Flaxseed (FS) is an oilseed containing two major bioactive anti-cancer components, FS oil (FO) and plant lignans(Reference Thompson, Thompson and Cunnane1–Reference Adlercreutz6). The FO, representing 40 % of FS, has more than 50 % as the n-3 fatty acid, α-linolenic acid(Reference Bougnoux, Chajes, Thompson and Cunnane2, Reference Cunnane, Thompson and Cunnane3). The predominant plant lignan in FS is secoisolariciresinol diglucoside (SDG) that can be metabolised by gut microbiota into mammalian lignans, enterodiol and enterolactone(Reference Thompson, Robb and Serraino4, Reference Thompson, Boucher and Liu5). Both enterodiol and enterolactone have weak oestrogenic or anti-oestrogenic activities due to their chemical structure which is similar to oestradiol (E2)(Reference Thompson, Thompson and Cunnane1, Reference Adlercreutz6).
Previous studies have shown that dietary FS can inhibit mammary tumourigenesis in carcinogen-treated rats(Reference Serraino and Thompson7–Reference Thompson, Seidl and Rickard10), reduce the tumour growth or metastasis in athymic mice with xenografts of oestrogen receptor-positive (ER+) or ER − human breast cancers(Reference Chen, Hui and Ip11–Reference Wang, Chen and Thompson19), reduce tumour cell proliferation and human epidermal growth factor receptor 2 (HER2) protein expression and increase apoptosis in postmenopausal breast cancer patients(Reference Thompson, Chen and Li20). Many breast cancer patients use complementary/alternative medicines, and of the dietary supplements consumed, FS is the third most commonly used just behind green tea and vitamin E(Reference Boon, Olatunde and Zick21). However, some patients find FS to be bulky for consumption; 25–50 g/d is the human equivalent to the intake of 10 % FS found effective in animal studies(Reference Thompson, Thompson and Cunnane1, Reference Chen, Stavro and Thompson17) and was used in clinical studies(Reference Thompson, Chen and Li20).
FS has been mechanically processed to yield two fractions: the inner cotyledon fraction (FC), which represents 72–83 % of the whole seed, and is rich in α-linolenic acid-rich oil but poor in lignan(Reference Boon, Olatunde and Zick21, Reference Oomah, Thompson and Cunnane22); and the outside hull fraction, which represents 17–28 % of the seed, and is rich in lignan content but low in α-linolenic acid-rich oil(Reference Boon, Olatunde and Zick21–Reference Madhusudhan, Wiesenborn and Schwarz24). If FC is found to be as effective as FS, consumption of FC will reduce the amount to be consumed by 17–28 % compared to the whole seed. However, its effect in regressing the growth of ER+ human tumours has not yet been tested.
Our previous studies have shown that FS can increase the effectiveness of tamoxifen (TAM) treatment by inhibiting tumour growth in ovariectomised athymic mice with established ER+ human breast cancer (MCF-7) at high or low circulating E2 concentration(Reference Chen, Hui and Ip11–Reference Chen, Power and Mann14). Both the FO and lignan components of FS are responsible for this effect, but FO exhibits a stronger effect than lignan when combined with TAM treatment(Reference Saggar, Chen and Corey25). This enhancing effect with TAM was attributed in part to the modulation of ER − and growth factor-related pathways(Reference Chen, Power and Mann13, Reference Saggar, Chen and Corey25). However, it is unknown whether the α-linolenic acid-rich FC fraction, also containing SDG, can similarly increase the effectiveness of TAM, and what may be the underlying mechanisms.
Hence, the objective of the present study was to determine the effect of FC, alone or in combination with TAM, on the growth of human breast tumour (MCF-7) in athymic mice with low circulating E2 levels, and to explore its potential mechanisms by investigating its effect on the expression of biomarkers in ER − and growth factor-mediated pathways. If FC is found effective, it may be used as an alternative to FS in potentially increasing the effectiveness of TAM.
Materials and methods
Cell line and cell culture
Human ER+ breast cancer cells, MCF-7 (ATCC, Manassas, VA, USA), were maintained in Dulbecco's modified Eagle' medium/F12 (Invitrogen Canada, Inc., Burlington, ON, Canada) supplemented with 10 % fetal bovine serum and 1 % antibiotics (Sigma-Aldrich, St Louis, MO, USA). For cell injection, the cells with 70–90 % confluency were harvested by trypsinisation and suspended in serum-free medium with 1:1 Matrigel at 2 × 107 cells/ml and placed on ice. Cell viability was over 90 % as determined by the trypan blue exclusion assay.
