Summations
-
The literature published within acute stress research over the last 20 years has an overall underrepresentation of women.
-
Articles incorporating only females were significantly underrepresented compared to articles incorporating only males.
-
More than half of the articles that included data from both females and males failed to analyse and interpret the results by sex, a significant methodological limitation.
Considerations
-
The current review included 9539 participants sourced from 124 original articles published over 20 years in recognised journals.
-
We did not consider studies on pregnant women, patient populations, post-menopausal women or older adults.
-
We did not provide additional demographic information. However, it is plausible that this information would demonstrate other biases in the field of acute social stress and warrants further investigation.
Introduction
There are notable differences between the sexes in their susceptibility and response to stress (Kirschbaum et al., Reference Kirschbaum, Kudielka, Gaab, Schommer and Hellhammer1999; Rohleder et al., Reference Rohleder, Wolf, Piel and Kirschbaum2003; Uhart et al., Reference Uhart, Chong, Oswald, Lin and Wand2006; Heck and Handa, Reference Heck and Handa2019). The coping mechanisms of men and women vary depending on the nature of the stressor (Kudielka and Kirschbaum, Reference Kudielka and Kirschbaum2005). Men show a more substantial response to stressors involving competition, whereas women react to interpersonal stressors such as social rejection (Kudielka and Kirschbaum, Reference Kudielka and Kirschbaum2005). In response to acute psychosocial stress, the differences between men and women are most pronounced when women are in the follicular phase of the menstrual cycle, characterised by low oestrogen levels (Heck and Handa, Reference Heck and Handa2019; Kudielka and Kirschbaum, Reference Kudielka and Kirschbaum2005). Women’s responses to stress are tightly connected with the gonadal hormones (Verma et al., Reference Verma, Balhara and Gupta2011) and thus vary considerably depending on menstrual phase and use of hormonal contraceptives (HCs) (Herman and Cullinan, Reference Herman and Cullinan1997; Kirschbaum et al., Reference Kirschbaum, Kudielka, Gaab, Schommer and Hellhammer1999). Furthermore, women are at a higher risk of developing stress-related diseases during adolescence and adulthood, supporting the idea that oestrogen and progesterone influence the manifestation of stress-related disorders in adult women (Heck and Handa, Reference Heck and Handa2019).
Original research should therefore be planned and powered to identify factors that may contribute to variations in psychological stress measures such as cortisol. Menstrual cycle phase and use of HCs are obvious candidates for scrutiny (Burrowes, Reference Burrowes2021). Women are underrepresented in a number of fields, such as cardiology (Kentner and Grace, Reference Kentner and Grace2017), sports medicine (Hagstrom et al., Reference Hagstrom, Yuwono, Warton and Ford2021), neuroscience (Beery and Zucker, Reference Beery and Zucker2011), autoimmune dysfunction (Lockshin, Reference Lockshin2006; Fish, Reference Fish2008), pharmacology (Beery and Zucker, Reference Beery and Zucker2011) and physiology (Beery and Zucker, Reference Beery and Zucker2011). The clinical studies focused only on males suggest the results to be applicable to both sexes. However, 80% of the drugs failing clinical trials from the US pharmaceutical market from 1997 to 2000 were due to side effects present solely in women (Burrowes, Reference Burrowes2021). In 1997, the U.S. Food and Drug Administration (FDA), implemented a rule according to which pharmaceutical companies need to ensure the safety of the manufactured drug for both genders (Verma et al., Reference Verma, Balhara and Gupta2011); stating that it is unethical to prescribe a drug to women unless they are included in the studies that aim to understand the disease mechanism. The outcome of such a policy has not gone unnoticed. It has made a difference by increasing the overall number of female participants in the clinical research funded by the US National Institutes of Health (NIH). However, the lack of funding for the medical fields focused on women, such as stress-related disorders or reproductive systems, remains a significant challenge (Burrowes, Reference Burrowes2021).
Researchers exploring the aspects of social stress commonly accept that there are physiological and psychological sex differences (Verma et al., Reference Verma, Balhara and Gupta2011). However, to the best of our knowledge, no authors have examined the number, ratio or percentage of male and female subjects participating in research in this field. Therefore, this study examines the sex of participants involved in social stress research published within the last 20 years.
Materials and methods
This is a semi-systematic review performed according to The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). In total, 124 publications were included in the quantitative synthesis.
Database search
A systematic literature search was performed using the electronic database MEDLINE® via PubMed®. The search was conducted in December 2021, with the following combined set of keywords: [((cortisol) OR (HPA) OR (neuroendocrine) OR (hydrocortisone) OR (psychoneuroimmunology) OR (psychoimmunology) OR (psychoneuroendocrinology)) AND ((“stress reactivity”) OR (“acute stress”) OR (“laboratory stress”) OR (“experimental stress”) OR (“psychological stress”) OR (“mood induction”) OR (“emotion”)) Filters: Abstract, Clinical Trial, Randomized Controlled Trial, from 2000 to 2021]. This search generated 1932 results.
