (Pridoli) beds of Old Red Sandstone facies (https://mapapps2.bgs.ac.uk/geoindex/home.html, accessed 26 March 2020; see also Bedrock map of Wales and adjacent area in Howells, Reference Howells2007).
Eight samples (CA 13-042 to CA 13-048 and TVDB 11-025) were collected from the Pibwr Member and ten samples (CA 13-006 to CA 13-013, CA 13-049, CA 13-050) from the Cwmffrⓦd Member, upstream from the point at which the stream passes under a bridge on a minor road (Fig. 3a).
3.a.3. Cwm yr Abbey
The upper Cwmffrⓦd Member, the Cym yr Abbey Member and the lower Afon Ffinnant Formation are exposed in the stream section of Cwm yr Abbey [SN 5002 1988–SN 5013 1943], about 9 km east of Carmarthen (Fig. 3). The section is oriented approximately N–S with the succession generally dipping northwards, although there is much minor folding and faulting (Owens, Reference Owens, Rushton, Owen, Owens and Prigmore1999, fig. 8.7).
One sample (TVDB 11-009) was collected from the upper Cwmffrⓦd Member, seven samples (TVDB 11-004 to TVDB 11-008, CA 13-059, CA 13-060) from the Cwm yr Abbey Member, and three samples (TVDB 11-001 to TVDB 11-003) from the base of the Afon Ffinnant Formation, downstream from the bridge crossing the stream on the minor B4300 road [SN 5002 1978] (Fig. 3b).
3.b. Whitland area
Samples were collected from the Castelldraenog Member of the Colomendy Formation and from the Cwmfelin Boeth, Pontyfenni and Llanfallteg formations in the Whitland area (Fig. 4). No samples were taken from the Blaencediw Formation or the Rhyd-Henllan and Whitland Abbey members of the Colomendy Formation.

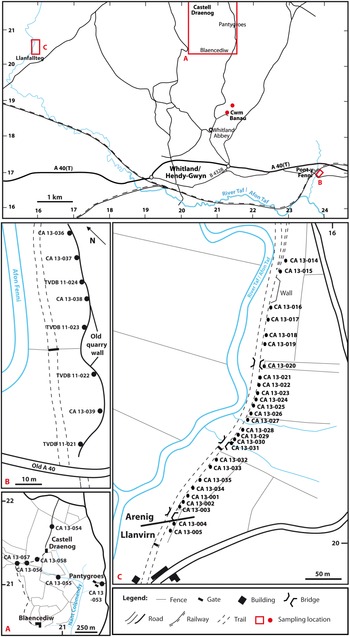
Fig. 4. Sample localities in the Whitland area, South Wales.
3.b.1. Castell Draenog
Four samples (CA 13-055 to CA 13-058; Fig. 4a) were collected from the Castelldraenog Member south of Castell Draenog [SN 2077 2139]. Other samples collected in the vicinity of Castell Draenog are CA 13-053, collected about 750 m SE of Castell Draenog in the vicinity of Pantygroes, about 50 m west of the minor road from Whitland Abbey to Llanboidy on the east side of Nant Colomendy, and CA 13-054, collected from the track approximately 260 m north of Castell Draenog. CA 13-053 is from beds mapped as Pontyfenni Formation by Fortey and Owens (Reference Fortey and Owens1987, fig. 2), and CA 13-054 from beds now placed in the Abergwilli Formation of Abereiddian (early Llanvirn) age (British Geological Survey, 2010; Burt et al. Reference Burt, Aspden, Davies, Hall, Schofield, Sheppard, Waters, Wilby and Williams2012).
3.b.2. Cwm Banau
Six samples (CA 13-040, CA 13-041, CA 13-051, CA 13-052, TVDB 11-019, TVDB 11-020) were collected from the Cwmfelin Boeth Formation at Cwm Banau [SN 2123 1862] (Fig. 4), NE of Whitland Abbey.
3.b.3. Pont-y-Fenni
Eight samples (CA 13-036 to CA 13-039, TVDB 11-021 to TVDB 11-024) were collected from the Pontyfenni Formation in a disused quarry at Pont-y-Fenni [SN 2379 1690–SN 2381 1693], the formation’s effective type locality, on the east bank of the Afon Fenni (Fig. 4b). The beds at Pont-y-Fenni comprise black to dark grey shale and blocky mudstone dipping northwards at about 60°, either on the northern limb of a fold that is subsidiary to an anticlinal area to the north, or on the overturned southern limb of the latter (Owens, Reference Owens, Rushton, Owen, Owens and Prigmore1999). The latter interpretation accords with the British Geological Survey’s (1975) map of the area and is adopted here. The southernmost sample, TVDB 11-021, is therefore placed highest in the succession.
3.b.4. Llanfallteg
The Llanfallteg section is along a disused and dismantled railway line, oriented NNE–SSW on the east bank of the Afon Taf (Fig. 4c). Beds at the northern end of the section are placed in the Pontyfenni Formation (locality 52Z of Fortey and Owens, Reference Fortey and Owens1987) but pass southwards into light grey mudstone and shale of the Llanfallteg Formation. The contact between the two formations is mapped as a fault north of locality 52Y of Fortey and Owens (Reference Fortey and Owens1987, figs2, 8; British Geological Survey, 1976). The Arenig–Llanvirn series boundary is placed towards the southern end of the section, within the Llanfallteg Formation, and is marked by pendent didymograptid graptolites that include Didymograptus artus, the eponymous index of the lowest Llanvirn graptolite biozone.
Twenty-seven samples (CA 13-001–CA 13-005, CA 13-014–CA 13-035) were collected from the Llanfallteg section over a distance of about 530 m [SN 1592 2058–SN 1567 2012] (Fig. 4c). Two samples at the northern end of the section (CA 13-014, CA 13-015) are from the Pontyfenni Formation. Twenty samples are from the Llanfallteg Formation, the most northerly of which, CA 13-021 (Fig. 4c), is from around locality 52Y of Fortey & Owens (Reference Fortey and Owens1987). Of these, 18 are from the Arenig part of the formation (CA 13-001–CA 13-003, CA 13-021–CA 13-035) and two from the Llanvirn (CA 13-004, CA 13-005). The remaining five samples (CA 13-016–CA 13-020) were collected between localities 52Y and 52Z of Fortey and Owens (Reference Fortey and Owens1987) and are therefore from either the upper Pontyfenni Formation or lower Llanfallteg Formation, most likely the former.
3.c. Arenig Fawr
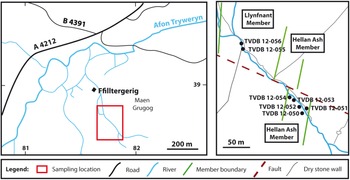
Seven samples (TVDB 12-050 to TVDB 12-056; Fig. 5) were collected along a stream section southeast from Hafotty Ffilltirgerig [SH 8184 3857], corresponding to the Llyfnant and Henllan Ash members of Zalasiewicz (Reference Zalasiewicz1984).

Fig. 5. Sample locality at Arenig Fawr, North Wales.
4. Methods
4.a. Sample preparation and analysis
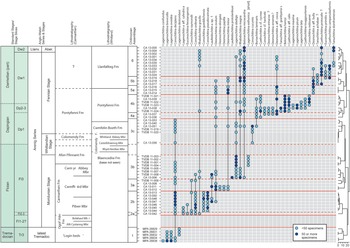
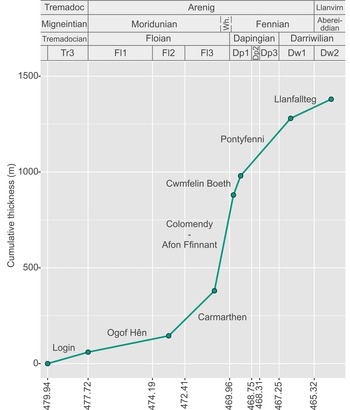
Ninety-three samples were prepared using standard palynological techniques. Between 40 and 60 g of rock were dissolved per sample. See Amberg et al. (Reference Amberg, Collart, Salenbien, Egger, Munnecke, Nielsen, Monnet, Hammer and Vandenbroucke2016) for the full procedure. The organic residues were sieved at 51 µm, and the top fraction was hand-picked under a stereomicroscope at ×50 magnification. More than 4400 specimens were obtained, and identifications are based on images taken with a FEI Scanning Electron Microscope (SEM) and a ZEISS LEO SEM. All figured material is stored and available for consultation in the collections of the UMR 8198 at the University of Lille. Ten genera and 44 species were identified from the sections and localities studied. Occurrences and ranges are shown in Figure 6.

Fig. 6. Chitinozoan occurrences and ranges in the highest Tremadoc – lowest Llanvirn (Tremadocian–Darriwilian) succession of South Wales, plotted against lithostratigraphy in the Carmarthen and Whitland areas, Anglo-Welsh series and stages and global stages and stage slices. Sampled lithostratigraphical units are indicated by bold typeface; unsampled or unproductive units are in italics. The dendrogram is from constrained hierarchical cluster analysis of binary (presence/absence) data (Jaccard dissimilarity index, rioja and vegan packages, R) and distinguishes the six assemblages identified in this paper (indicated by solid lines across the range chart). Sub-assemblages are based on successive lowest occurrences of chitinozoan species (indicated by dashed lines).
4.b. Data analysis
R version 3.6.2 (R Core Team, 2019) was used for data analysis and visualization. Stratigraphically constrained hierarchical cluster analysis was carried out on a distance matrix using the ‘rioja’ package (Juggins, Reference Juggins2017) and the CONISS method. The distance matrix was computed from presence–absence data of species occurrences per sample using the Jaccard index for binary data in the ‘vegan’ package (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O’Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2019). Ranges, stratigraphic columns and the dendrogram were plotted using the ‘ggplot2’ and ‘gridExtra’ packages (Wickham, Reference Wickham2016; Auguié, Reference Auguié2017). A broken-stick model (Bennett, Reference Bennett1996) was applied to the cluster analysis to determine the significance of each cluster. Clusters identified as significant using this method form the basis of assemblages identified in the succession.
5. Preservation, abundance and species richness
Preservation is variable between localities and sections, depending on the degree of metamorphism. Robinson and Bevins (Reference Robinson and Bevins1986, fig. 2) and Merriman (Reference Merriman2006) delineated zones of incipient metamorphism in the Welsh Basin based on clay mineral assemblages and illite crystallinity, with the Carmarthen–Whitland area in the diagenetic zone to low anchizone and Arenig Fawr at a higher grade in the epizone. Intense small-scale folding affects the shale and mudstone units in the Carmarthen area (Fortey & Owens, Reference Fortey and Owens1978), and small-scale reverse faults occur in the Whitland area (Fortey & Owens, Reference Fortey and Owens1987), especially around Castelldraenog, where a NNE–SSW fault along Nant Colomendy pushes a block of the Colomendy Formation up in contact with the Pontyfenni Formation, and around Llanfallteg. The deformation results in generally only slight distortion of the macrofauna and mineralization (Fortey & Owens, Reference Fortey and Owens1987).
Abundance and species richness also vary between sections and within sections themselves. For this study, we consider a number of 1 to 50 specimens per sample of dissolved rock (about 40 g) to be low abundance, 51 to 150 specimens to be moderate abundance and over 150 specimens to be high abundance.