Animals and diets
BALB/c, nu/nu, athymic mice (Charles River Canada, St-Constant, PQ, Canada), 5–6 weeks old and ovariectomised, were maintained in micro-isolator cages (four per cage). The mice were housed in a pathogen-free isolation facility under a 12 h light–dark cycle at 22–24°C and 50 % humidity, and were fed ad libitum diets and water. The experimental protocol (no. 20006109) was approved by the University of Toronto Animal Care Committee, and the animal care was in accordance with the Guide to the Care and Use of Experimental Animals(26).
Two experimental diets were prepared: a basal diet (BD, control) based on the AIN-93G diet(Reference Reeves, Nielsen and Fahey27) modified to contain 20 % maize oil instead of soyabean oil as in our previous studies(Reference Chen, Hui and Ip11, Reference Saarinen, Power and Chen12, Reference Dabrosin, Chen and Wang16–Reference Wang, Chen and Thompson19, Reference Saggar, Chen and Corey25), and a FC diet as the BD supplemented with 82 g/kg FC. The FC fraction was prepared by Pizzey's Nutritionals (Angusville, MB, Canada), and its amount in diet was equivalent to that in a 10 % FS diet assuming that this fraction represents 82 % (w/w) of the whole FS(Reference Oomah, Mazza and Kenaschuk23). The FC contains 40·7 % oil, 18·8 % protein, 20·6 % dietary fibre, 13·7 % available carbohydrate and 0·8 % SDG. Hence, the FC diet formulation was adjusted for the fat, dietary fibre, available carbohydrate and protein contents such that its macronutrient and energy values were the same as in BD (Table 1). Diets were prepared by Dyets, Inc. (Bethlehem, PA, USA) and sterilised by Isomedix Corporation (Whitby, ON, Canada).
Table 1 Composition of diets
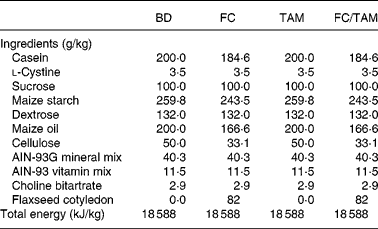
BD, basal diet; FC, flaxseed cotyledon; TAM, tamoxifen.
Experimental design
After 1-week acclimatisation with the BD, mice were anaesthetised with a mixture of isoflurane and oxygen. They were then injected subcutaneously with a 50 μl cell suspension containing 1 × 106 cells into each of four mammary fat pads (right and left thoracic and right and left abdominal), producing four sites of mammary tumours. To promote tumour growth, a sterilised 17-β E2 pellet (0·36 mg, 60 d release; producing 150–200 pg/ml E2 blood level; Innovative Research of America, Sarasota, FL, USA) was implanted in the intrascapular region via a 2–3 mm incision that was then sealed with Vetbond. Mice were fed with the BD.
At week 7, when tumour average area reached about 30 mm2, four groups were formed such that the mean tumour size and mouse weight did not significantly differ from one another. All mice received further surgery to remove the E2 pellet to simulate the postmenopausal condition (27–38 pg/ml plasma E2 in mice; 10–40 pg/ml in women). Two groups of mice were then implanted with a TAM pellet (5 mg, 60 d release, producing 3–4 ng/ml blood level; Innovative Research of America). Group 1 was fed the BD (control; n 13); group 2 was fed the FC diet (8·2 % FC; n 13); group 3 with TAM implant was fed the BD (n 12); and group 4 with the TAM implant was fed with the FC diet (n 13). Food intake, body weight and palpable tumour area were monitored weekly with the mouse cage coded. The palpable tumour area was calculated as length/2 × width/2 × π. At 8 weeks post-treatment, mice were killed with CO2 asphyxiation followed by cervical dislocation. Tumours were excised and weighed. A portion of the tumour tissue was frozen in liquid N2 and stored at − 80°C for quantitative real-time PCR analyses. A small piece of tumour tissue was fixed in a 10 % buffered formalin solution for immunohistochemical analysis. The major organs including the uterus were excised and weighed as markers of oestrogenicity.
