The most common mental disorders, depression and anxiety, are global health problems in both developed and developing countries( Reference Baxter, Scott and Vos 1 , Reference Steel, Marnane and Iranpour 2 ). Major depression and anxiety affect about 4·7 and 7·3 % of people worldwide, respectively( Reference Baxter, Scott and Vos 1 , Reference Ferrari, Somerville and Baxter 3 ). Reports from Iran suggest that about 21·0 and 20·8 % of adults have anxiety and depressive symptoms, respectively( Reference Noorbala, Yazdi and Yasamy 4 ). On average, mental health disorders occur in Iranian women 1·7 times higher than men (29 v. 16 %)( Reference Noorbala, Yazdi and Yasamy 4 ). Several studies have shown a significant association among chronic diseases, depression and anxiety( Reference Vaccarino, McClure and Johnson 5 – Reference van Reedt Dortland, Giltay and Van Veen 7 ). Since mental disorders impose a significant economic burden, and contribute to disability and premature death( Reference Olesen, Gustavsson and Svensson 8 , Reference Sobocki, Jönsson and Angst 9 ), it is important to find appropriate strategies to prevent these disorders.
A poor dietary pattern and physical inactivity are modifiable risk factors which may improve mental health outcomes( Reference Popa and Ladea 10 , Reference Quirk, Williams and O’Neil 11 ). Among the dietary factors, foods rich in phytochemical compounds including fruits, vegetables, nuts, legumes, soya, whole grains and olive oil have been associated with lower risk of mental health disorders( Reference Hosseinzadeh, Vafa and Esmaillzadeh 12 – Reference Su, Yu and He 16 ).
Phytochemicals are plant-based non-nutritive bioactive compounds including phenolic compounds (flavonoids, phenolic acids, hydroxycinammic acids, lignans, tyrosol esters, stilbenoids), isoperenoids, organosulphur compounds (allyl sulphurs, isothiocyanate)( Reference Han, Shen and Lou 17 – Reference Tucci 19 ). Interestingly, intake of some of the phytochemicals have been associated with decreased risk of mortality( Reference Grosso, Micek and Godos 20 ), CVD( Reference Wang, Ouyang and Liu 21 ), diabetes( Reference Liu, Zhan and Liu 22 ) and several cancers( Reference Grosso, Godos and Lamuela-Raventos 23 ). Determining the amount of phytochemical compounds in the food supply or in human tissue samples is expensive, laborious and impractical for large populations. Therefore, alternative methods should be used to determine the phytochemical content of the foods. The dietary phytochemical index (DPI), which was presented for the first time by McCarty( Reference McCarty 24 ), is defined as the percentage of energy intake derived from phytochemical-rich foods. This index is an easy and practical method for the assessment of phytochemical intake and diet quality in clinical practice. Several epidemiologic studies have investigated the association between DPI and CVD risk factors including oxidative stress, obesity, blood pressure, blood sugar, lipid profile and cancer( Reference Bahadoran, Golzarand and Mirmiran 25 – Reference Abshirini, Mahaki and Bagheri 33 ). These studies showed the DPI was inversely related to obesity, oxidative stress, hypertriacylglycerolaemia, hypercholesterolaemia, insulin resistance, prediabetes, hypertension and breast cancer.
We are not aware of any publications regarding DPI and mental health. Therefore, this study aimed to determine the association between DPI and mental health in Iranian women.