Five of the 10 samples from the Login beds yielded chitinozoans, mainly large lagenochitinids, belonging to between one and four species in a maximum of two genera. They are not abundant, but are relatively well preserved, although flattened.
Six of the 18 samples from the Carmarthen Formation at Allt Pen-y-Coed were unproductive, two from the Pibwr Member and four from the Cwmffrⓦd Member. The remainder, six each from the Pibwr and Cwmffrⓦd members, yielded abundant and diverse chitinozoans. Productive samples from the Pibwr Member consistently yielded five to six species belonging to two to four genera. Most of the productive samples from the Cwmffrⓦd Member were similar, with four or five species belonging to one or two genera, mostly conochitinids in the lower part, but desmochitinids higher in the succession. There is a marked increase in species richness in the uppermost sample from the Cwmffrⓦd Member, CA 13-006, however, with seven species of five genera. Both three-dimensional and flattened specimens were found, some being pyritized. Except for the largest specimens, chitinozoans are generally complete.
The samples from the upper Carmarthen Formation (Cwmffrⓦd and Cwm yr Abbey members) and lower Afon Ffinnant Formation in the Cwm yr Abbey section produced moderately abundant and diverse faunas dominated by small lagenochitinids and a few conochitinids. Three of the 11 samples were barren. The rest yielded between two and five species belonging to between one and five genera, the most diverse assemblages being from the Cwm yr Abbey Member. All specimens are relatively poorly preserved, however, the majority being flattened, although a few specimens of Conochitina are preserved in 3D.
The samples from the Castelldraenog Member at Castell Draenog and the Cwmfelin Boeth Formation at Cwm Banau were less productive. Five out of the 12 samples were barren and the rest yielded very sparse faunas, although large rock samples were dissolved (about 100 g). Most productive samples contained two or three species belonging to one or two genera. The exception is sample CA 13-051 from the Cwmfelin Boeth Formation, which yielded eight species of six genera.
The most diverse and abundant faunas are from the samples of the Pontyfenni Formation collected at Pont-y-Fenni. All eight samples yielded chitinozoans, and the specimens are well preserved, although often flattened. Yields are variable, from a minimum of three species in three genera, to a maximum for the area of 14 species belonging to seven genera in sample CA 13-038. Assemblages are dominated by large cyathochitinids with lagenochitinids and desmochitinids.
Abundance and species richness are variable throughout the Llanfallteg section. The six samples (CA 13-014 to CA 13-019) from the northern part of the section, and therefore definitely or probably from the Pontyfenni Formation, yielded assemblages of moderate abundance and diversity, with five to seven species and two to six genera. In contrast, the middle part of the section (11 samples, CA 13-020 to CA 13-030) is almost barren, despite some samples being re-processed twice to enhance recovery (totalling up to about 150 g of dissolved rock for these samples). Only one sample from this part of the section (CA 13-024) yielded chitinozoans, albeit moderately diverse with five species and four genera. Nine of the ten samples from the southern part of the section, however, including those from the lowermost Llanvirn Series, yielded assemblages of moderate abundance and diversity, with between three and eight species in two to six genera. The faunas from the Llanfallteg section are dominated by desmochitinids, belonechitinids and cyathochitinids. The specimens are often pyritized or broken, and both 3D and flattened specimens were found.
The samples from the Arenig Fawr section yielded the poorest assemblages with low abundance and diversity. Only two of the seven samples, both from the Henllan Ash, yielded chitinozoans and the specimens are severely broken. Three species belonging to three genera were determined, with most specimens being lagenochitinids.
6. Chitinozoan assemblages from South Wales
The broken-stick model applied to the constrained hierarchical cluster dendrogram distinguished six significant chitinozoan assemblages, here numbered 1–6 from the base of the succession upwards (Fig. 6). Assemblages 2–5 are further subdivided, based on stratigraphic changes in the composition of their faunas. Correlation of the assemblages and sub-assemblages with global series and stages, Anglo-Welsh series and stages, and graptolite, chitinozoan and conodont biozones, is shown in Fig. 7.

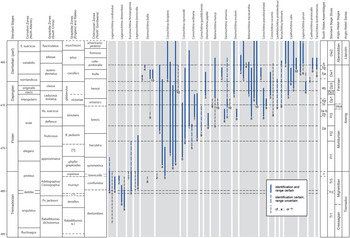
Fig. 7. Ranges of selected species from South Wales recorded elsewhere in (1) low palaeolatitude Gondwana (Australia): Quintavalle & Playford (Reference Quintavalle and Playford2006); (2) Perigondwana (Megumia, Avalonia or Ganderia: UK, Belgium): Jenkins (Reference Jenkins1967); Samuelsson & Verniers (Reference Samuelsson and Verniers2000); Herbosch & Verniers (Reference Herbosch and Verniers2014); Amberg et al. (Reference Amberg, Vandenbroucke, Molyneux and Servais2017); (3) Baltica: Grahn (Reference Grahn1984); Nõlvak & Grahn (Reference Nõlvak and Grahn1993); Grahn et al. (Reference Grahn, Nõlvak and Paris1996); Hints & Nõlvak (Reference Hints and Nõlvak2006); Grahn & Nõlvak (Reference Grahn and Nõlvak2007); Tammekand et al. (Reference Tammekänd, Hints and Nõlvak2010); Nõlvak et al. (Reference Nõlvak, Liang and Hints2019); (4) Bohemia: Paris & Mergl (Reference Paris and Mergl1984); Fatka (Reference Fatka1993, Reference Fatka2003); (5) Laurentia: Achab (Reference Achab1980, Reference Achab1989); Achab & Maletz (Reference Achab and Maletz2021); (6) Qaidam: W Wang et al. (Reference Wang, Zhao, Muir, Li and Tan2018); (7) South America (Gondwana): Heuse et al. (Reference Heuse, Grahn and Erdtmann1999); Achab et al. (Reference Achab, Rubinstein and Astini2006); Grahn (Reference Grahn2006); de la Puente & Rubinstein (Reference de la Puente and Rubinstein2009, Reference de la Puente and Rubinstein2013); Toro et al. (Reference Toro, de la Puente and Rubinstein2010); (8) South China: Brocke et al. (Reference Brocke, Li and Wang2000); X Wang, et al. (Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005); Tang et al. (Reference Tang, Paris, Geng and Zhu2007); Chen et al. (Reference Chen, Paris and Miao2008, Reference Chen, Paris, Wang and Zhang2009); Liang et al. (Reference Liang, Servais, Tang, Liu and Wang2017, Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018, 2009); W Wang et al. (Reference Wang, Feng, Vandenbroucke, Li and Verniers2013); (9) high southern palaeolatitude Gondwana (North Africa, southern Europe; North Gondwana of Paris, Reference Paris1990): Paris (Reference Paris1981, Reference Paris1990); Elaouad-Debbaj (Reference Elaouad-Debbaj1984, Reference Elaouad-Debbaj1988); Soufiane & Achab (Reference Soufiane and Achab1993); Oulebsir & Paris (Reference Oulebsir and Paris1995); Nowak et al. (Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016); (10)) middle palaeolatitude western Gondwana (Iran, Oman, Saudi Arabia, Pakistan): Al-Hajri (Reference Al-Hajri1995); Quintavalle et al. (Reference Quintavalle, Tongiorgi and Gaetani2000); Le Herisse et al. (2007); Sansom et al. (Reference Sansom, Miller, Heward, Davies, Booth, Fortey and Paris2009), Rickards et al. (Reference Rickards, Booth, Paris and Heward2010); Ghavidel-syooki et al. (Reference Ghavidel-Syooki, Popov, Álvaro, Ghobadi Pour, Tolmacheva and Ehsani2014). Left-hand columns are from TimeScale Creator v. 7.4 (https://timescalecreator.org/index/index.php) and are calibrated to the age model in Ogg et al. (Reference Ogg, Ogg and Gradstein2016). Right-hand columns: chitinozoan assemblages (this paper) correlated with standard stage slices and Anglo-Welsh series and stages, based on chitinozoan ranges depicted in this figure and discussed in the text.
6.a. Assemblage 1
Assemblage 1 is restricted to the Login beds and comprises Conochitina decipiens, Lagenochitina brevicollis (Fig. 8s, t), L. conifundus (Fig. 9, o), L. destombesi (Fig. 8a, b) and L. ovoidea (Fig. 9a, b). The species of Lagenochitina have only been recorded from the Login beds in South Wales, whereas C. decipiens ranges at least as high as the basal Pontyfenni Formation (Fig. 6).