Study selection
One investigator selected the manuscripts based on the title and abstract, followed by a full-text assessment. See Fig. 1 for a graphical representation of the search and selection process. All selected papers met the following inclusion criteria: 1) healthy adult participants, 2) 18–50 years of age, 3) medication-free participants, 4) acute psychological stress, 5) salivary or blood cortisol and 6) at least two measurement points for cortisol. Papers were excluded if they contained at least one of the exclusion criteria such as: 1) pregnant women, 2) subjects under 18 or above 50 years of age, including menopausal women or post-menopausal women, 3) physical or psychological illnesses, 4) chronic stressor studies, 5) physical stressors, 6) physical–psychological combined stressor and 7) urinary or hair cortisol.
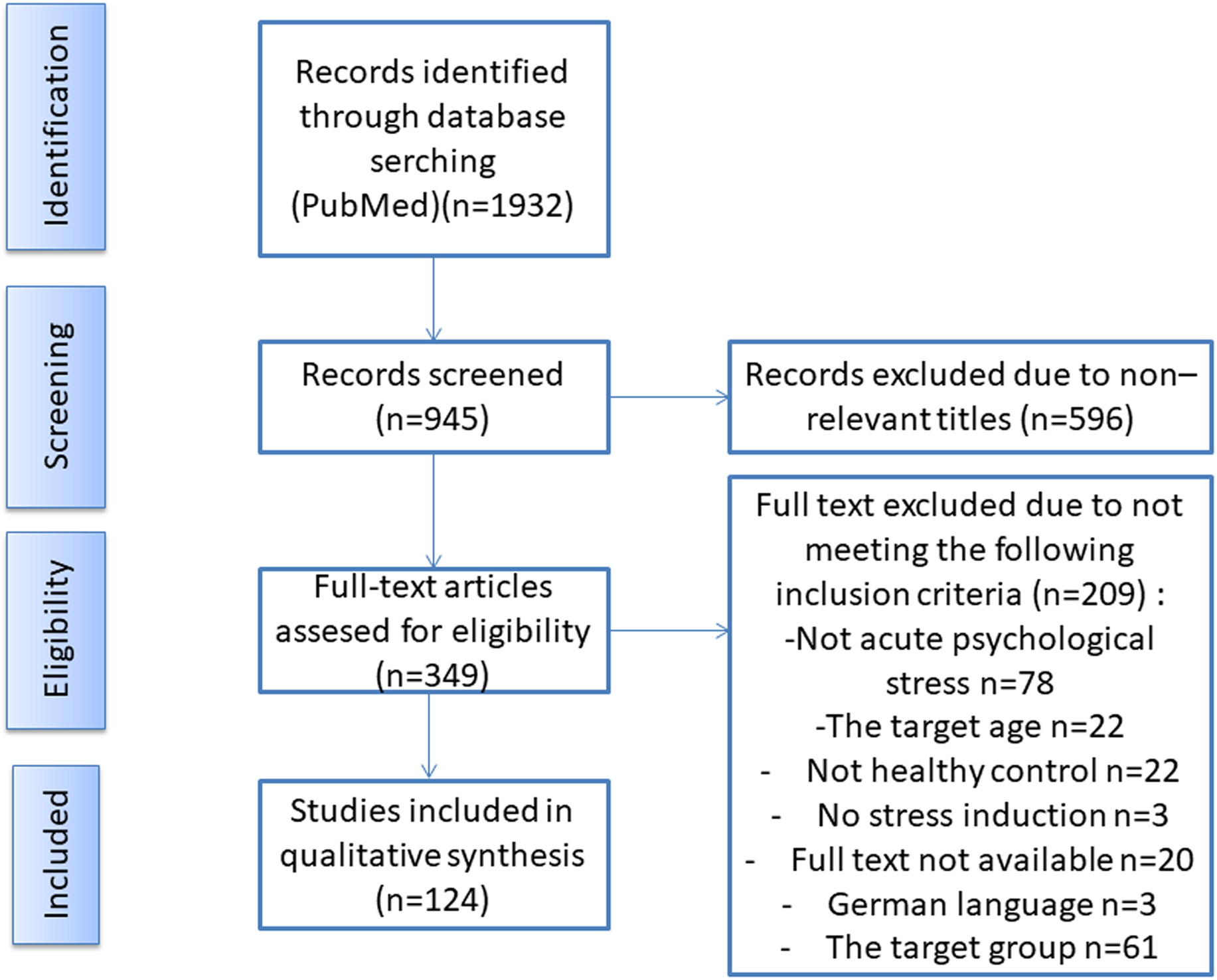
Figure 1. The flow chart illustrates the search and selection process of studies for this semi-systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The process includes an initial database search, followed by the screening of titles and abstracts for relevance, and then full-text screening of potentially relevant studies. The excluded studies at each stage are documented along with the reasons for their exclusion. Finally, the included studies are assessed for quality, and the relevant data is extracted for the present semi-systematic review.