RNA preparation and real-time PCR
Tumours representing the mean area for each group (BD, n 10; FC, n 8; TAM, n 9; FC/TAM, n 9) were selected for analysis. The protocol for RNA preparation and real-time PCR was as described previously(Reference Saggar, Chen and Corey25, Reference Saggar, Chen and Corey28). The mRNA expressions of the following were analysed: Bcl2, ERα, ERβ, progesterone receptor, pS2, HER2 and insulin-like growth factor 1 receptor (IGF-1R). Briefly, tumours were homogenised in TRI Reagent solution (Sigma-Aldrich) and centrifuged in 0·5 ml of isopropanol at 12 000 g for 10 min at 4°C, followed by washing the RNA pellet with 1 ml of 75 % ethanol. After centrifugation, the RNA pellet was air-dried, dissolved in RNase-free distilled water and stored at − 80°C. RNA concentration was measured at 260 and 280 nm to ensure a ratio of >1·7 indicating pure and non-contaminated RNA.
The QIAGEN multiplex real-time PCR kit (Qiagen, Valencia, CA, USA) was used to generate complementary DNA for real-time PCR analysis according to the manufacturer's instructions. All the analyses were done using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Austin, TX, USA). The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase, was quantified as control. All genes were analysed using the Taqman® gene expression Assay-on-Demand kits (Applied Biosystems). Complementary DNA production was performed by incubation of RNA ( < 125 ng/25 μl reaction) at 50°C for 20 min, 95°C for 15 min, then forty cycles of 45 s at 94°C and 45 s at 60°C.
Quantification of expression of the target gene in samples was accomplished by measuring the fractional cycle number at which the amount of expression reaches a fixed cycle threshold (C T). The relative expression level of target gene was determined as 2− ΔΔC T, where ΔΔC T is the tumour sample ΔC T minus the reference ΔC T (control sample) with triplicate runs(Reference Saggar, Chen and Corey25, Reference Saggar, Chen and Corey28, Reference Livak and Schmittgen29).
Immunohistochemistry
The immunohistochemical staining was as described previously(Reference Chen, Hui and Ip11–Reference Chen, Saggar and Corey15, Reference Saggar, Chen and Corey25, Reference Saggar, Chen and Corey28). Briefly, tumour sections (n 10 except n 9 for Ki-67) were treated in Tris–EDTA buffer (10 mmol Tris–HCl and 1 mm-EDTA at pH 9) in a microwave to retrieve antigen. The primary antibodies were rabbit antihuman mono- or polyclonal antibody and they were diluted as follows: Ki-67 at 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), amplified in breast 1 (AIB1) at 1:200 (Santa Cruz Biotechnology), ERα at 1:400 (Santa Cruz Biotechnology), HER2 at 1:400 (Dako Cytomation, Mississauga, ON, Canada), phosphospecific ERα at 1:50 (all phosphospecific antibodies from Cell Signalling, Pickering, ON, Canada), phosphospecific HER2 at 1:100, phosphospecific mitogen-activated protein kinase (MAPK) at 1:100 and phosphospecific Akt 1:100. They were diluted in Diluent buffer (Dako Cytomation) to block any non-specific antigens. The sections with specific antibody were incubated at 4°C overnight, then after washing, incubated with biotinylated swine antirabbit IgG (Dako Cytomation). Streptavidin–horseradish peroxidase and aminoethylcarbazole (AEC) chromogen (Dako Cytomation) were used to demonstrate the site of the target antigen. The slides were observed blindly with a light microscope at 400 × magnification. For Ki-67, over 1000 cells from five different fields were counted, and the labelling index was calculated as percentage of positive cells over total cells counted. For assessing expression of other biomarkers, the Allred scoring method(Reference Allred, Harvey and Berardo30) was used, where intensity score (range: 0 = negative to 3 = strong staining) and proportion score (range: 0 = 0 % positive to 5 = 100 % positive) were combined to a total score up to 8 (range: 0–8).