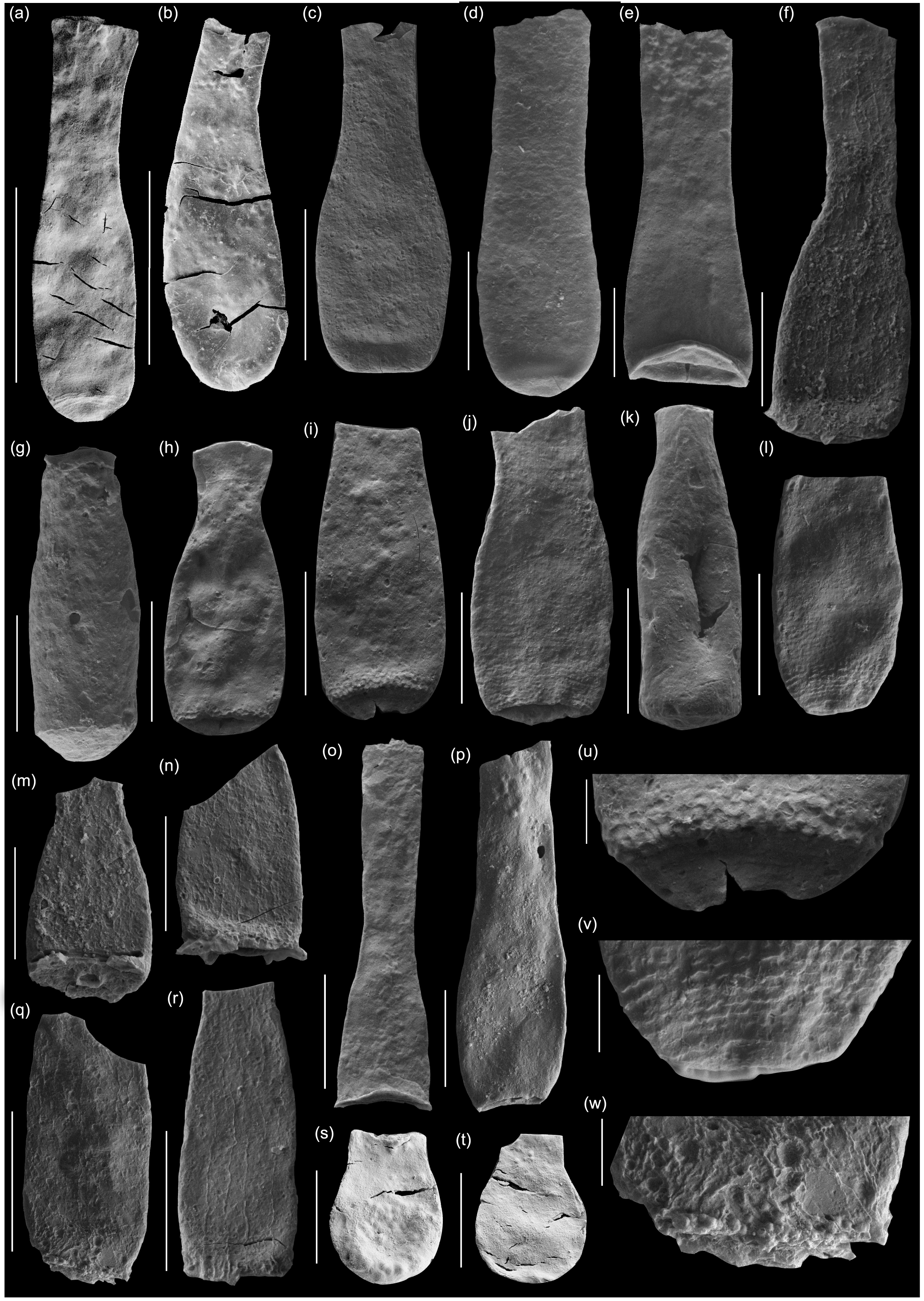
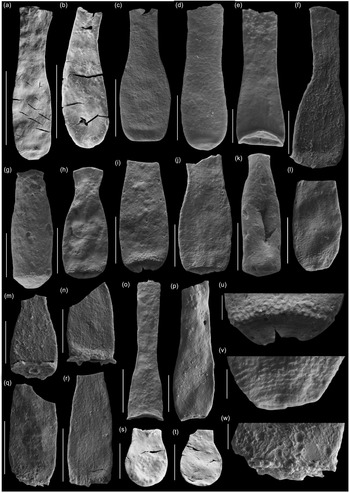
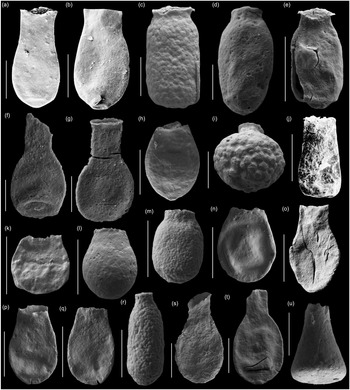
Fig. 8. Scanning electron micrographs of selected chitinozoans with location, formation and sample number. (a, b) Lagenochitina destombesi Elaouad-Debbaj (Heol Login, ‘Login Beds’, MPA 26829/ MPA 26831); (c) Lagenochitina pirum Achab (Pont-y-Fenni Old Quarry, Pont-y-Fenni Fm., TVDB 11-023); (d) Conochitina raymondii (Achab) (Cwm Yr Abbey, Afont Ffinnant Fm., TVDB 11-009); (e) Cyathochitina cf. cycnea (Vandenbroucke, Hennissen & Servais) (Pont Y Fenni Old Quarry, Pont-Y-Fenni Fm., TVDB 11-023); (f, m, n, q, r, w) Laufeldochitina sp. 1 (Cwm Yr Abbey, Carmarthen Fm., TVDB 11-008); (g) Conochitina pseudocarinata Paris (Allt Pen-y-Coed, Carmarthen Fm., TVDB 11-007); (h–j), l, u, v) Conochitina gueddichensis Oulebsir & Paris (Allt Pen-y-Coed, Carmarthen Fm., TVDB 11-007), where (u) is an enlargement of (i), (v) is an enlargement of (l) and (w) is an enlargement of (q); (k) Lagenochitina aff. cylindrica Eisenack (Allt Pen-y-Coed, Carmarthen Fm., CA 13-044); (o) Cyathochitina aff. calix (Eisenack) (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., TVDB 11-022); (p) Laufeldochitina protolardeuxi Soufiane & Achab (Allt Pen-y-Coed, Carmarthen Fm., CA 13-006); (s, t) Lagenochitina brevicollis Taugourdeau & de Jekhowsky (Heol Login, ‘Login Beds’, MPA 26838). Scale bars = 100 µm except for (a), (b), (c), (o), (r) = 200 µm and (u) (v) (w) = 20 µm.

Fig. 9. Scanning electron micrographs of selected chitinozoans with location, formation and sample number. (a, b) Lagenochitina ovoidea Benoit & Taugourdeau (Heol Login, ‘Login Beds’, MPA 26838); (c, m) Desmochitina minor Paris (Allt Pen-y-Coed, Carmarthen Fm., CA 13-014); (d) Desmochitina ovulum (Eisenack) (Allt Pen-y-Coed, Carmarthen Fm., CA 13-012); (e) Desmochitina papilla Grahn (Allt Pen-y-Coed, Carmarthen Fm., CA 13-012); (f, g) Lagenochitina esthonica Eisenack (short forms, Cwm yr Abbey, Afont Ffinnant Fm., TVDB 11-002); (h, i) Desmochitina aff. bulla Taugourdeau & de Jekhowsky (Llanfallteg Old Railwaiy, Llanfallteg Fm., CA 13-015/13-018); (j) Euconochitina fenxiangensis Chen, Paris & Zhang (Allt Pen-y-Coed, Carmarthen Fm., CA 13-042); (k) Desmochitina erinacea? Eisenack (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., CA 13-037); (l) Desmochitina ornensis? Paris (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., CA 13-037); (n) Desmochitina aff. cocca (Eisenack) (Llanfallteg Old Railway, Llanfallteg Fm., CA 13-014); (o) Lagenochitina conifundus (Poumot) (Heol Login, ‘Login Beds’, MPA 26838); (p, q) Bursachitina laminaris Tang, Paris, Geng & Zhu (Pont-Y-Fenni Old Quarry, Pont-Y-Fenni Fm., TVDB 11-024); (r) Desmochitina elongata Eisenack (Allt Pen-y-Coed, Carmarthen Fm., CA 13-008); (s, t) Lagenochitina obeligis Paris (19, Arenig Fawr, Carned Iago Fm., TVDB 12-052; 20, Cwm yr Abbey, Afont Ffinnant Fm., TVDB 11-002); (u) ?Conochitina primitiva Eisenack (Llanfallteg Old Railway, Llanfallteg Fm., CA 13-019). All scale bars = 100 µm.
The species of Lagenochitina all have Tremadocian records or affinities with Tremadocian species. Lagenochitina ovoidea, described from the Ordovician of the Sahara by Benoît and Taugourdeau (Reference Benoît and Taugourdeau1961), has perhaps the longest range, with records from the Dapingian and Darriwilian stages of Baltica (Nõlvak et al. Reference Nõlvak, Liang and Hints2019) as well as the Tremadocian of South China (Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017). The other three Lagenochitina spp. are all zonal indicators for Tremadocian biozones in Gondwana.
Lagenochitina destombesi has its lowest occurrence in the middle Tremadocian of Morocco (Elaouad-Debbaj, Reference Elaouad-Debbaj1988; Paris, Reference Paris1990) and South China (Chen et al. Reference Chen, Paris and Miao2008; Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013), and ranges into the upper Tremadocian Araneograptus murrayi graptolite Biozone in Morocco (Nowak et al. Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016), South China (Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013) and NW England (Amberg et al. Reference Amberg, Vandenbroucke, Molyneux and Servais2017). It gives its name to the Lagenochitina destombesi chitinozoan Biozone of high palaeolatitude Gondwana (Fig. 7), stated in its original description as having a likely late early Tremadoc – early late Tremadoc age (Paris, Reference Paris1990, p. 188).
The destombesi Biozone is succeeded in Paris’s (Reference Paris1990) Gondwanan scheme by the L. conifundus chitinozoan Biozone (previously known as the Amphorachitina conifundus Biozone). Lagenochitina conifundus was depicted as having an upper Tremadocian to lower Floian range by Paris (Reference Paris1990), but the lower Floian record was based on the occurrence of abundant specimens, recorded as Amphorachitina conifundus, in sample 2 of Paris and Mergl (Reference Paris and Mergl1984; Paris, Reference Paris1990) from the lowermost Klabava Formation of the Prague Basin. The locality was reported by Paris and Mergl (Reference Paris and Mergl1984) to be from ‘the lowermost part of the Corymbograptus v. similis Zone; assemblage with Clonograptus’ and was correlated with the Tetragraptus approximatus Graptolite Zone. Fatka (Reference Fatka1993) reported L. conifundus from the same locality (his sample KL-7) but pointed out that an absence of graptolites at the level of his sample precluded direct correlation with the graptolite zonation.
In revisions of the Gondwanan biozonation scheme, the conifundus Biozone has been replaced either wholly (Paris et al. Reference Paris, Le Hérissé, Monod, Kozlu, Ghienne, Dean, Vecoli and Günay2007; Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010) or in part (Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012) by the brevicollis Biozone. Where it replaces the conifundus Biozone entirely, the brevicollis Biozone is correlated with the upper Tremadocian Araneograptus murrayi and Hunnegraptus copiosus graptolite biozones (Paris et al. Reference Paris, Le Hérissé, Monod, Kozlu, Ghienne, Dean, Vecoli and Günay2007; Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010). In other instances (Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012, fig. 20.1), the brevicollis Biozone is correlated with the uppermost Tremadocian Hunnegraptus copiosus Biozone and the conifundus Biozone with the Araneograptus murrayi Biozone (Fig. 7). In both instances, the brevicollis Biozone is overlain by the symmetrica Biozone. Recent revision of the symmetrica Biozone has placed its base below instead of at the base of the Floian Stage (i.e. below the base of the Arenig Series; Nowak et al. Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016; Amberg et al. Reference Amberg, Vandenbroucke, Molyneux and Servais2017; Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017; Achab & Maletz, Reference Achab and Maletz2021) so that the conifundus and brevicollis biozones now lie entirely within the upper Tremadocian Stage (Webby et al. Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004; Paris et al. Reference Paris, Le Hérissé, Monod, Kozlu, Ghienne, Dean, Vecoli and Günay2007; Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010; Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012).
The lowest occurrence of Lagenochitina brevicollis in South China is in the Araneograptus murrayi graptolite Biozone of the Jiangnan Slope (Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013) and the upper Tremadocian part of the Paroistodus proteus conodont Biozone on the Yangtze Platform (Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017). In both cases, correlation of the lowest occurrence with a level in the upper Tremadocian Stage Slice Tr3 is indicated (Fig. 7). The species’ highest occurrence there is in the Acrograptus filiformis graptolite Biozone of the Yangtze Platform (Liang et al. Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018), which correlates with a level in the middle Floian, either in the upper part of Stage Slice Fl1 (Zhang et al. Reference Zhang, Chen and Goldman2007, Reference Zhang, Chen, Goldman, Zhang, Cheng and Song2010; Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013) or in Fl2 (Zhang et al. Reference Zhang, Chen, Goldman, Zhang, Cheng and Song2010).