Data extraction and analysis
The extracted data are number of women included, number of men included, year of publication and whether or not sex, menstrual cycle phase and HC were taken into account when analysing data. Data were analysed via Chi-square tests. p-Values < 0.05 were considered a priori as significant. Data are presented as percentages.
Results
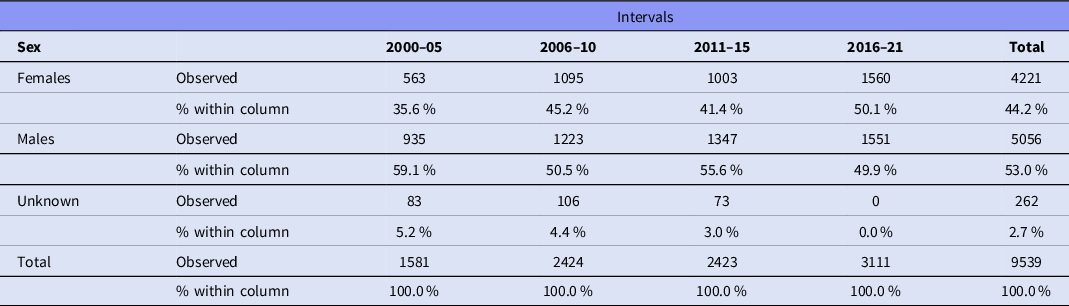
Data were extracted from 124 articles, listed in Table 1, involving 9539 participants. A total of 4221 (44.2%) participants were female, and 5056 were male (53.0%); the sex of 262 (2.7%) individuals was not reported (Table 2). Females were significantly underrepresented (χ2 = 75.283, df = 1, p < .001). Figure 2 shows the sex of participants from 2000 to 2021.
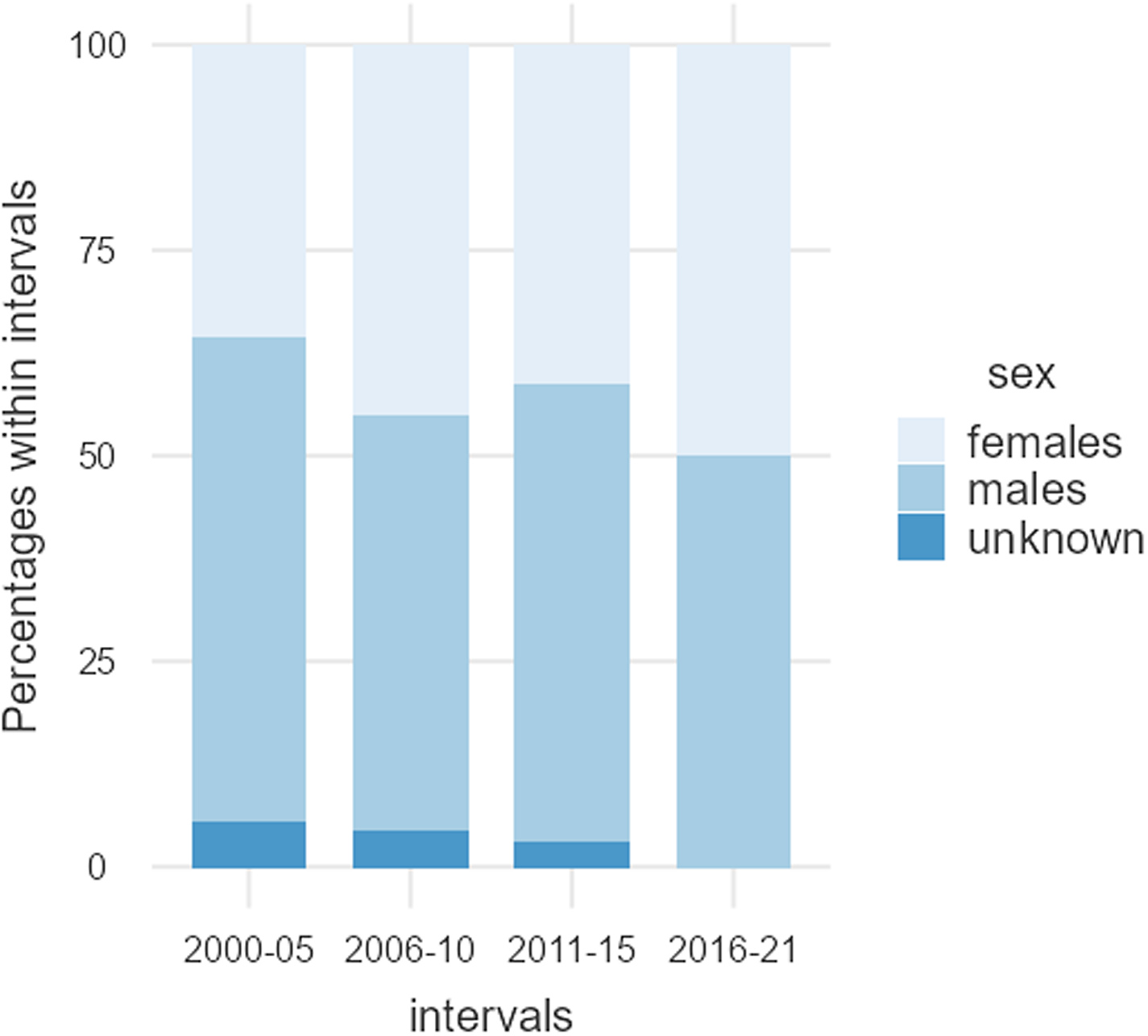
Figure 2. Male and female participants, as well as individuals where the sex was not reported. Between 2000 and 2021, the percentage of male participants remained relatively stable, while the percentage of individuals where the sex was not reported appeared to decrease slightly, in favour of female participants.