Methods
Study design and participants
In this cross-sectional study, 488 women from ten health centres affiliated with the Tehran University of Medical Sciences (TUMS) were recruited using multistage cluster sampling. The study sample size was determined using the prevalence of mental disorders( Reference Noorbala, Yazdi and Yasamy 4 ) as the dependent variable, employing the formula: N=[(Z 1−α /2)2 P(1−P)]/d 2. Using P=29, d=4·03 and α=0·05, we obtained 487 subjects. Women were included if they were between 20 and 50 years old, Iranian, not pregnant or lactating, not postmenopausal, not suffering from depression diagnosed by a psychiatrist or using anti-depressive drugs in the past 12 months, no history of an unfortunate events (such as severe financial problems, death of first-degree relatives or friends, love failure) in the past 6 months, not history of chronic diseases such as diabetes, cardiovascular, cancer, liver, kidney, pulmonary, thyroid, hypertension, epilepsy, multiple sclerosis disease, and were not following a specialised diet. In the current study, we excluded twenty-five participants whose total daily energy intake was outside the range of 2092–14644 kJ/d. Each participant provided informed written consent. In total, 488 participants were included in the statistical analysis. The study received ethics approval by the Medical Research Ethics Committee of TUMS.
Dietary assessment
Dietary intake was assessed using a 168-item semi-quantitative FFQ, which has been previously assessed for reliability and validity( Reference Azadbakht, Mirmiran and Esmaillzadeh 34 ). All FFQ were completed through face-to-face interviews by a trained nutritionist. To convert the portion sizes of the consumed foods to grams, household measures were used. Mean energy and nutrient intakes from the FFQ were calculated using a modified version of NUTRITIONIST IV software for Iranian foods (version 7.0; N-Squared Computing).
Phytochemical index calculation
The DPI was determined based on method developed by McCarty; (DPI=(daily energy derived from phytochemical-rich foods (kJ)/total daily energy intake (kJ))×100)( Reference McCarty 24 ). Fruits, vegetables, legumes, whole grains, nuts, soya products, seeds and olive oil were considered as phytochemical-rich foods. Potatoes were not included due to their poor source of phytochemicals. Natural fruit and vegetable juices as well as tomato sauces were considered in the fruit and vegetable groups due to their high phytochemical content.
Assessment of psychological profile
The psychological status of the participants was assessed by the validated depression, anxiety and stress scales (DASS-21) questionnaire( Reference Henry and Crawford 35 ). Each items of DASS-21 (depression, anxiety, psychological distress) contains seven questions. The answers were divided into four categories: zero, low, medium and high with a score between 0 and 3, respectively. DASS-21 is the summarised form of DASS-42. So, the total points for each item should be multiplied by 2. Based on the total score, the subjects were ranked into five groups of normal (0–9), mild (10–13), moderate (14–20), severe (21–27) and very severe (>27) for depressive symptoms. These descriptions for five groups of anxiety and stress were 0–7, 8–9, 10–14, 15–19, >20 and 0–14, 15–18, 19–25, 26–33, >33, respectively. However, due to the limited number of cases in some groups, they were divided into two groups of normal and mild/moderate/severe/very severe. Depressive symptoms, anxiety and stress were defined as scores of equal or greater than 10, 8, and 15, respectively.
Assessment of other variables
The required information on other variables including age, marital status, socio-economic status (SES), smoking status, pregnancy or lactating, number of births, chronic diseases (diabetes, cardiovascular, cancer, liver, kidney, pulmonary, thyroid, hypertension, epilepsy, multiple sclerosis disease), family history of chronic disease, drug and supplement use, adherence to a specialised diet, menopause status, sleep duration, time away from home, stressful events in the past 6 months, and body size satisfaction was obtained from demographic questionnaires. SES score was computed as an index of SES based on family situation (head of family, self-care, under supervision), family size (≤4, >4 people), education (≤diploma, >diploma), head of family education, occupation (housewife/unemployed/other, worker/free job, retired/employee, manager), head of family job, number of employed member(s) of the family, number of rooms in the home, children under 18-year-old (yes/no), welfare status and amount of travel within and outside Iran (yes/no).
Body weight was measured to the nearest 0·1 kg, while participants wore minimal clothing and barefoot, using a digital scale (SECA). Height was measured to the nearest millimeter with an inflexible measuring rod. BMI calculated as weight in kg divided by height in m2. Physical activity was determined based on metabolic equivalents×h/d (MET-h/d) by recording physical activity over 24 h and the level of physical activity of individuals was calculated as MET-h/d( Reference Seyed, Gholi and Saraf 36 ).