Lagenochitina brevicollis is possibly also present in the upper Tremadocian of South America (Fig. 7). De la Puente and Rubinstein (Reference de la Puente and Rubinstein2009) recorded a single specimen from the Tremadocian A. murrayi graptolite Biozone of the Parcha Formation, NW Argentina. They also noted that the holotype of Lagenochitina brevicollis Taugourdeau and de Jekhowsky (Reference Taugourdeau and de Jekhowsky1960) was likely to be a specimen of Lagenochitina with a broken neck whereas the paratype displayed characteristics of Desmochitina. Consequently, they referred to their specimen as Desmochitina sp. cf. L. brevicollis. Furthermore, de la Puente and Rubinstein (Reference de la Puente and Rubinstein2009, Reference de la Puente and Rubinstein2013) regarded specimens recorded by Heuse et al. (Reference Heuse, Grahn and Erdtmann1999) as Desmochitina sp. gr. minor from the upper Tremadocian A. murrayi and H. copiosus biozones of south Bolivia to be conspecific with the paratype of L. brevicollis and with their Desmochitina sp. cf. L. brevicollis.
The specimen of Desmochitina sp. cf. L. brevicollis from the Parcha Formation is accompanied by Euconochitina paschaensis, Lagenochitina conifundus and L. cf. longiformis. Wang et al. (Reference Wang, Feng, Vandenbroucke, Li and Verniers2013) suggested that the last species might be equivalent to L. destombesi. Specimens referred to Lagenochitina cf. longiformis also occur in an assemblage from the Leetse Formation, in the proteus conodont Biozone of the Hunneberg Stage of Estonia, close to the Tremadocian–Floian stage boundary (Hints & Nõlvak, Reference Hints and Nõlvak2006). The specimens of L. cf. longiformis from the Leetse Formation are similar to the specimens of L. aff. destombesi from Assemblage 1 and are suggested here to be conspecific.
Conochitina decipiens has widespread lowest occurrences in the Floian Stage (Fig. 7), perhaps as low as the basal Floian approximatus graptolite Biozone in Bolivia (Heuse et al. Reference Heuse, Grahn and Erdtmann1999) and Bohemia (Paris & Mergl, Reference Paris and Mergl1984). Achab and Maletz (Reference Achab and Maletz2021), however, recorded its lowest occurrence in Québec at a level in the symmetrica chitinozoan Biozone that is now placed in the highest Tremadocian. Amberg et al. (Reference Amberg, Vandenbroucke, Molyneux and Servais2017) recorded a similar form as Conochitina aff. decipiens from the murrayi graptolite Biozone in the Tremadocian of NW England (Fig. 7).
The co-occurrences of Conochitina decipiens and Lagenochitina destombesi suggest a latest Tremadocian, Tr3 age for Assemblage 1 (Figs 6, 7), confirming and perhaps further restricting the latest Tremadocian or earliest Floian age indicated for the same beds by acritarchs (Molyneux & Dorning, Reference Molyneux and Dorning1989; Molyneux et al. Reference Molyneux, Raevskaya and Servais2007).
6.b. Assemblage 2
Assemblage 2 occurs in Allt Pen-y-Coed, where it extends from sample CA 13-042 in the Pibwr Member to CA 13-013 in the lower part of the Cwmffrⓦd Member (Fig. 6). Species of Conochitina dominate the faunas. Conochitina decipiens and C. ordinaria are present throughout, and C. gueddichensis (Fig. 8h–j, l, u, v) and C. pseudocarinata (Fig. 8g) occur in all except the lowest sample. Conochitina raymondii (Fig. 8d) occurs more sporadically.
The assemblage is subdivided into a lower Assemblage 2a, comprising the fauna from the lowest sample of the Pibwr Member in Allt Pen-y-Coed (CA 13-042), and a higher Assemblage 2b comprising chitinozoans from the rest of the samples from the Pibwr Member and the lower Cwmffrⓦd Member in the section (Fig. 6). Neither subdivision contains index species of Gondwanan chitinozoan biozones, which precludes direct correlation and introduces some uncertainty regarding their ages.
Assemblage 2a is characterized by the association of Euconochitina fenxiangensis (Fig. 9j) with Conochitina decipiens, C. ordinaria, C. raymondii (Fig. 8d), Lagenochitina aff. cylindrica (Fig. 8k) and Rhabdochitina gracilis. All range into Assemblage 2b, although E. fenxiangensis and L. aff. cylindrica are restricted to its lower part.
Euconochitina fenxiangensis was described by Chen et al. (Reference Chen, Paris and Miao2008) from the upper Fenxiang and lower Honghuayuan formations on the Yangtze Platform of South China. Chen et al. (Reference Chen, Paris and Miao2008) indicated that it ranged from the upper Lagenochitina destombesi Biozone into the Euconochitina symmetrica Biozone and therefore, based on correlations accepted at the time, from upper Tremadocian into lower Floian strata. Liang et al. (Reference Liang, Servais, Tang, Liu and Wang2017), however, recalibrated the symmetrica Biozone on the Yangtze Platform, placing it entirely within the uppermost Tremadocian Stage (Fig. 7). This might be taken to indicate that Euconochitina fenxiangensis is restricted to the Tremadocian Stage in South China, but Chen et al. (Reference Chen, Paris and Miao2008) also noted its coexistence with conodonts of the Paroistodus proteus and Prioniodus elegans biozones, which supports its occurrence in the lower Floian Stage. Subsequent records, however, have been from the upper Tremadocian of South China (Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013; Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017, Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018), including beds of the murrayi graptolite Biozone, and from the upper Tremadocian copiosus graptolite Biozone of NW Argentina (Toro et al. Reference Toro, de la Puente and Rubinstein2010; E. cf. fenxiangensis).
The species of Conochitina in Assemblage 2a all have lower Floian or upper Tremadocian lowest occurrences. That of Conochitina decipiens is noted under discussion of Assemblage 1 above. Conochitina raymondii was described by Achab (Reference Achab1980) from the Lévis Formation of Québec, where its lowest occurrence is in zone A of Raymond (Reference Raymond1914) with Tetragraptus approximatus (Achab & Maletz, Reference Achab and Maletz2021, fig. 2). Chen et al. (Reference Chen, Paris, Wang and Zhang2009) designated a Conochitina raymondii Biozone in the Yichang area of South China at a level that correlates with the Oepikodus communis conodont Biozone. This in turn equates with the lower Floian Stage Slice Fl1 (pre-evae Biozone; Wang et al. Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005, Reference Wang, Stouge, Chen, Li, Wang, Finney, Zeng, Zhou, Chen and Erdtmann2009). Chen et al. (Reference Chen, Paris, Wang and Zhang2009) further noted that the lowest occurrence of C. raymondii was in Floian strata older than their sampled level, indicating an earlier Floian age.
Conochitina ordinaria was also described by Achab (Reference Achab1980) from the Lévis Formation, Québec, but its lowest occurrence there is a little higher than that of C. raymondii, in Zone B of Raymond (Reference Raymond1914) with the graptolites Phyllograptus typus, Tetragraptus quadribrachiatus and Dichograptus octobrachiatus. Raymond’s (Reference Raymond1914) Zone B has been correlated with the Tshallograptus fruticosus graptolite Biozone (Achab & Maletz, Reference Achab and Maletz2021, fig. 2) in the middle Floian Stage. The lowest occurrence of Conochitina ordinaria in the Yichang area of South China is at about the base of the Lagenochitina lata Sub-biozone of the Clavachitina langei chitinozoan Biozone. Conodonts of the lower Oepikodus evae Biozone and graptolites of the Didymograptus [Didymograptellus] bifidus Biozone provide independent evidence for a middle to late Floian age (Chen et al. Reference Chen, Paris, Wang and Zhang2009; Fig. 7. See Toro & Herrera Sánchez (Reference Toro and Herrera Sánchez2019), for correlation of the D. bifidus Biozone with Stage Slice Fl3 and with the eobifidus, deflexus and probably the lower part of the suecicus biozones in South China).
Of the other two forms included in Assemblage 2a, Lagenochitina aff. cylindrica has affinities with a species that ranges in South China from the Euconochitina symmetrica chitinozoan Biozone (Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017) into the Sagenachitina dapingensis chitinozoan Biozone (Chen et al. Reference Chen, Paris, Wang and Zhang2009), respectively of latest Tremadocian and Dapingian age. Rhabdochitina gracilis is a long-ranging and widespread Ordovician species that has lowest occurrences in the upper Tremadocian of Morocco (Nowak et al. Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016) and close to the Tremadocian–Floian boundary in the Paraistodus proteus conodont Biozone and Hunneberg Regional Stage of Estonia (Hints & Nõlvak, Reference Hints and Nõlvak2006), but ranges into the Upper Ordovician (e.g. Grahn & Nõlvak, Reference Grahn and Nõlvak2007).
The base of Assemblage 2b is marked by the lowest occurrences of Conochitina gueddichensis and C. pseudocarinata (Fig. 6). Desmochitina ovulum and Laufeldochitina sp. 1 (Fig. 8f, m, n, q, r, w) have lowest occurrences midway through the interval (Fig. 6). Paris (Reference Paris1981) described Conochitina pseudocarinata from the Armorican Massif, where it occurs in the same samples as Desmochitina ornensis and is one of the index species of his middle Arenig Desmochitina ornensis – Conochitina pseudocarinata Biozone. The latter biozone was not included in Paris’s (Reference Paris1990) scheme, but C. pseudocarinata is listed as one of the associated species of his Desmochitina ornensis Biozone (Fig. 7).
In South China, Conochitina pseudocarinata gives its name to the Conochitina pseudocarinata Biozone of Wang et al. (Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005, Reference Wang, Stouge, Chen, Li, Wang, Finney, Zeng, Zhou, Chen and Erdtmann2009) and the Conochitina pseudocarinata Sub-biozone of Chen et al. (Reference Chen, Paris, Wang and Zhang2009). In both instances, the base of the unit, defined by the lowest occurrence of C. pseudocarinata, is in the upper part of the Oepikidus evae conodont Biozone and the lower part of the Azygograptus suecicus graptolite Biozone. These correlations indicate that the lowest occurrence of C. pseudocarinata in South China is in the upper part of the Floian Stage (Stage Slice Fl3; Fig. 7). Zhang et al. (Reference Zhang, Chen, Goldman, Zhang, Cheng and Song2010, Fig. 2) correlated the base of the suecicus Biozone with the base of the Australian Castlemainian Stage, which in turn lies in the upper part of Stage Slice Fl3 (Bergström et al. Reference Bergström, Chen, Gutiérrez-Marco and Dronov2009; Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012, fig. 20.9). Records of the species from Belgium (Samuelsson & Verniers, Reference Samuelsson and Verniers2000; Herbosch & Verniers, Reference Herbosch and Verniers2014) and NW France (Paris, Reference Paris1981, Reference Paris1990) are also from the ‘middle’ Arenig or higher.
Conochitina gueddichensis and Desmochitina ovulum support a later Floian age for Assemblage 2b. Conochitina gueddichensis was described by Oulebsir and Paris (Reference Oulebsir and Paris1993) from the Eremochitina brevis chitinozoan Biozone of Algeria, which is correlated with the upper Floian Stage, equivalent to the upper part of Stage Slice Fl2 and most of Fl3 (Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012, figs 20.1, 20.9) (Fig. 7). Desmochitina ovulum is generally found in deposits of Darriwilian and younger age (Paris, Reference Paris1981; Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Oulebsir & Paris, Reference Oulebsir and Paris1995; Tammekand et al. Reference Tammekänd, Hints and Nõlvak2010; Nõlvak et al. Reference Nõlvak, Liang and Hints2019), but was recorded by Liang et al. (Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018) from the lower Azygograptus suecicus graptolite Biozone in the upper Floian Stage (Stage Slice Fl3) of South China.