Table 1. Description of studies (N = 124)

The number of female and male participants is listed. Publication year indicates when the manuscript was first made available, for example, online, and may vary from reference. The extent that sex, menstrual cycle phase or hormonal contractions (HC) was included in the analysis is indicated with yes or no when applicable.
Table 2. The sex of participants from 2000 to 2021
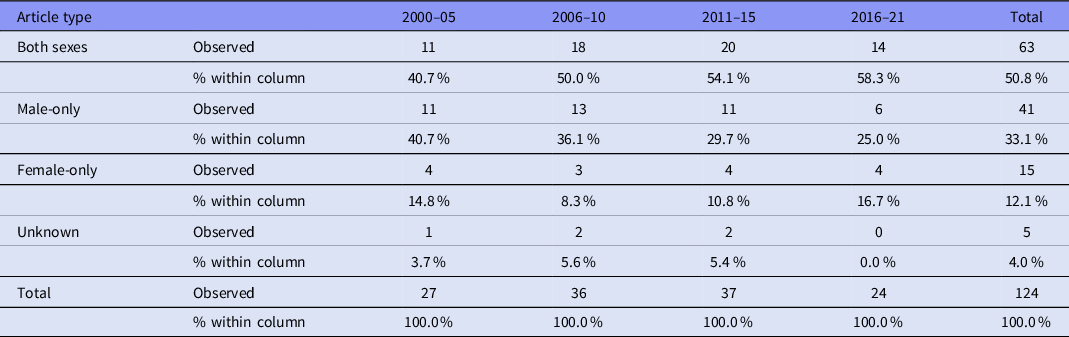
Overall, only 15 (12.1%) of the articles incorporated only females, 41 (33.1%) included only males and 63 (50.8%) included both males and females; five articles (4.0%) did not report the sex of their participants (Table 3). Articles incorporating only females were significantly underrepresented compared to articles incorporating only males (χ2 = 29.109, df = 1, p < .001). Figure 3 shows data of the use of male-only, female-only and both sexes as participants from 2000 to 2021.