Statistical analysis
General characteristics across tertiles of DPI were expressed as means and standard deviations for continuous variables and numbers and percentages for categorical variables. To examine the differences across tertiles, we used an ANOVA for continuous variables and a χ 2 test for categorical variables. Dietary intakes of study participants across tertiles of DPI were compared using ANCOVA and all values were adjusted for energy intake. We used binary logistic regressions to estimate OR and 95 % CI for psychological profile across tertiles of DPI in crude and multivariable-adjusted models. In these analyses, age, total energy intake, SES (low, medium and high), marital status (married, single), physical activity, supplement use (yes/no), drug use (yes/no), family history of chronic disease (yes/no), sleep duration, time away from home, body size image (normal, abnormal) and BMI were controlled in the adjusted model. P trend was determined by considering tertiles of DPI as ordinal variables in the logistic regression analysis. All statistical analyses were completed using the Statistical Package for Social Sciences (version 21; SPSS Inc.). P<0·05 was considered to be statistically significant.
Results
The general characteristics of the study participants across tertiles of DPI are presented in Table 1. Compared with those in the first tertile, the number of participants in the highest tertile of DPI were lower in the low SES level, and more likely to be married and were older. No other significant difference was seen in the general characteristics across tertiles of DPI.
Table 1 General characteristics of participants across the tertiles (T) of dietary phytochemical index (DPI) (Mean values and standard deviations; numbers and percentages)
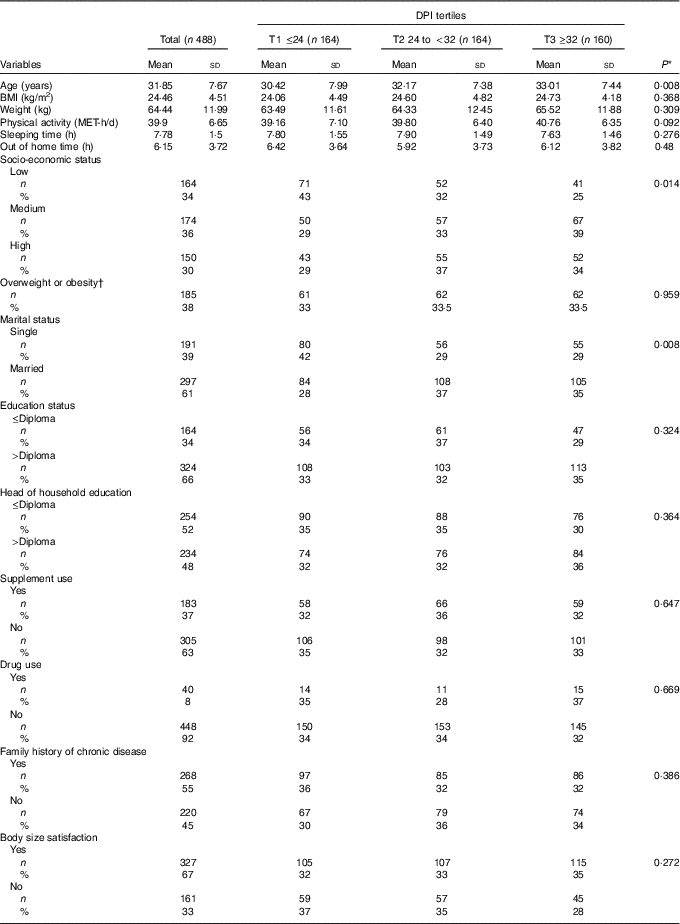
MET, metabolic equivalents.
* Obtained from ANOVA for continuous variables and χ 2 test for categorical variables.
† BMI ≥25 kg/m2.