It seems unlikely that Assemblage 2b is much older than Fl3 given the First Appearance Datums (FADs) of Conochitina gueddichensis, C. pseudocarinata and Desmochitina ovulum (Fig. 7). The possibility then is that Assemblage 2a is not much older than Assemblage 2b, either Fl3 or possibly Fl2. This suggestion is supported by the continuity of lithostratigraphy and lithofacies between assemblages 2a and 2b and records of the graptolites Phyllograptus cf. densus and Pseudophyllograptus aff. angustifolius from lower in the Pibwr Member at Glan Pibwr (Fortey & Owens, Reference Fortey and Owens1978; Owens, Reference Owens, Rushton, Owen, Owens and Prigmore1999). Pseudophyllograptus angustifolius has been reported in England and Wales from the middle Floian jacksoni graptolite Biozone to the middle Darriwilian artus graptolite Biozone, and Phyllograptus densus only from the Dapingian victoriae graptolite Biozone (Zalasiewicz et al. Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009). Webby et al. (Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004) correlated the Phyllograptus densus and Pseudophyllograptus angustifolius elongatus biozones of Baltoscandia with their Time Slice 2c, which correlates in turn with the upper Floian Stage. Against this, the occurrence of Euconochitina fenxiangensis in Assemblage 2a and its co-occurrence with C. pseudocarinata and C. gueddichensis in the lower part of Assemblage 2b introduces some uncertainty, given that E. fenxiangensis has not been recorded with confidence from such a high level in the Floian Stage. Nevertheless, a middle to late Floian, Fl2–Fl3 age is suggested here for Assemblage 2a, albeit with a degree of uncertainty, and an Fl3 age for Assemblage 2b (Figs 6, 7). The suggestion of a middle to late Floian age for Assemblage 2a implies the possibility of significant hiatuses lower in the succession. Either the Ogof Hên Formation covers a significant amount of time (Fl1–Fl2?), or there are breaks at the base, top and/or within that formation.
6.c. Assemblage 3
Assemblage 3 covers much of the middle Arenig succession in South Wales, from the upper Moridunian Stage to the basal Fennian Stage, and is subdivided into assemblages 3a, 3b and 3c by successive lowest occurrences of chitinozoan species (Fig. 6). Assemblages 3a and 3b are found in the Carmarthen area and Assemblage 3c in the Whitland area.
Assemblage 3a is restricted to four samples from the upper part of the Cwmffrⓦd Member in Allt Pen-y-Coed and Cwm yr Abbey (Fig. 6). It is characterized by a change from conochitinid to desmochitinid-dominated faunas, with the lowest occurrences of Desmochitina elongata (Fig. 9r), D. minor (Fig. 9c, m) and D. papilla (Fig. 9e) at its base. Desmochitina ovulum ranges up from Assemblage 2b and is relatively common in the lowest two samples. Conochitina decipiens, C. ordinaria, C. raymondii and Laufeldochitina sp. 1 also range up into Assemblage 3a, but the species of Conochitina only occur in the highest two samples and are the only forms recorded in those samples.
The records of Desmochitina ovulum documented above indicate an Fl3 age or younger for Assemblage 3a. There is nothing in the assemblage to limit it to the upper Floian, but it is most likely to correlate with the Fl3 Stage Slice (Figs 6, 7) given the ages suggested below for assemblages higher in the South Wales succession. Of the other desmochitinids in the assemblage, records of D. elongata are from the Darriwilian and Sandbian stages (Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Tammekand et al. Reference Tammekänd, Hints and Nõlvak2010; Nõlvak et al. Reference Nõlvak, Liang and Hints2019), and Desmochitina papilla was described by Grahn (Reference Grahn1984) from Estonia where its lowest occurrence is at the base of the Vaana Substage in the middle of the regional Volkhov Stage. This in turn suggests correlation with a level in the lower Dapingian Stage (Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012, fig. 20.9). In South China, the lowest occurrence of D. papilla is in the Didymograptellus bifidus graptolite Biozone and the lower part of the Oepikidus evae conodont Biozone (Wang et al. Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005), and therefore in the upper Floian Stage (Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012).
Assemblage 3b covers the rest of the sampled succession in the Carmarthen district, from the upper Cwmffrⓦd Member into the basal Afon Ffinnant Formation in Allt Pen-y-Coed and Cwm yr Abbey (Fig. 6). Its base is marked by the lowest occurrences of Belonechitina micracantha, Lagenochitina obeligis (Fig. 9s, t), Laufeldochitina protolardeuxi (Fig. 8p) and Rhabdochitina magna (Fig. 10l). Conochitina decipiens (Fig. 10g, h), C. ordinaria, Desmochitina ovulum, Laufeldochitina sp. 1 and Rhabdochitina gracilis are also present in the lower part of the assemblage, some ranging through. Short forms of Lagenochitina esthonica (Fig. 9f, g) are consistently present at the top of the succession in samples collected across the contact of the Carmarthen and Afon Ffinnant formations.

Fig. 10. Scanning electron micrographs of selected chitinozoans with location, formation and sample number. (a, b) Conochitina cf. redouanei Oulebsir & Paris (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., TVDB 11-022); (c) d) Belonechitina henryi Paris (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., TVDB 11-022); (e) Cyathochitina touggourtensis Oulebsir & Paris (Pont-y-Fenny Old Quarry, Pont-y-Fenny Fm., CA 13-038); (f, q, s) Cyathochitina protocalix? Paris (Llanfallteg Old Railwaiy, Llanfallteg Fm., (f, q) CA 13-017, (s) CA 13-031); (g, h) Conochitina decipiens Taugourdeau & de Jekhowsky, (g) Allt Pen-y-Coed, Carmarthen Fm., CA 13-045, (h) Arenig Fawr, Carned Igo Fm., TVDB 12-052); (i, j) Conochitina cf. havliceki Paris & Mergl (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., CA 13-038); (k) Conochitina cucumis Grahn (Cwm Banau, Cwmfelin Boeth Fm., CA 13-051); (l) Rhabdochitina magna Eisenack (Cwm Yr Abbey, Carmarthen Fm., CA 13-006); (m) Lagenochitina maxima Taugourdeau & de Jekhowsky (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., TVDB 11-023); (n, r) Tanuchitina granbyensis Grahn, Nõlvak & Paris (Pont-y-Fenni Old Quarry, Pont-Y-Fenni Fm., TVDB 11-024); (o, p): Tanuchitina achabae? Paris (Llanfallteg Old Railwaiy, Llanfallteg Fm., CA 13-018); (t, u) Tanuchitina domfrontensis Paris (Llanfallteg Old Railwaiy, Llanfallteg Fm., CA 13-004). Scale bars = 100 µm except (i), (j), (l) = 200 µm and (m) = 300 µm.
Of the four species with lowest occurrences at the base of Assemblage 3b, Lagenochitina obeligis occurs in all samples and is particularly common in those from the upper part of the Cwm yr Abbey Member and the Afon Ffinnant Formation in Cwm yr Abbey. It is a characteristic species of Assemblage 3b but is nevertheless long-ranging. It was described by Paris (Reference Paris1981) from Brittany and depicted by Paris (Reference Paris1990) as ranging from the middle Arenig Eremochitina brevis Biozone into the Llanvirn Series in southern Gondwanan terranes. Other records are from the Tremadocian Stage of South China (Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013; Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017, Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018) and the Darriwilian Stage in South America (Grahn Reference Grahn2006), Belgium (Herbosch & Verniers, Reference Herbosch and Verniers2014) and Oman (Sansom et al. Reference Sansom, Miller, Heward, Davies, Booth, Fortey and Paris2009; Heward et al. Reference Heward, Booth, Fortey, Miller and Sansom2018), with similar forms (L. cf. obeligis) recorded from the upper Tremadocian of Morocco (Nowak et al. Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016) and NW England (Amberg et al. Reference Amberg, Vandenbroucke, Molyneux and Servais2017) and the Darriwilian Stage in Iran (Ghavidel-syooki et al. Reference Ghavidel-Syooki, Popov, Álvaro, Ghobadi Pour, Tolmacheva and Ehsani2014).
Laufeldochitina protolardeuxi, only recorded from the lowest sample of Assemblage 3b, was originally described from the middle Arenig of Morocco (Soufiane & Achab, Reference Soufiane and Achab1993), and Rhabdochitina magna was reported as an associated species of the lower–middle Arenig Eremochitina baculata Biozone (Fl1–Fl2; Fig. 7) of Gondwana (Paris, Reference Paris1990). Other records of R. magna, however, are from the Dapingian (Liang et al. Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018), Darriwilian (Jenkins, Reference Jenkins1967; Grahn et al. Reference Grahn, Nõlvak and Paris1996; Rickards et al. Reference Rickards, Booth, Paris and Heward2010; Tammekand et al. Reference Tammekänd, Hints and Nõlvak2010; Wang et al. Reference Wang, Zhao, Muir, Li and Tan2018; Nõlvak et al. Reference Nõlvak, Liang and Hints2019) and higher stages (Paris, Reference Paris1990; Oulebsir & Paris, Reference Oulebsir and Paris1995; Vandenbroucke, Reference Vandenbroucke2008 a, b). Similar forms have been reported as Rhabdochitina cf. magna from the middle Floian of Argentina (de la Puente & Rubinstein, Reference de la Puente and Rubinstein2013) and the Tremadocian of Morocco (Nowak et al. Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016).
Other species from Assemblage 3b are long-ranging, but Belonechitina micracantha corroborates the evidence of Desmochitina ovulum to indicate that this part of the succession is not older than late Floian. Belonechitina micracantha is only present in the lowest sample from Assemblage 3b but reoccurs higher in the South Wales succession. Elsewhere, its lowest occurrence is in the lower Azygograptus suecicus graptolite Biozone of the upper Floian Stage (upper Fl3) in South China (Wang, X. et al. Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005; Liang et al. Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018; Fig. 7). As with Assemblage 3a, there is nothing to limit the assemblage to the late Floian, but the base of the Dapingian Stage is placed at a higher level in the Whitland area. Assemblage 3b is correlated accordingly with the Fl3 Stage Slice (Figs 6, 7).
Assemblage 3c comprises chitinozoans from the Castelldraenog Member of the Colomendy Formation, the Cwmfelin Boeth Formation and the basal Pontyfenni Formation in the Whitland area, and therefore spans the Whitlandian–Fennian stage boundary (Fig. 6). The lowest sample, from the Castelldraenog Member, yielded only three species, Conochitina cucumis (Fig. 10k), Conochitina decipiens and Lagenochitina obeligis, and the base of the assemblage is marked by the lowest occurrence of C. cucumis.
Conochitina cucumis is the eponymous species of the cucumis Biozone of Baltoscandia (Nõlvak & Grahn, Reference Nõlvak and Grahn1993). The base of the biozone was originally placed in the upper Volkhov Stage of Baltoscandia, at a level that correlates approximately with the basal Darriwilian Stage (Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Webby et al. Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004). Nõlvak et al. (Reference Nõlvak, Liang and Hints2019), however, repositioned its base to a lower level, within the Dapingian Stage. Furthermore, a form from the lower unit of the Dawan Formation in the Huanghuachang section of South China, designated as Conochitina cf. cucumis by Chen et al. (Reference Chen, Paris, Wang and Zhang2009), has its lowest occurrence in the upper Floian, Stage Slice Fl3, in the lower Azygograptus suecicus graptolite Biozone and upper Oepikodus evae conodont Biozone (cf. sample positions in Wang et al. Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005, fig. 8; 2009, fig. 5).