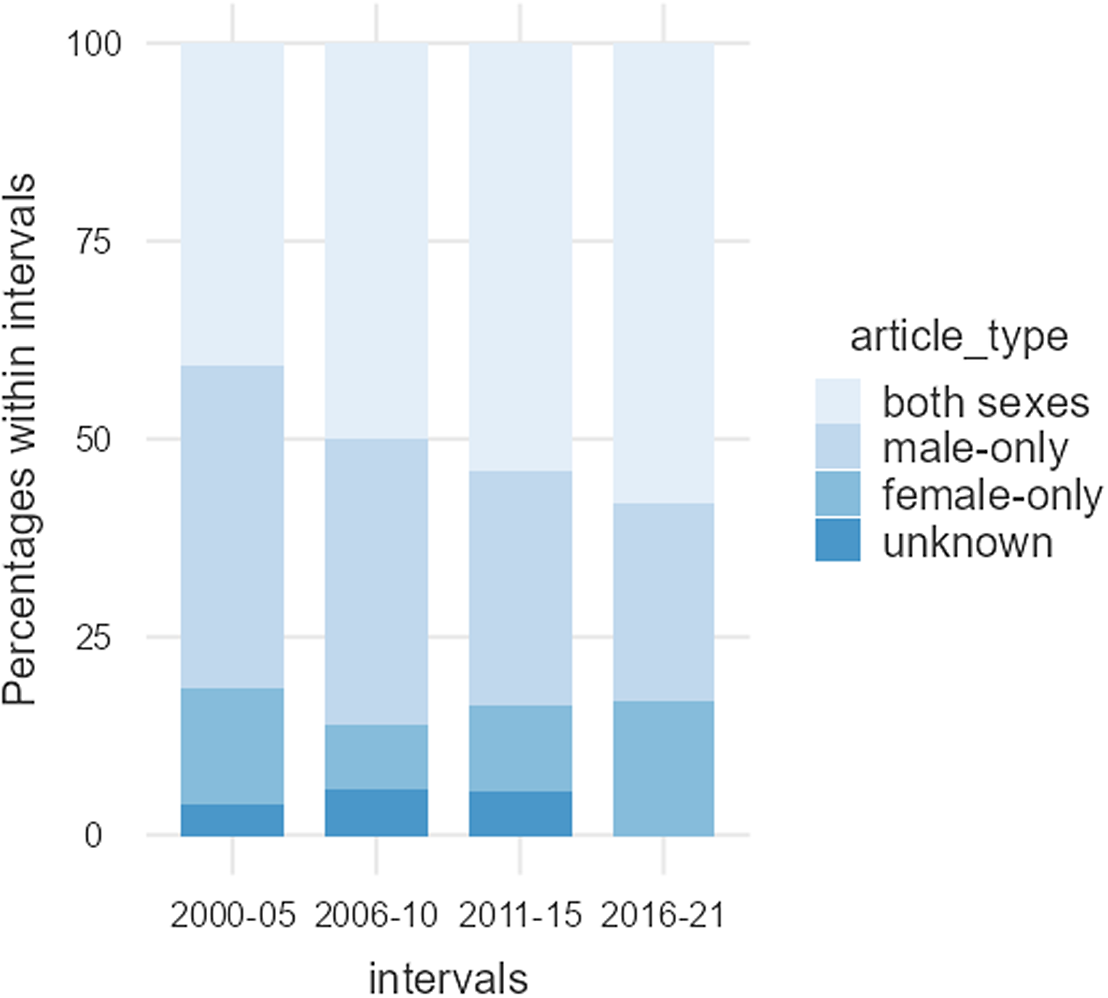
Figure 3. Articles using male-only, female-only, and both sexes as participants between 2000 and 2021. The percentage of female-only articles remained relatively stable, while those using only males appeared to decrease slightly, in favour of articles that included participants of both sexes.
Table 3. Use of male-only, female-only and both sexes as participants from 2000 to 2021
Only 22 articles (36.5%), which presented data from both females and males, included sex as an experimental variable.
Twenty-six articles (33.3%) on females-only and both females and males included the stage of the participants' oestrous or menstrual cycle phase. Of the articles reporting womens' menstrual cycle phase, 13 articles (50.0%) only included women in the luteal phase, and 4 articles (15.4%) included women in both the follicular and luteal phases. Fifty-two articles (66.7%) did not report menstrual cycle phase, nor was it an inclusion or exclusion criteria.
Forty-two articles, corresponding to 53.8% of all studies on females-only and both females and males, excluded women using HCs. Seven studies (9.0%) included women using HCs, of which only two studies (2.6%) analysed the stress response of women using HCs separately from freely cycling (FC) women; 29 (37.2%) did not mention HCs as inclusion or exclusion criteria.
Discussion
To our knowledge, this is the first study to examine the sex of participants in the acute social stress literature. Here, we present evidence that female participants are significantly underrepresented in the research from the last 20 years. In the studies where females are represented, severe methodological limitations are apparent, including lack of information of menstrual cycle phases or whether participants were HC users.
Research suggests that men and women may differ in their response to acute social stress, both in behaviour and physiology (Otte et al., Reference Otte, Hart, Neylan, Marmar, Yaffe and Mohr2005). For example, men tend to exhibit “fight or flight” responses, which is characterised by increased arousal and readiness for physical action. This response may be due to the influence of higher levels of testosterone. On the other hand, women have a greater likelihood of exhibiting the “tend and befriend” response, which is characterised by increased social behaviour and affiliation (Taylor et al., Reference Taylor, Klein, Lewis, Gruenewald, Gurung and Updegraff2000). This response is mediated by the release of oxytocin and is characterised by seeking support and protection of family and friends, nurturing and caring for others, and increased prosocial behaviour. This response may be due to the influence of higher levels of oestrogen and progesterone, which are associated with social behaviour and attachment to others (Lighthall et al., Reference Lighthall, Sakaki, Vasunilashorn, Nga, Somayajula, Chen, Samii and Mather2012). Furthermore, research suggests that there may be differences in the hypothalamic–pituitary–adrenal (HPA) axis between men and women, which may contribute to behavioural differences triggered by acute social stress. For example, studies have shown that women tend to have higher baseline cortisol levels than men, which may make them more sensitive to stressors. Additionally, women may have a more sensitive HPA axis overall, with greater responsiveness to stressors and a more rapid return to baseline cortisol levels following a stressor (Kajantie and Phillips, Reference Kajantie and Phillips2006).