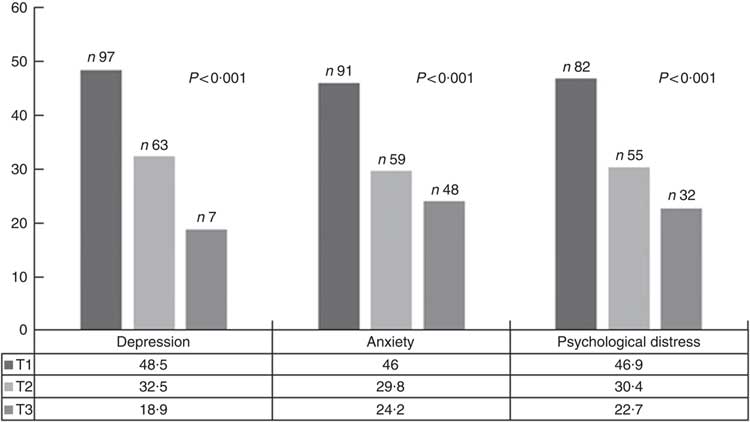
The prevalence of symptoms of depression, anxiety and psychological distress were 34·6, 40·6 and 42·4 % of the study participants, respectively. The prevalence of depressive symptoms, anxiety and psychological distress across tertiles of DPI is shown in Fig. 1. Symptoms of depression, anxiety and psychological distress were significantly less prevalent among individuals in the top tertile of DPI compared with those in the bottom tertile.
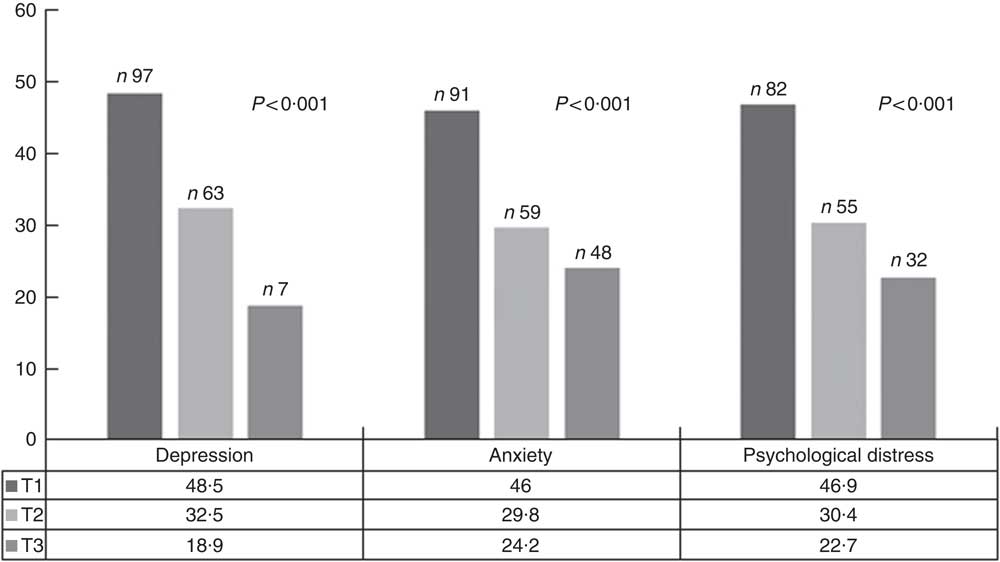
Fig. 1 Prevalence of depressive symptoms, anxiety and psychological distress across tertiles (T) of dietary phytochemical index. ![]() , T1;
, T1; ![]() , T2;
, T2; ![]() , T3.
, T3.