Apoptosis (n 9) was measured by the ApopTag Detection kit (Chemicon, Temecula, CA, USA)(Reference Chen, Hui and Ip11–Reference Chen, Saggar and Corey15, Reference Saggar, Chen and Corey25). Briefly, proteinase K (20 μg/ml) was used to pretreat deparaffinised and rehydrated sections. The sections were then incubated with terminal transferase and digoxigenin deoxyuridine triphosphate at 37°C for 1 h, followed by incubation with antidigoxigenin antibody coupled to horseradish peroxidase for 30 min. The slides were then incubated with diaminobenzidine for 6 min, and counter-stained with methyl green. The number of tumour cells showing positive nuclear immunoreactivity was counted blindly and expressed as apoptotic cell number per mm2 at 400 × magnification with a microscopic grid.
Statistical analysis
All data showed no departure from normality and are presented as means with their standard errors. Palpable tumour area was calculated as the mean of all tumours in the mouse, i.e. mouse as the unit. The paired Student's t test was used to compare the difference in palpable tumour area between week 0 (pre-treatment) and week 7 within the same group. A 2 × 2 factorial analysis was used to determine the main (overall) and interaction effects of FC and TAM on the extent of tumour regression (tumour area at week 0 minus tumour area at week 7), and the biomarkers such as uterine weight, ER − and growth factor-related genes and proteins. A significant main effect of one factor (FC or TAM) indicates that the overall direction of effect (increase or decrease) of that factor is consistent across the level (e.g. presence or absence) of the other factor. An interaction effect indicates that the effect of one factor depends on the effect of the other factor. The significance level was set at P < 0·05, but for interaction, P < 0·10 is acceptable among statisticians. Tukey's pairwise comparison test was used to compare groups with each other including the effect of TAM alone v. the TAM/FC combination. Group differences in food intake, body weight and other organ weights were analysed by one-way ANOVA followed by Tukey's test to determine differences among groups. All analyses were done using SigmaStat version 3.5 (Systat Software, Inc., San Jose, CA, USA).
Results
Food intake, body weight gain and organ weight
There were no significant differences among groups in food intake, weight gain or major organ weights (data not provided). TAM caused a significant overall effect in increasing the uterine weight (P = 0·001) (Table 2), indicating an oestrogenic effect. FC tended to interact with TAM (P = 0·07), so that the uterine weight in the TAM/FC group did not differ from the BD control. The uterine weight in the FC/TAM group tended to be lighter (24 %; P = 0·07) than that in TAM group alone (Table 2).
Table 2 Effects of flaxseed cotyledon (FC) fraction and tamoxifen (TAM) on uterus and tumour biomarkers
(Mean values with their standard errors)

ER, oestrogen receptor; PgR, progesterone receptor; pERα, phosphospecific oestrogen receptor α; AIB1, amplified in breast 1; HER2, human epidermal growth factor receptor 2; IGF-1R, insulin-like growth factor 1 receptor; pHER2, phosphospecific HER2.
a,b Mean values with unlike superscript letters within a row were significantly different (P < 0·05) among the four groups.
* Unit: arbitrary unit with mRNA expression in basal diet as 1.
† Unit: score (scores of intensity and proportion of immunostaining by the Allred scoring method).
Palpable tumour growth, cell proliferation and apoptosis
The mean palpable tumour area before treatment at week 0 (approximately 30 mm2) did not significantly differ among groups. At week 7, after E2 pellet removal, tumour area in the BD, FC and FC/TAM groups significantly decreased (P < 0·001) by 43, 56 and 43 %, respectively, while that in the TAM group did not significantly change (Fig. 1). The FC and TAM had significant main (overall) effects (P = 0·002 and 0·006, respectively) on the extent of tumour regression, but there was no significant interaction between FC and TAM. However, the extent of regression was higher when FC was combined with TAM treatment (FC/TAM group) compared to TAM treatment alone (P = 0·011).