Females account for 44.2% of the total number of participants in the 124 articles reviewed. Interestingly, the average percentage of female participants was relatively consistent from 2000 to 2021, although both the number of articles and the total number of participants have consistently increased over time, see Table 2 and Fig. 1. Despite the fact that 50.8% of the articles published over this period incorporated both male and female participants, women are underrepresented. This is mainly due to the greater volume of articles consisting of male-only data (33.1%), compared to female-only data (12.1%), and secondly, articles reporting data from both sexes tend to overrepresent male participants and limit the number of female participants. The proportion of female-only articles was relatively consistent from 2000 to 2021, whereas the proportion of male-only articles seems to have experienced a slight decrease in favour of articles incorporating participants of both sexes, see Table 3 and Fig. 2. Furthermore, most of the studies conducted on males do not give a thorough justification of why females were excluded. Only two of the publications stated that females were excluded from the studies due to challenges in studying all women at the same phase of the menstrual cycle (Honk et al., Reference Honk, Tuiten, van den Hout, Koppeschaar, Thijssen, Haan and Verbaten2000; Jönsson et al., Reference Jönsson, Wallergård, Österberg, Hansen, Johansson and Karlson2010). Another publication mentions that females are excluded because the menstrual cycle and birth control pills alter the level of glucocorticoids corticosterone and cortisol (CRT) (Montoya et al., Reference Montoya, Bos, Terburg, Rosenberger and van Honk2014). Only 37.1% of the articles that incorporated male and female participants over the last 20 years included sex as an experimental variable. Thus, most articles failed to recognise potential sex-related differences in susceptibility and response to acute social stress, inadvertently adding variability to their data in cases where sex-related differences exist. Although the increased inclusion of both sexes in the most recent years is encouraging, most of these studies still failed to investigate potential sex differences; they missed the opportunity to uncover how sex influences physiological parameters, for example, cortisol release.
According to Genazzini and co-workers (Genazzani et al., Reference Genazzani, Lemarchand-Béraud, Aubert, Felber, Muller, Lavanchy and Gomez1975), adrenocorticotropic hormone (ACTH) vary across the menstrual cycle phase. Women in the follicular and luteal phases do not have the same salivary-free cortisol response (Maki et al., Reference Maki, Mordecai, Rubin, Sundermann, Savarese, Eatough and Drogos2015). In order to compare the findings between men and women, it is first necessary to specify the phase of the menstrual cycle so the results can be interpreted. Progesterone has an inhibitory role in the HPA axis in women. In other words, the menstrual cycle phase impacts the response of the HPA axis to stress. However, it is still unclear how the sex hormones throughout the menstrual cycle affect the activity of the HPA axis. Consequently, there is a need for further studies to investigate the interaction between sex hormones and the HPA axis. This will facilitate the design of future stress-related experiments. Twenty-six articles (33.3%) on females-only and both females and males included the stage of the participants' oestrous or menstrual cycle phase. Fifty-two articles (66.7%) did not report menstrual cycle phase, nor was it an inclusion or exclusion criteria. Seven studies (Gold et al., Reference Gold, Zakowski, Valdimarsdottir and Bovbjerg2003; Raspopow et al., Reference Raspopow, Abizaid, Matheson and Anisman2010; Zöller et al., Reference Zöller, Maroof, Weik and Deinzer2010; Almela et al., Reference Almela, Hidalgo, Villada, van der Meij, Espín, Gómez-Amor and Salvador2011; Bostock et al., Reference Bostock, Hamer, Wawrzyniak, Mitchell and Steptoe2011; Heimgartner et al., Reference Heimgartner, Meier, Grolimund, Ponti, Arpagaus, Kappeler and Gaab2021; Kothgassner et al., Reference Kothgassner, Goreis, Glenk, Kafka, Beutl, Kryspin-Exner, Hlavacs, Palme and Felnhofer2021) conducted solely in female groups did not provide information about the phase of the menstrual cycle. On the other hand, 40 studies conducted in both sexes did not specify women’s menstrual cycle phase. It is possible for researchers to include only women in a selected phase of the menstrual cycle to compare results between sexes while minimising cost and time, such as the study by Spanakis et al. (Reference Spanakis, Wand, Ji and Golden2016) that selected women during the follicular phase where sex hormone levels are thought to be comparable between men and women. In order to detect the differences between men and women, it has been recommended to compare the male group to two or more groups of women at different menstrual cycle phases (Rich-Edwards et al., Reference Rich-Edwards, Kaiser, Chen, Manson and Goldstein2018). Of the articles reporting womens' menstrual cycle phase, 13 articles (50.0%) only included women in the luteal phase, 9 (34.6%) only included women in the follicular phase and 4 (15.4%) included women in both the follicular and luteal phases.