Assemblage 3c is a low-diversity assemblage. Conochitina decipiens occurs in all samples but was not recorded from any of the overlying assemblages. Belonechitina micracantha, Desmochitina ovulum, Rhabdochitina magna and Lagenochitina obeligis range through Assemblage 3c, occurring in one or more samples, but the most diverse microfauna with all five of these species plus Bursachitina laminaris, relatively common Conochitina cucumis and Desmochitina minor is from the Cwmfelin Boeth Formation (sample CA 13-051). The lowest occurrence of Bursachitina laminaris (Fig. 9p, q) is at this level.
Bursachitina laminaris was described by Tang et al. (Reference Tang, Paris, Geng and Zhu2007) from South China and is shown as occurring in only one sample from the lower Darriwilian Stage on their range charts (austrodentatus Biozone, Dianbatou section: Tang et al. Reference Tang, Paris, Geng and Zhu2007, fig. 5). It was reported in the text of their paper, however, as occurring in the ‘3rd Stage’ (i.e. Dapingian) and lower Darriwilian Stage, and as ranging from the Azygograptus suecicus graptolite Biozone to the Undulograptus austrodentatus graptolite Biozone. As the suecicus Biozone spans the Floian–Dapingian boundary in South China (Zhang et al. Reference Zhang, Chen and Goldman2007, Reference Zhang, Chen, Goldman, Zhang, Cheng and Song2010), it follows that the lowest occurrence of Bursachitina laminaris there is likely to be close to the base of the Dapingian Stage (Fig. 7).
Based on their previous records, the occurrences of Bursachitina laminaris and Conochitina cucumis in Assemblage 3c are taken here to indicate a level close to the Floian–Dapingian stage boundary. Possible positions for the base of the Dapingian Stage are at (1) the lowest occurrence of C. cucumis and therefore correlating with a level in the Whitlandian Stage (Fig. 6); (2) the lowest occurrence of B. laminaris and therefore within the lowest Fennian Stage; or (3) within the assemblage, somewhat arbitrarily at a level that coincides with the base of the Fennian Stage. There is no conclusive evidence to favour one of these options over the others, but the lowest occurrence of C. cucumis and the base of Assemblage 3c is adopted here. Assemblage 3c is thus provisionally interpreted as being early Dapingian in age (Figs 6, 7). This position also suggests correlation of Assemblage 3c, at least in part, with the Gondwanan Desmochitina ornensis Biozone (Fig. 7), following the correlations depicted by Webby et al. (Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004) and Cooper and Sadler (Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012), although the index species is not present at this level in South Wales.
6.d. Assemblage 4
There is a significant change in the upper part of the succession that distinguishes assemblages 4–6 from assemblages 2 to 3. It coincides with division of the dendrogram into two high-level clusters (Fig. 6), the change taking place within the Pontyfenni Formation. Chitinozoan faunas from assemblages 4–6 contain Gondwanan index species, and these are accompanied by graptolites to aid correlation. The dating and the correlation of assemblages 4–6 are consequently more secure.
Assemblage 4 corresponds to the chitinozoan fauna from the Pontyfenni Formation at Pont-y-Fenni. Its base is marked by the lowest occurrence of Cyathochitina cf. cycnea (Fig. 8e) Vandenbroucke et al. (Reference Vandenbroucke, Hennissen and Servais2013), which ranges through but is restricted to the assemblage. It also contains Desmochitina ornensis? (Fig. 9l) and Belonechitina henryi (Fig. 10c, d), both of which are Gondwanan index species, and is subdivided into assemblages 4a and 4b at the lowest occurrence of the latter. Cyathochitina cycnea is a replacement name in Vandenbroucke et al. (Reference Vandenbroucke, Hennissen and Servais2013) for Cyathochitina giraffa Hennissen et al. (Reference Hennissen, Vandenbroucke, Chen, Tang and Verniers2010), described from the Darriwilian–Sandbian section at Dawangou in the Tarim Basin of NW China, but a junior homonym of Cyathochitina giraffa Grahn and Nõlvak (Reference Grahn and Nõlvak2010) from the Upper Ordovician (Sandbian) of Sweden.
Assemblage 4a is based on the two samples considered to be the lowest from the section at Pont-y-Fenni (CA 13-036, CA 13-037). Sample CA 13-036 yielded only Bursachitina laminaris, Cyathochitina cf. cycnea and Desmochitina ovulum (Fig. 9d). The second sample also has Bursachitina laminaris, accompanied by Conochitina cf. havliceki (Fig. 10i, j), Desmochitina erinacea? (Fig. 9k), D. ornensis? (Fig. 9l), relatively common D. aff. cocca (Fig. 9n) and Rhabdochitina magna.
The characteristic rugged, scaly wall of D. ornensis was rarely observed on specimens from South Wales, hence the question over the identification. Nevertheless, the specimens from South Wales are similar to D. ornensis in size and morphology, and the difference in wall ornamentation could be the effect of alteration. Despite the uncertain identification, the occurrence of D. ornensis? suggests correlation of Assemblage 4a with the Gondwanan Desmochitina ornensis Biozone (Paris, Reference Paris1990; Fig. 7). This biozone is correlated in turn with the highest Floian and lower Dapingian stages in some schemes (Webby et al. Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004); Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012), although Videt et al. (Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010) showed a higher correlation with stage slices Dp2 and Dp3. Interpretation of the age of Assemblage 3c as early Dapingian places Assemblage 4a within the Dapingian Stage, and in Stage Slice Dp1 following the correlations of Cooper & Sadler (Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012) and Webby et al. (Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004), or possibly Dp2 after Videt et al. (Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010).
Associated species from the ornensis Biozone of Gondwana are Conochitina pseudocarinata, Lagenochitina obeligis, Sagenachitina oblonga, Tanuchitina achabae and Velatachitina veligera. None of these occur in Assemblage 4a, although C. pseudocarinata, L. obeligis and T. achabae are all present at other levels in South Wales.
Of the other chitinozoan species present in Assemblage 4a, Bursachitina laminaris and Rhabdochitina magna range through. Conochitina havliceki was described by Paris & Mergl (Reference Paris and Mergl1984) from the upper Arenig Tetragraptus cf. pseudobigsbyi graptolite Biozone of the Prague Basin, Bohemia. Specimens from South Wales do not have a foveolated surface, as originally described by Paris and Mergl (Reference Paris and Mergl1984) and are here designated C. cf. havliceki. Specimens similarly designated as Conochitina cf. havliceki from South America, but not necessarily the same as those from South Wales, range from the lower Floian Tetragraptus phyllograptoides graptolite Biozone (Heuse et al. Reference Heuse, Grahn and Erdtmann1999) into the lower–middle Floian Conochitina decipiens Interval Zone of Grahn (Reference Grahn2006) and possibly into the lower Darriwilian dentatus graptolite Biozone (Grahn, Reference Grahn2006, fig. 2). Desmochitina erinacea has Darriwilian and Sandbian records from Baltoscandia (Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Tammekand et al. Reference Tammekänd, Hints and Nõlvak2010; Nõlvak et al. Reference Nõlvak, Liang and Hints2019) and South China (Tang et al. Reference Tang, Paris, Geng and Zhu2007). The other species of Desmochitina are long-ranging, from at least the Floian Stage to the Darriwilian Stage, and higher in the case of D. cocca (Paris et al. Reference Paris, Le Hérissé, Monod, Kozlu, Ghienne, Dean, Vecoli and Günay2007; Rickards et al. Reference Rickards, Booth, Paris and Heward2010; Tammekand et al. Reference Tammekänd, Hints and Nõlvak2010; Liang et al. Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018).
Assemblage 4b is defined by the total range of Belonechitina henryi and is the most diverse chitinozoan assemblage recorded from the Arenig Series of South Wales. In South China, the base of the Dapingian Stage was correlated with the base of the Belonechitina cf. henryi Biozone by Wang et al. (Reference Wang, Stouge, Chen, Li, Wang, Finney, Zeng, Zhou, Chen and Erdtmann2009), but the FAD of the species is usually taken to be above the base of the stage. The Belonechitina henryi chitinozoan Biozone of Gondwana (Paris, Reference Paris1990) thus succeeds the ornensis Biozone in the Dapingian Stage (Fig. 7), but there are differences of correlation between different schemes. The base of the henryi Biozone was placed in Stage Slice Dp1 by Cooper and Sadler (Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012) and its top in Stage Slice Dp3. In contrast, Videt et al. (Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010) placed the base of the henryi Biozone in Stage Slice Dp3 and its top in the lower Darriwilian Dw1 Stage Slice.
Other species restricted to Assemblage 4b include Conochitina redouanei (Fig. 10a, b), Cyathochitina aff. calix (Fig. 8o), Cy. touggourtensis (Fig. 10e), Lagenochitina lata, L. maxima (Fig. 10m), L. pirum (Fig. 8c) and Tanuchitina aff. granbyensis (Fig. 10n, r). Cyathochitina calix and Lagenochitina pirum have lowest occurrences in the Dapingian Stage elsewhere. Cyathochitina calix, for example, has lowest occurrences in the Dapingian Stage of Baltoscandia and South China (Grahn, Reference Grahn1984; Liang et al. Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018; Nõlvak et al. Reference Nõlvak, Liang and Hints2019) and has been recorded from the Darriwilian Stage of Baltoscandia, Avalonia and South China (Jenkins, Reference Jenkins1967; Grahn, Reference Grahn1984; Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Grahn & Nõlvak, Reference Grahn and Nõlvak2007; Tammekand et al. Reference Tammekänd, Hints and Nõlvak2010; Herbosch & Verniers, Reference Herbosch and Verniers2014; Liang et al. Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018; Nõlvak et al. Reference Nõlvak, Liang and Hints2019). It is the index species for the early Darriwilian Cyathochitina calix Biozone (Paris, Reference Paris1990; Webby et al. Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004; Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012). Lagenochitina pirum has its lowest occurrence in the upper Dapingian Stage of South China (Brocke et al. Reference Brocke, Li and Wang2000; Chen et al. Reference Chen, Paris, Wang and Zhang2009), and has been recorded from the Darriwilian Stage of South China (Chen et al. Reference Chen, Paris, Wang and Zhang2009), Qaidam (Wang et al. Reference Wang, Zhao, Muir, Li and Tan2018, including forms recorded as ‘cf.’), Australia (Quintavalle & Playford, Reference Quintavalle and Playford2006) and eastern Laurentia (Achab, Reference Achab1989). In the last of these, Lagenochitina pirum is the index species of the Darriwilian C. pirum Biozone (Achab, Reference Achab1989). L. maxima is very similar to Conochitina ulsti sp. nov. described by Nõlvak et al. (2022) from the Kunda Stage (lower Darriwillian) in central Latvia.