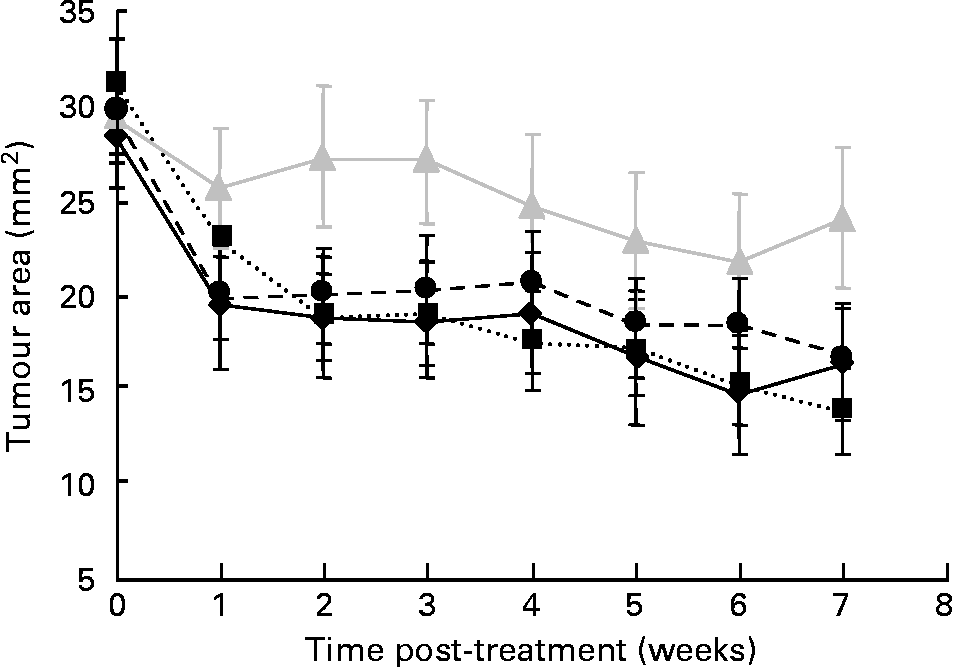
Fig. 1 Effect of flaxseed cotyledon (FC) and tamoxifen (TAM), alone or in combination, on regression of palpable MCF-7 breast tumour over treatment time in ovariectomised athymic mice. Data are means with their standard errors. Extent of regression: main effect: FC, P = 0·002; TAM, P = 0·006; interaction: FC × TAM, P = 0·572. –♦–, basal diet; ···■···, FC;![]() , TAM; - -●- -, FC/TAM.
, TAM; - -●- -, FC/TAM.
A significant overall effect of FC in lowering (P = 0·001) and of TAM in increasing (P = 0·021) cell proliferation (Ki-67 index) was observed (Fig. 2(a)). Compared to the BD group, the FC group had a lower (P = 0·007) Ki-67 index, while the TAM group had a higher (P = 0·009) Ki-67 index. There was no significant interaction between FC and TAM in cell proliferation; however, compared with TAM treatment alone, the combination of FC and TAM (FC/TAM group) induced lower cell proliferation (P < 0·001). In contrast, compared to the BD, FC had no significant effect, while TAM significantly lowered the apoptosis index (P = 0·021) (Fig. 2(b)). The FC/TAM group tended to have an effect intermediate between the FC and TAM groups (v. FC, P = 0·063; v. TAM, P = 0·074). Consistent with decreasing apoptosis, a significant overall effect of increasing Bcl2 mRNA expression (P = 0·016) was noted in the TAM group but not in the FC group (Table 2).

Fig. 2 Effect of flaxseed cotyledon (FC) and tamoxifen (TAM), alone or in combination, on (a) cell proliferation (Ki-67-labelling index) and (b) apoptotic index of MCF-7 breast tumours in ovariectomised athymic mice. Data are means with their standard errors. (a) Main effect: FC, P < 0·001; TAM, P = 0·021; interaction: FC × TAM, P = 0·134. (b) Main effect: FC, P = 0·032; TAM, P = 0·004; interaction: FC × TAM, P = 0·718. BD, basal diet.
Expression of oestrogen receptor-related tumour biomarkers
For systematic analysis of mechanism of action, we tested the effect of the treatments on the expression of ER at transcription (mRNA), translation (protein) and phosphorylation (functional status). FC only tended to induce (P = 0·053) the expression of ERα mRNA (Table 2), but it caused a significant overall reduction in ERα (P = 0·019) and phosphospecific ERα (P = 0·032) proteins (Table 2), indicating that ERα played a role in the FC effect. TAM had no overall effect on the expression of ERα protein and mRNA, but its interaction tendency with FC (P = 0·08) caused a large drop in ERα protein from 4·74 to 3·67 (P = 0·005) (Table 2). The FC diet did not change the ERβ and progesterone receptor mRNA expressions, but had an overall effect in suppressing pS2 mRNA (P = 0·041) (Table 2). However, TAM had an overall effect in suppressing ERβ mRNA expression (P = 0·01) and increasing progesterone receptor mRNA expression (P = 0·009), but had no effect on pS2 mRNA expression (Table 2). TAM has a significant overall effect in decreasing ERβ mRNA and significantly interacted with FC (P = 0·034) to reverse the effect. Further, FC had an overall effect in decreasing AIB1 protein expression (P = 0·002) (Table 2).