The women on HCs have a significantly different cortisol rhythm than FC women. In healthy adults, the cortisol level reaches a peak in the morning, approximately 30 minutes after waking up (Boisseau et al., Reference Boisseau, Enea, Diaz, Dugué, Corcuff and Duclos2013; Roche et al., Reference Roche, King, Cohoon and Lovallo2013). On the other hand, the women on HCs have lower cortisol peaks; therefore, the cortisol level does not experience a significant drop (Boisseau et al., Reference Boisseau, Enea, Diaz, Dugué, Corcuff and Duclos2013). In addition, the daily cortisol rhythms are blunted in pill-taking women; consequently, the daily cortisol curve seems to be flatter compared to naturally cycling women (Boisseau et al., Reference Boisseau, Enea, Diaz, Dugué, Corcuff and Duclos2013). The high level of corticosteroid-binding globulins in HC users blunt the cortisol release in response to the stress (Wiegratz et al., Reference Wiegratz, Kutschera, Lee, Moore, Mellinger, Winkler and Kuhl2003; Kumsta et al., Reference Kumsta, Entringer, Hellhammer and Wüst2007). It is suggested that birth control pills hyperactivate the HPA axis, and this causes the HPA axis to shut down. In reality, the pattern of HPA axis in pill-taking women is very similar to women with chronic stress (Miller et al., Reference Miller, Chen and Zhou2007; Hertel et al., Reference Hertel, König, Homuth, van der Auwera, Wittfeld, Pietzner, Kacprowski, Pfeiffer, Kretschmer, Waldenberger, Kastenmüller, Artati, Suhre, Adamski, Langner, Völker, Völzke, Nauck, Friedrich and Grabe2017). However, this remains a hypothesis and requires research to identify the role that HCs may play and the potential reversibility of effects upon cessation of HC use. Recent studies have found that hormonal contraception may impact the oxytocin system (Garforth et al., Reference Garforth, Degnbol, Terris, Zak and Winterdahl2020); however, more research is needed to understand the nature and magnitude of these effects on acute social stress, behaviour and cognition (Byg et al., Reference Byg, Dioni and Winterdahl2023). Forty-two articles, corresponding to 53.8% of all studies on females-only and both females and males, excluded women using HCs. Four studies (Walser et al., Reference Walser, Fischer, Goschke, Kirschbaum and Plessow2013; Abelson et al., Reference Abelson, Erickson, Mayer, Crocker, Briggs, Lopez-Duran and Liberzon2014; Creswell et al., Reference Creswell, Pacilio, Lindsay and Brown2014; Meier et al., Reference Meier, Wirz, Dickinson and Pruessner2021) had HC use as an exclusion criterion for women but did not exclude men on exogenous androgens and anabolic steroids. Surprisingly, 29 studies (37.2%) did not mention HCs as inclusion or exclusion criteria. Only seven studies (9.0%) included women using HCs (Rohleder et al., Reference Rohleder, Wolf, Piel and Kirschbaum2003; Hammerfald et al., Reference Hammerfald, Eberle, Grau, Kinsperger, Zimmermann, Ehlert and Gaab2006; Kumsta et al., Reference Kumsta, Entringer, Hellhammer and Wüst2007; Pilgrim et al., Reference Pilgrim, Ellenbogen and Paquin2014; Herbison et al., Reference Herbison, Henley, Marsh, Atkinson, Newnham, Matthews, Lye and Pennell2016; Ditzen et al., Reference Ditzen, Germann, Meuwly, Bradbury, Bodenmann and Heinrichs2019; Sandner et al., Reference Sandner, Lois, Streit, Zeier, Kirsch, Wüst and Wessa2020) of which only two studies (2.6%) analysed the stress response of women using HCs (Boisseau et al., Reference Boisseau, Enea, Diaz, Dugué, Corcuff and Duclos2013; Hertel et al., Reference Hertel, König, Homuth, van der Auwera, Wittfeld, Pietzner, Kacprowski, Pfeiffer, Kretschmer, Waldenberger, Kastenmüller, Artati, Suhre, Adamski, Langner, Völker, Völzke, Nauck, Friedrich and Grabe2017) separately from FC women and concluded that HC use impacts the HPA axis response to stress.