The other forms restricted to Assemblage 4b have so far only been recorded lower or higher in the Ordovician succession. Conochitina redouanei and Cyathochitina touggourtensis were both described by Oulebsir and Paris (Reference Oulebsir and Paris1993) from the Eremochitina brevis Biozone (upper Floian) of Algeria; Lagenochitina lata has been recorded from the Tremadocian of Baltoscandia (Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017; Nõlvak et al. Reference Nõlvak, Liang and Hints2019), and the Tremadocian and Floian of South China (Wang et al. Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005; Chen et al. Reference Chen, Paris, Wang and Zhang2009; Liang et al. Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018); and Tanuchitina granbyensis has been described and recorded from the Darriwilian Stage of Sweden (Grahn et al. Reference Grahn, Nõlvak and Paris1996) and Latvia (Nõlvak et al. Reference Nõlvak, Liang and Hints2021). There are no complete specimens of T. granbyensis in the Welsh assemblage, but the largest specimen measures more than 600 µm and the specimens have the typical ovoid apex with a carina that corresponds to the description given by Grahn et al. (Reference Grahn, Nõlvak and Paris1996). Conochitina cucumis and Tanuchitina granbyensis highlight increasing affinities between the Welsh and Baltoscandian assemblages during the Dapingian and early Darriwilian, in addition to Cyathochitina calix, although the latter was not found in association with the others in central Latvia (Nõlvak et al. Reference Nõlvak, Liang and Hints2021).
Belonechitina micracantha, Desmochitina aff. cocca, D. minor, D. ovulum, Lagenochitina obeligis, Rhabdochitina gracilis and R. magna all range through the assemblage. Bursachitina laminaris, Conochitina cf. havliceki, Cyathochitina cf. cycnea, Desmochitina erinacea? and D. ornensis? range into and have their local highest occurrences in Assemblage 4b.
The relatively common occurrence of Belonechitina henryi throughout the Pont-y-Fenni section is taken to indicate correlation of Assemblage 4b with the henryi Biozone and therefore with a level that is probably in the upper part of the Dapingian Stage, perhaps Dp2–Dp3, or the basal Darriwilian Stage (Dw1). This correlation is corroborated and perhaps further restricted by graptolites from the Pontyfenni Formation at Pont-y-Fenni, which are reported to indicate the Undulograptus sinicus Subzone of the basal Darriwilian U. austrodentatus Biozone (Owens, Reference Owens, Rushton, Owen, Owens and Prigmore1999).
6.e. Assemblage 5
Assemblage 5 straddles the Pontyfenni–Llanfallteg formation boundary in the lower part of the Llanfallteg section and comprises samples CA 13-014 to CA 13-019 (Fig. 6). Its base is marked by the lowest occurrence of Desmochitina aff. bulla (Fig. 9h, i), which is present in all samples and relatively common in three (CA 13-016 to CA 13-018). The lowest occurrences of Cyathochitina protocalix? (Fig. 10f, q, s) and Tanuchitina achabae? (Fig. 10o, p) in sample CA 13-017, midway through Assemblage 5 and low in the Llanfallteg Formation, are used to subdivide the assemblage into 5a and 5b.
Assemblage 5a is a low-diversity assemblage in the lower three samples (CA 13-014–CA 13-016). It consists mainly of Desmochitina spp., with D. aff. cocca, D. minor and D. ovulum in addition to D. aff. bulla. Belonechitina micracantha is the only other species present. All occur in all three samples.
Desmochitina bulla is the index species of the bulla Biozone (Paris, Reference Paris1990), which succeeds the henryi Biozone in the Gondwanan Arenig biozonal scheme and is placed in the lower Darriwilian Stage Slice Dw1 (Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010; Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012; Fig. 7). Desmochitina aff. bulla differs from D. bulla in having a wider opening and a less sub-spherical shape. Nevertheless, correlation of Assemblage 5a with Stage Slice Dw1 is consistent with previous correlations of the highest part of the Anglo-Welsh Arenig Series with the lower Darriwilian Stage.
Cyathochitina protocalix was described by Paris (Reference Paris1981) from the Armorican Massif, where it was shown as ranging across the Arenig–Llanvirn series boundary and was described as a good marker for the boundary interval between the two series. It is the marker species for the protocalix Biozone of Paris (Reference Paris1990), which was regarded as the lowest Llanvirn biozone. More recently published schemes, however, have placed the protocalix Biozone in the upper Arenig (Webby et al. Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004) or have shown it spanning the Arenig–Llanvirn series boundary (Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010; Cooper & Sadler, Reference Cooper, Sadler, Gradstein, Ogg, Scmitz and Ogg2012). As the base of the Llanvirn Series is placed higher in the succession, a Dw1 age is indicated here for Assemblage 5b.
Cyathochitina protocalix was not positively identified in Assemblage 5b due to preservation, but specimens referred to as Cyathochitina protocalix? nevertheless display the restriction just above the base of the vesicle that is typical of the species (Fig. 10f, q, s). Tang et al. (Reference Tang, Paris, Geng and Zhu2007) described a similar form from the lower Darriwilian (Dw1) of South China, but as there were only three poorly preserved specimens and they were significantly smaller than those from the Armorican Massif (France), they were kept in open nomenclature as Hyalochitina cf. protocalix.
Tanuchitina achabae was described by Paris (Reference Paris1981) from the base of the Pissot Formation in the Armorican Massif as an accessory species of the lower Dapingian Desmochitina ornensis Biozone, to which it was restricted (Paris, Reference Paris1990, fig. 3). Higher in the Pissot Formation, however, Paris (Reference Paris1981) described a second form as Tanuchitina sp. aff. achabae, with a longer carina and ranging from the Belonechitina henryi Biozone into the Cyathochitina protocalix Biozone. As no entire specimen was found in the Welsh material, we cannot determine which form is the closest.
6.f. Assemblage 6
Assemblage 6 was identified as one of the significant clusters on the dendrogram (Fig. 6), but its base is not marked by any lowest occurrences. It comprises ten samples in the upper part of the Llanfallteg Formation, from CA 13-024 at the base to CA 13-005 at the top. Most species range up from underlying assemblages. Belonechitina micracantha, Cyathochitina protocalix?, Desmochitina aff. cocca, Rhabdochitina gracilis and R. magna range through all or most of the assemblage; Conochitina primitiva (Fig. 9u), Cyathochitina campanulaeformis, Desmochitina aff. bulla and Tanuchitina achabae? have highest occurrences in the lower part of the assemblage; and Lagenochitina obeligis returns in sample CA 13-001, above its absence from Assemblage 5. The only lowest occurrence within Assemblage 6 is that of Tanuchitina domfrontensis (Fig. 10t, u), which is present in samples CA 13-033 and CA 13-035 in the lower half of the assemblage and again in the top two samples (CA 13-004, CA 13-005) where it is relatively common.
Tanuchitina domfrontensis was described by Paris (Reference Paris1981) from the Pissot Formation of the Armorican Massif, where its lowest occurrence is in the protocalix Biozone at a level that coincides with the lowest occurrence of pendant graptoloids (Paris, Reference Paris1981, fig. 7). This led Paris (Reference Paris1981) to observe that the FAD of Tanuchitina domfrontensis might provide a useful criterion for the position of the Arenig–Llanvirn boundary in other successions, notably in the Cacemes Group of Portugal, from which it was also recorded. The successive lowest occurrences of Cyathochitina protocalix and Tanuchitina domfrontensis in the Pissot Formation further led Paris (Reference Paris1981) to propose a subdivision of the Cyathochitina protocalix Biozone into a lower subzone of Rhabdochitina ?gracilis – Cyathochitina protocalix and an upper subzone of Cyathochitina protocalix – Tanuchitina domfrontensis.
Tanuchitina domfrontensis is more bulbous than T. achabae and smaller. Very few complete specimens were recovered from Wales, but based on measurement and morphology, both T. achabae? and T. domfrontensis are present in Assemblage 6, albeit not continuously and their ranges do not overlap. Tanuchitina achabae? occurs in one sample at the base of Assemblage 6 (Fig. 6). Tanuchitina domfrontensis occurs higher in the Llanfallteg Formation, from a few metres below the Arenig–Llanvirn boundary as defined by Fortey and Owens (Reference Fortey and Owens1987) on graptolite evidence. Consequently, either the base of the Llanvirn Series is slightly lower than previously located in the section, or the range of T. domfrontensis straddles the Arenig–Llanvirn boundary rather than its FAD pinpointing the boundary. Either way, Assemblage 6 spans the boundary between the Arenig and Llanvirn series and between stage slices Dw1 and Dw2 (Figs 6, 7).
6.g. Sample CA 13-054
Sample CA 13-054 was collected north of Castell Draenog [c. SN 208 217] at a locality mapped as the Abergwilli Formation of Abereiddian (early Llanvirn) age (British Geological Survey, 2010; Burt et al. Reference Burt, Aspden, Davies, Hall, Schofield, Sheppard, Waters, Wilby and Williams2012). It yielded Bursachitina laminaris, Conochitina decipiens, Desmochitina ornensis?, D. aff. cocca, D. minor and Lagenochitina obeligis. Other samples in which Bursachitina laminaris and Desmochitina ornensis? occur together are from the top of Assemblage 4a and the base of Assemblage 4b in the Pontyfenni Formation (Fig. 6). Most of the other species range through that interval. If the beds at the locality are from the Llanvirn Series, it implies a recurrence at a higher stratigraphic level of a chitinozoan assemblage associated with the Pontyfenni Formation. An alternative explanation is that the sample is from an outcrop of the Pontyfenni Formation north of and overlying or in faulted contact with the Whitland Shale Member (see Fortey & Owens, Reference Fortey and Owens1987, Fig. 2).
7. Chitinozoan assemblage from Arenig Fawr
Three species were identified from the two productive samples of the Henllan Ash Member: Conochitina decipiens, Lagenochitina obeligis (Fig. 9s) and Rhabdochitina magna from sample TVDB 12-052, and L. obeligis and R. magna from sample TVDB 12-054. All three species range through long intervals in the South Wales succession, but their ranges overlap in assemblages 3b and 3c (Fig. 6). Applying the same ranges to the sections around Arenig Fawr would indicate a late Moridunian to early Fennian age and correlation with the late Floian Stage Slice Fl3 or early Dapingian Stage Slice Dp1. This is consistent with the late Moridunian age shown for the Henllan Ash by Fortey et al. (Reference Fortey, Harper, Ingham, Owen, Parkes, Rushton and Woodcock2000), or with the Whitlandian age supported by the graptolite fauna (Zalasiewicz, Reference Zalasiewicz1984, Reference Zalasiewicz1986; Zalasiewicz et al. Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009). But although they support previous interpretations of the age of the Henllan Ash, all three species have longer stratigraphic ranges elsewhere so their evidence must be considered permissive rather than conclusive.
8. Discussion
The chitinozoans recorded from Arenig sections in the Carmarthen and Whitland areas of South Wales indicate correlation with stage slices from the upper Tremadocian through the Floian and Dapingian stages to the middle Darriwilian Stage, but with a degree of certainty that varies through the succession. Assemblage 1 is certainly close to the Tremadocian–Floian boundary and probably late Tremadocian (Stage Slice Tr3). The age and correlation of Assemblage 2a has the greatest uncertainty, but it is suggested to correlate with the middle to upper Floian Stage (Fl2–Fl3). There is more certainty over correlation of assemblages 2b, 3a, 3b and 3c. The occurrences of Conochitina gueddichensis, Conochitina pseudocarinata and Desmochitina ovulum in Assemblage 2b all suggest that the assemblage is not older than the late Floian Fl3 Stage Slice. Correlation of the overlying assemblages is constrained by their observed or, in the case of Assemblage 3c, inferred superpositional relationships and by the occurrence of species that support a late Floian or younger age. The certainty of correlation increases upwards, with that of assemblages 4, 5 and 6 and their sub-assemblages supported by the presence of index species, or related forms, and by the occurrence of graptolites to corroborate the chitinozoan evidence, particularly in the Pontyfenni and Llanfallteg formations.