Expression of growth factor-related biomarkers
HER2 mRNA expression was not modulated by the treatments (Table 2). However, FC had an overall effect in reducing the level of HER2 (P = 0·001) and phosphospecific HER2 (P = 0·044) proteins (Table 2). In contrast to FC, TAM tended to have an overall effect in increasing HER2 protein level (P = 0·059) and had a significant effect in elevating phosphospecific HER2 protein (P = 0·009) (Table 2). Both FC and TAM had an overall effect in modulating IGF-1R mRNA expression, decreasing it in the case of FC (P = 0·012) and increasing it in the case of TAM (P = 0·023) (Table 2). FC demonstrated an overall effect in suppressing the phosphorylation of MAPK (P = 0·002) and Akt (P = 0·036), while TAM showed no effect (Fig. 3).

Fig. 3 Effect of flaxseed cotyledon (FC) and tamoxifen (TAM), alone or in combination, on the expression of (a) phosphospecific mitogen activated protein kinase (pMAPK) and (b) phosphospecific Akt (pAkt). Data are means with their standard errors. (a) Main effect: FC, P = 0·002; TAM, P = 0·272; interaction: FC × TAM, P = 0·663. (b) Main effect: FC, P = 0·036; TAM, P = 0·598; interaction: FC × TAM, P = 0·943. BD, basal diet.
There were no significant interactions between FC and TAM in all ER-related and growth factor-related tumour biomarkers, except in ERβ mRNA (P = 0·034) and a tendency in ERα protein (P = 0·080). However, when compared to TAM treatment alone, there were reductions in many biomarkers when FC was combined with TAM treatment, e.g. reductions of 24 % in phosphospecific ERα (P = 0·023), 25 % in AIB1 (P = 0·009), 23 % in HER2 protein (P = 0·009), 22 % in phosphospecific HER2 (P = 0·02) and 50 % in IGF-1R (P = 0·01). Combining FC diet with TAM treatment also reduced phosphospecific MAPK by 25 % (P = 0·044).
Discussion
The present study demonstrated that, after removal of the E2 pellet to reduce circulating E2 to simulate the postmenopausal situation, established MCF-7 tumours in the BD, FC and FC/TAM groups, but not in the TAM group, significantly regressed in size. Dietary FC, at the level present in 10 % FS, alone or combined with TAM treatment, did not promote tumour growth, in agreement with our previous studies, which showed that the whole FS does not promote tumour growth and does not interfere but rather strengthens the TAM effect(Reference Chen, Hui and Ip11, Reference Chen, Power and Mann13). Moreover, the present study suggests that FC acted in part through down-regulation of ER − and growth factor-mediated signalling pathways.
Because the FC fraction represents about 82 % of the whole FS, the FC amount added to the diet was 82 g/kg diet, equivalent to amounts in a 10 % FS diet found effective in our previous studies. The regression of pre-treatment tumour size caused by the FC diet (56 %) was close to that caused by the 10 % FS diet (62 %)(Reference Chen, Saggar and Corey15) and pure FO at 40 g/kg diet (58 %)(Reference Saggar, Chen and Corey28), but less than that caused by pure SDG at 1 g/kg diet (70 %)(Reference Chen, Saggar and Corey15), which were all tested for 8 weeks under the same conditions used in the present study. The FC in the present study and the FO were prepared from the same batch of FS used in the previous study(Reference Saggar, Chen and Corey25, Reference Saggar, Chen and Corey28). The FC/TAM group had 43 % reduction in tumour size, very similar to the reductions seen with the FS/TAM (47 %)(Reference Chen, Hui and Ip11) and FO/TAM (44 %)(Reference Saggar, Chen and Corey25) groups, but higher than that seen with the SDG/TAM (24 %)(Reference Saggar, Chen and Corey25) groups. All these indicate that FC has similar effect to diets supplemented with whole FS or pure FO, when used either alone or combined with TAM. Thus, the FC at levels equivalent to that in 10 % FS diet may potentially be consumed as an alternative to FS or FO.