It is difficult to provide a specific rationale for the sex bias in the acute social stress literature, and a range of physiological and methodological issues likely contribute. Women have been excluded from biomedical research over the years under the assumption that the results from studies on men apply to females or that the variation of sex hormones throughout the menstrual cycle makes the interpretation challenging. However, other studies do not justify the exclusion of women (Beery and Zucker, Reference Beery and Zucker2011). Furthermore, there is still a misconception that women are more variable than men. Both males and females have variations in gonadal steroid hormones. Testosterone has a circadian rhythm, and its level is affected by factors such as physical exercise and age (Smith et al., Reference Smith, Coward, Kovac and Lipshultz2013). In comparison, reproductive females have a fluctuation of gonadal hormones throughout the phases of the menstrual cycle (Rich-Edwards et al., Reference Rich-Edwards, Kaiser, Chen, Manson and Goldstein2018). Treating males as the norm comes with the consequence of placing women’s health at risk. It is beneficial to science to include both sexes in order to get a full insight into the mechanisms of the HPA axis. It is not possible to elucidate stress response mechanisms by studying only males. The consideration of both sexes in stress-related research will advance our knowledge and lead to the development of safer products and therapies for stress-related disorders, minimising their side effects. The very least, the presentation of the data of both sexes can improve the design of future studies. The differences in methodological design are thought to be a main reason for inconsistent results across studies (Rich-Edwards et al., Reference Rich-Edwards, Kaiser, Chen, Manson and Goldstein2018).
Original research should be planned and powered to identify factors that may contribute to variations in acute social stress measures such as cortisol, including sex, menstrual cycle and oral contraceptive use. By doing so, the findings will be more robust, generalisable and informative. Ensuring that a study has enough statistical power to distinguish different stress responses between, for example, men vs women, different phases of the menstrual cycle and hormonal contraception users vs natural cycling women, requires careful planning and consideration of several factors. Some key considerations include the following: using the correct statistical model, both analysis of variance (ANOVA) and a general linear model (GLM) can be used to model the relationship between a continuous dependent variable (such as cortisol levels or responses) and one or more independent variables (such as sex, menstrual cycle phase and hormonal contraception use). By including these variables in the model, the study would be able to test for main effects of sex, menstrual cycle phase and hormonal contraception use on the measures of choice, as well as for any interactions between these variables. The main difference between ANOVA and GLM approach is that ANOVA is used to compare the means of two or more groups and assumes normality and homogeneity of variances, while GLM can be used to model a relationship between a continuous dependent variable and one or more independent variables and assumes linearity and normality of residuals. GLM can also be extended to include other explanatory variables such as age, BMI and lifestyle factors that may influence, for example, cortisol levels and can be used to model non-normal distributions of the response variable, and it allows to include categorical and continuous variables. Furthermore, the sample size of a study is one of the most critical factors in determining statistical power. Larger sample sizes increase the likelihood of detecting significant differences between groups, so it is important to ensure that the study has a large enough sample size to detect differences in stress hormones between men and women. It is important to control for any confounding variables that may affect stress hormones such as age, lifestyle and other hormones. Finally, as multiple comparisons are made with biological samples across different groups, it may be important to adjust the significance level to take into account the increased chance of finding false positives.
Limitations and future research
A strength of the current study is the number of participants (9539) sourced from original articles published over 20 years in recognised journals. However, the current review did not consider the following in the analysis: studies involving multiple publications based on data from the same population/sample, studies on pregnant women or patient populations or studies on post-menopausal women and older adults. Finally, due to the large number of participants, we did not provide additional demographic information. It is plausible that this information would demonstrate other biases in the field of acute social stress and warrants further investigation.
Conclusion
The current study demonstrated that female participants are still underrepresented in the acute social stress literature. Absolute numbers and percentage of female participants are significantly lower than males. More importantly, the ratio of articles that included only males to articles that only included females is two-fold greater across the literature. We stress the importance of reporting menstrual cycle phase information and HC use in studies presenting female data, and we encourage the comparison of male and female data in order to uncover potential important sex differences, which may be of utmost importance for drug development and providing appropriate medical care to both sexes.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgements
We thank Marie Vadstrup Pedersen for assistance and feedback.
Author contributions
AR and MR conceptualised the study. AR extracted data, analysed and wrote the first draft under the supervision of MW and AL. All authors have edited and approved the submitted version and take responsibility for the integrity of the findings.
Funding statement
AR’s salary was supported by the ERASMUS programme at University of Wrocław.
Conflict of interest
None.