Chitinozoan zonal schemes covering the same stratigraphic interval (Fig. 7) have been developed for South Gondwana (Paris, Reference Paris1990), based on successions in North Africa, SW and Central Europe, with a separate scheme for the upper Tremadocian of Morocco published by Nowak et al. (Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016); South America (Grahn, Reference Grahn2006), with a separate scheme for NW Argentina (de la Puente & Rubinstein, Reference de la Puente and Rubinstein2013); Baltoscandia (Nõlvak & Grahn, Reference Nõlvak and Grahn1993; Grahn et al. Reference Grahn, Nõlvak and Paris1996), Estonia (Nõlvak et al. Reference Nõlvak, Liang and Hints2019) and Sweden (Grahn & Nõlvak, Reference Grahn and Nõlvak2007); South China (X Wang et al. Reference Wang, Stouge, Erdtmann, Chen, Li, Wang, Zeng, Zhou and Chen2005, Reference Wang, Stouge, Chen, Li, Wang, Finney, Zeng, Zhou, Chen and Erdtmann2009; Chen et al. Reference Chen, Paris, Wang and Zhang2009; W Wang et al. Reference Wang, Feng, Vandenbroucke, Li and Verniers2013; Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017); and Laurentia (Achab, Reference Achab1989). Each of these regions and zonal schemes has species in common with South Wales, but the regions with the highest numbers in common are South China, South Gondwana and Baltica. South China has the most in common overall. For assemblages 2 and 3, 16 of the 19 named species from South Wales (84 %) are also known from South China, 10 (53 %) are known from South Gondwana, and another 10 are known from Baltica. For assemblages 4–6, 18 of the 24 named species (75 %) are known from South China, 18 from South Gondwana and 13 (54 %) from Baltica.
The lack of Gondwanan index species in assemblages 2 and 3 is notable. The absence of Conochitina symmetrica and Eremochitina baculata, the index fossils of the lowest two biozones in the Floian Stage of Gondwana, might be explained by an absence of strata or a lack of samples (Figs 6, 7), but this does not explain the absence of Eremochitina brevis. This species is the eponymous index of Paris’s (Reference Paris1990) upper Floian brevis Biozone with which assemblages 2 and 3 are mainly correlated (Fig. 7). Furthermore, Eremochitina brevis has been widely reported from North Africa (Paris, Reference Paris1990; Oulebsir & Paris, Reference Oulebsir and Paris1995; Videt et al. Reference Videt, Paris, Rubino, Boumendjel, Dabard, Loi, Ghienne, Marante and Gorini2010; Nowak et al. Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016), South China (Liang et al. Reference Liang, Servais, Tang, Liu and Wang2017, Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018) and NW Argentina (de la Puente & Rubinstein, Reference de la Puente and Rubinstein2013). Avalonia rifted away from Gondwana at around the beginning of the Ordovician Period (Domeier, Reference Domeier2016), but there is no indication of increased faunal provincialism following rifting to explain the absence of the species from South Wales. Moreover, Avalonia’s trajectory only served to increase the distance from Gondwana during the Fennian Stage, whereas Gondwanan index species are present at that level.
The Eremochitina brevis Biozone is itself in need of revision. It was originally defined as a total range biozone (Paris, Reference Paris1990), but Nowak et al. (Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016) recorded Eremochitina brevis from the upper Tremadocian of Morocco and furthermore introduced an Eremochitina brevis Biozone (Fig. 7) at a much lower level than the brevis Biozone of other schemes (Paris, Reference Paris1990; Grahn, Reference Grahn2006; de la Puente & Rubinstein, Reference de la Puente and Rubinstein2013). The occurrence of Eremochitina brevis in Morocco is accompanied by graptolites of the Araneograptus murrayi Biozone, acritarchs of the messaoudensis–trifidum microflora, and other chitinozoans that are consistent with a late Tremadocian age such as Lagenochitina cf. destombesi and the Euconochitina paschaensis–symmetrica group. Nor is this Tremadocian record of E. brevis the only one to come to light recently, as Liang et al. (Reference Liang, Servais, Tang, Liu and Wang2017, Reference Liang, Hints, Luan, Tang, Nõlvak and Zhan2018) recorded the species from the upper Tremadocian Tungtzu Formation of South China. This indicates a downward extension of the species range in both areas and necessitates a redefinition of Paris’s (Reference Paris1990) brevis Biozone from a total range to a partial range biozone. It is nevertheless unfortunate that Nowak et al. (Reference Nowak, Servais, Pittet, Vaucher, Akodad, Gaines and Vandenbroucke2016) chose to use the same species as the index of a Tremadocian biozone, bringing with it the potential for confusion.
The absence of Gondwanan index species from the Moridunian succession in South Wales perhaps has a parallel with graptolite faunas in South Wales. In their synoptic history of the Arenig Series in South Wales, Fortey and Owens (Reference Fortey and Owens1987) noted that ongoing transgression in the early Arenig resulted in soft, muddy sea-floor conditions represented by the Pibwr Member, but while oceanic connections were sufficiently developed for the appearance of a few graptolites at one level, a fully oceanic suite of species is not known. Fortey and Owens (Reference Fortey and Owens1987) postulated that the subsequent development of a probable barrier to circulation further west, during deposition of the Cwmffrⓦd and Cwm yr Abbey members, then produced a stagnant basin with restricted circulation in the Carmarthen region. This interpretation was augmented by Traynor’s (Reference Traynor1988) investigation of sedimentary processes during deposition of the Arenig Series in South Wales, which concluded that the Arenig deposits across South Wales were ponded in small interconnected marine sub-basins with facies and facies distributions controlled by intra-Arenig tectonic activity during an overall sea-level rise. Graptolites first become locally numerous in the Afon Ffinnant, Blaencediw and Colomendy formations, with species of Azygograptus and Expansograptus, and together with cyclopygid trilobites bring the first indications of oceanic conditions. Graptolites from the Pontyfenni and Llanfallteg formations are yet more cosmopolitan and oceanic, the Pontyfenni Formation perhaps representing the most oceanic conditions (Fortey & Owens, Reference Fortey and Owens1987, p. 104). It is very likely that the change from a restricted marine basin to the more oceanic setting documented by Fortey and Owens (Reference Fortey and Owens1987) affected chitinozoans as much as the graptolites, and offers an explanation for the presence of Gondwanan index species in the higher parts of the succession, in contrast to their absence from the lower part.
The results from this study have implications for the extent of the regional Whitlandian Stage. The chitinozoan assemblages from the Carmarthen and Afon Ffinnant formations suggest correlation of much of the Moridunian Stage succession in the Carmarthen area with the Fl3 Stage Slice (Figs 6, 7). As the base of the regional Fennian Stage is placed at a level low in the Dapingian Stage, based on recognition of the ornensis and henryi biozones higher in the succession, the interval available for correlation with the Whitlandian Stage is restricted to the upper part of the Fl3 Stage Slice and the lower part of the Dp1 Stage Slice (Figs 6, 7).
A consequence of re-correlating the Whitlandian Stage with the upper part of the Fl3 Stage Slice and the lower part of Dp1 is that Whitlandian strata in South Wales would have been deposited in a relatively short period of time. A cumulative thickness curve (Fig. 11) shows trends from the Tremadocian Stage to the mid Darriwilian Stage that take account of the revised correlation. Although the curve is not corrected for compaction or tectonic thickening, it steepens in the upper Floian and lowest Dapingian stages, represented by the 500 m minimum thickness of the Colomendy Formation (Table 1). Similarly, cumulative thickness curves published by Verniers et al. (Reference Verniers, Pharaoh, André, Debacker, De Vos, Everaerts, Herbosch, Samuelsson, Sintubin, Vecoli, Winchester, Pharaoh and Verniers2002, fig. 3) and Linnemann et al. (Reference Linnemann, Herbosch, Liégeois, Pin, Gärtner and Hofmann2012, fig. 6) for the Lower Palaeozoic of the Brabant Massif, also part of Avalonia, show increases in cumulative thickness around 470 Ma. The Brabant formation associated with this, however, is the Tribotte Formation (Linnemann et al. Reference Linnemann, Herbosch, Liégeois, Pin, Gärtner and Hofmann2012, fig. 6), which is dated biostratigraphically as early Darriwilian (Herbosch & Verniers, Reference Herbosch and Verniers2014).

Fig. 11. Cumulative thickness curve for the upper Tremadocian to middle Darriwilian succession in South Wales based on thicknesses in Table 1.
Table 1. Thicknesses of formations and members from the Arenig Series of South Wales used to compile the cumulative thickness curve in Figure 11

An assessment of global chitinozoan distribution patterns in the Lower and Middle Ordovician is not an aim of this paper, but it has been shown that such patterns respond to large-scale environmental changes related to palaeoclimate (Vandenbroucke et al. Reference Vandenbroucke, Armstrong, Williams, Paris, Sabbe, Zalasiewicz, Nõlvak and Verniers2010 a, b). Several studies using proxy data, sequence stratigraphy and Global Circulation Models have suggested important global cooling during the Early–Middle Ordovician (Trotter et al. Reference Trotter, Williams, Barnes, Lécuyer and Nicoll2008), with ice caps present from the Darriwilian if not earlier (Turner et al. Reference Turner, Armstrong and Holt2011, Reference Turner, Armstrong, Wilson and Makhlouf2012; Dabard et al. Reference Dabard, Loi, Paris, Ghienne, Pistis and Vidal2015; Pohl et al. Reference Pohl, Donnadieu, Le Hir, Ladant, Dumas, Alvarez-Solas and Vandenbroucke2016 a, b; Rasmussen et al. Reference Rasmussen, Ullmann, Jakobsen, Lindskog, Hansen, Hansen, Eriksson, Dronov, Frei, Korte, Nielsen and Harper2016). The data presented here has the potential to contribute to a better understand of chitinozoan distribution patterns and migrations during this period in the development of Earth’s climate system, as well as augmenting stratigraphic correlations.
Acknowledgements
This work is part of a PhD study financed by the Ecole Doctorale 104 Sciences de la Matière, du Rayonnement et de l’Environnement (SMRE) of Lille University. TRAV and CEAA acknowledge financial support from the CNRS (INSU, action SYSTER), the French ‘Agence Nationale de la Recherche’ through [grant ANR-12-BS06-0014] ‘SeqStrat-Ice’, and the SMRE doctoral school of the University of Lille. SGM publishes by courtesy of the Executive Director, British Geological Survey, NERC, UK. We thank Richard Ramsey and Catherine Russell for their assistance with the collection of field samples, Laurence Debeauvais for laboratory preparations and Philippe Recourt for SEM imaging.
Conflict of interest
None.