TAM is the primary drug for ER+ breast cancer(Reference Furr and Jordan31–Reference MacGregor and Jordan33). However, due to its anti- and weak oestrogenic properties, TAM treatment induces side effects including menopausal-like symptoms and increased risk of endometrial carcinogenesis. TAM resistance also occurs, i.e. tumours regrow, after prolonged treatment(Reference MacGregor and Jordan33, Reference Ring and Dowsett34). Therefore, many patients taking TAM use complementary dietary supplements including phyto-oestrogen-rich foods to alleviate menopausal-like symptoms and supplement the tumour-reducing effect of TAM(Reference Duffy and Cyr35). Because FS is the third most commonly used dietary supplement by patients practising alternative/complementary therapies(Reference Boon, Olatunde and Zick21), the effect of TAM in combination with 10 % FS, or pure SDG and FO, at equivalent levels in 10 % FS has been tested(Reference Chen, Hui and Ip11, Reference Chen, Power and Mann13, Reference Chen, Power and Mann14, Reference Saggar, Chen and Corey25). These studies have shown that 10 % FS and its components, particularly FO, can enhance the tumour-inhibitory effect of TAM at a low E2 level in ovariectomised athymic mice with established ER+ human breast cancer (MCF-7)(Reference Chen, Hui and Ip11, Reference Chen, Power and Mann13, Reference Saggar, Chen and Corey25). The present study showed that FC, the FO-rich fraction of FS, combined with TAM similarly regressed tumour size better than TAM treatment alone.
TAM is antagonistic to E2 by competing for binding with ER, inhibiting its transcriptional activation, thereby decreasing cell proliferation(Reference MacGregor and Jordan33, Reference Osborne, Bardou and Hopp36). However, at low circulating E2 levels or low tumour E2 synthesis, TAM binding to ER may also induce a weak oestrogenic/tumour-promoting effect after prolonged exposure(Reference Osborne, Bardou and Hopp36). Hence, diminishing the oestrogenicity caused by TAM would prolong the effect of TAM and delay the development of resistance. Although the present study was carried out for only 8 weeks, not long enough to induce TAM resistance, TAM treatment overall exhibited higher oestrogenicity in some biomarkers, such as uterus weight and expression of ERβ and progesterone receptor. TAM alone also did not significantly regress the tumour size over the treatment period. However, the FC diet combined with TAM treatment induced significant tumour regression, and FC showed overall effect in decreasing the expression of oestrogen-related biomarkers, such as ERα and phosphospecific ERα proteins, pS2 mRNA and AIB1 protein. Moreover, the FC/TAM treatment had a tendency to interactively reduce the uterine weight, which is similar to the results of FS/TAM treatment(Reference Chen, Hui and Ip11). All of these suggest that FC enhanced TAM effectiveness at least in part through the down-regulation of the oestrogen-sensitive ERα-mediated pathway.
Although FC had a marginal effect in increasing the expression of ERα mRNA, it had a significant overall effect in decreasing the ERα protein and its phosphorylation. This suggests that FC may directly affect ERα either through degradation or through reduced translation and activation in MCF-7 tumours, which is similar to the findings with FS treatment(Reference Chen, Power and Mann13). pS2 mRNA expression is positively associated with oestrogenicity because its transcription is a primary response to oestrogen in breast cancer cells(Reference Soto, Fernandez and Luizzi37, Reference Van Meeuwen, Ter Burg and Piersma38). pS2 gene transcription was reduced by FC, indicating a decrease in oestrogenicity that then led to the tumour regression.
The overexpression and phosphorylation of AIB1, an ERα co-activator, can lead to ERα-mediated transcription, which confers resistance in in vitro and in xenograft models(Reference Osborne, Bardou and Hopp36, Reference Ali and Coombes39), and is associated with reduced responsiveness to TAM in patients(Reference Le Romancer, Treilleux and Leconte40). Knockdown of AIB1 can restore the antitumour effects of TAM(Reference Anzick, Kononen and Walker41). The dietary FC suppressed the expression of AIB1 protein, which is similar to the effect of the lignan SDG(Reference Saggar, Chen and Corey25). A previous study showed that pure SDG can suppress the expression of AIB1, while FO cannot down-regulate it(Reference Saggar, Chen and Corey25). Therefore, the lignan component probably contributed to the effect of FC in AIB1 down-regulation. It underlines the significance of SDG in FC and also suggests that FS with a higher SDG level than FC may have a greater effect on A1B1 regulation than FC.
Down-regulation of the growth factor signalling pathway is another strategy to regress tumour growth and enhance the responsiveness to TAM treatment. Increased expression of epidermal growth factor receptor (EGFR), HER2 and IGF-1R can elicit TAM resistance(Reference Ring and Dowsett34, Reference Osborne, Bardou and Hopp36, Reference Shou, Massarweh and Osborne42, Reference Nicholson, Hutcheson and Britton43), similar to its ability to activate the components of their downstream signalling pathways, particularly the MAPK and PI3K pathways(Reference Ghayad, Vendrell and Larbi44–Reference Knowlden, Hutcheson and Jones46). The present study found that TAM tended to increase the expression of HER2 protein and significantly increased the IGF-1R mRNA, and phosphorylation of HER2, while FC treatment had an overall opposite effect and also suppressed the activation of MAPK and Akt. These results are in agreement with our previous findings that dietary FS can reduce the protein expression of EGFR, HER2, IGF-1 or IGF-1R in mice with ER − human tumours(Reference Chen, Stavro and Thompson17), in TAM-treated MCF-7 tumours(Reference Chen, Power and Mann13, Reference Chen, Power and Mann14, Reference Saggar, Chen and Corey25) and in breast cancer patients(Reference Thompson, Chen and Li20). Hence, the down-regulation of growth factor receptors, such as EGFR, HER2 and IGF-1R, can suppress the activation of protein kinases MAPK and Akt downstream to the cascade of cyclin-dependent kinases(Reference Butt, McNeil and Musgrove47, Reference Mester and Redeuilh48), resulting in decreased cell proliferation and increased apoptosis found in the present study. Because ER can also be activated as a consequence of signalling events downstream of tyrosine kinase receptors such as EGFR, HER2 and IGF-1R(Reference Osborne, Bardou and Hopp36, Reference Nicholson, Hutcheson and Britton43, Reference Song, Chen and Zhang49–Reference Gee, Robertson and Gutteridge51), the down-regulation of the signalling pathway will also block the crosstalk between ER and these receptors to inhibit the activation of transcription. Therefore, the dietary FC in the present study improved the responsiveness of TAM in reducing tumour growth through, at least in part, the down-regulation of HER2 and IGF-1R expression and the activation of the MAPK and Akt cascade.
In the present study, the FC fraction was administered at a level found in a 10 % FS diet, which is about 18 % less than the amount in the whole seed. The 10 % FS is equivalent to the human consumption of 25–50 g (2·5–5 tablespoons) of FS per day depending on the person's food consumption(Reference Thompson, Seidl and Rickard10). Thus, 20–40 g of FC (2–4 tablespoons) can be consumed by those who cannot tolerate the large amount of FS. The present study did not reveal any side or oestrogenic effects when FC was used alone or in combination with TAM, which is similar to our previous FS studies(Reference Chen, Hui and Ip11–Reference Wang, Chen and Thompson19). It further suggests that dietary FS or its fractions may be safe when used as a complementary therapy for breast cancer.
In conclusion, FC did not promote but rather had a main effect in reducing the growth of ER+ human breast tumours at low circulating levels of E2. It enhanced the regression of TAM-treated tumours and, alone or combined with TAM treatment, reduced cell proliferation and increased apoptosis in part through modulation of ER − and growth factor-mediated signalling pathways. The effect of FC was similar to those previously observed with FS in increasing the effectiveness of TAM, suggesting that FC may potentially substitute for FS.
Acknowledgements
The present study was financially supported in part by Natural Sciences and Engineering Research Council of Canada, Canadian Institute of Health Research and Canadian Breast Cancer Research Alliance. J. C., P. C. and L. U. T. designed the research; J. C. and J. K. S. conducted the research; J. C. and J. K. S. analysed data; J. C. and L. U. T. wrote the paper with comments from other authors; L. U. T. had primary responsibility for the final content. All the authors read and approved the final manuscript. The authors also wish to thank Pizzey's Nutritionals for kindly providing the samples of FC fraction. None of the authors had any personal or financial conflict of interest.