Dietary intakes of study participants across tertiles of DPI are provided in Table 2. Participants in the top tertile had higher intakes of fruits, whole grain, vegetables, legumes, nuts, olive oil, Zn, K, Mg, vitamin C, vitamin E, vitamin A, total fibre, total carotenoids, carbohydrate (P<0·001), seeds (P=0·004) compared with those in the bottom tertile. Participants in the highest DPI consumed lower fat (P=0·02), PUFA (P=0·03) and SFA (P<0·001). In addition, there were no significant differences across tertiles of DPI for soya (P=0·66), energy (P=0·81), protein (P=0·08), MUFA (P=0·54), cholesterol (P=0·13) and Ca (P=0·11).
Table 2 Multivariable-adjusted dietary intakes across the tertiles (T) of dietary phytochemical index (DPI)Footnote * (Mean values with their standard errors)
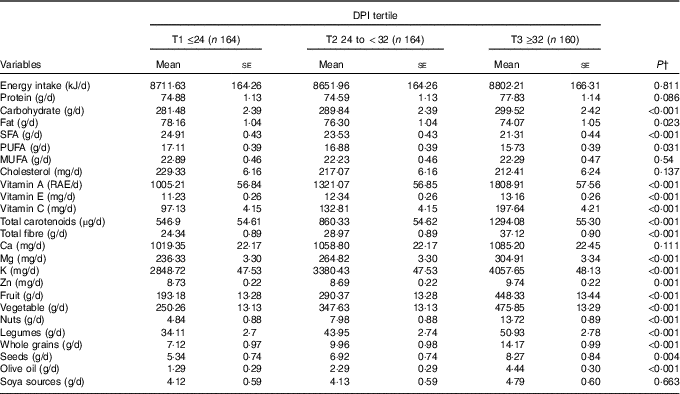
RAE, retinol activity equivalents.
* All values are adjusted for energy intake, except for total energy.
† Obtained from ANCOVA.
Crude and multivariable-adjusted OR (95 % CI) for mental health across tertiles of DPI for the whole population are presented in Table 3. After controlling for potential confounders, a significant association was observed between DPI and prevalence of symptoms of depression (OR 0·22; 95 % CI 0·12, 0·38; P<0·001) and anxiety (OR 0·33; 95 % CI 0·20, 0·55; P<0·001), as well as psychological distress (OR 0·30; 95 % CI 0·18, 0·49; P<0·001) among those in the highest v. lowest tertile of DPI.
Table 3 Symptoms of depression, anxiety and psychological distress across tertiles (T) of dietary phytochemical index (DPI) (Multivariable-adjusted odds ratios and 95 % confidence intervals)
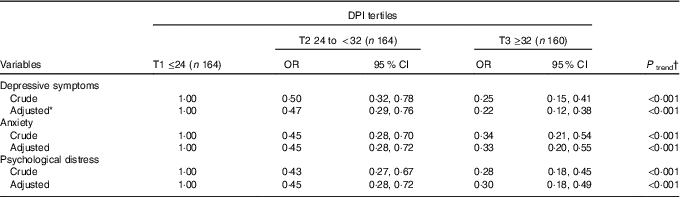
* Adjusted for age, energy intake, socio-economic status, physical activity, marriage status, supplement use, drug use, family history of chronic disease, satisfaction with the physical form, number of sleeping hours, and number of outside hours, BMI.
† Obtained from logistic regression by considering tertiles of DPI as ordinal variable.
Discussion
This cross-sectional study showed that the highest DPI was associated with decreased prevalence of symptoms of depression, anxiety and psychological stress in adult women.
Several studies have shown that plant foods are good sources to increase serum phytochemicals. Lipsky et al. ( Reference Lipsky, Cheon and Nansel 37 ) successfully demonstrated whole plant foods (whole grains, vegetables, whole fruit, legumes, nuts, seeds) were positively related to serum carotenoids. Two studies showed serum carotenoids are considered a reliable biomarker of fruit and vegetable intake( Reference van Kappel, Steghens and Zeleniuch-Jacquotte 38 , 39 ). In fact, foods rich in phytochemicals are able to increase plasma phytochemicals after consumption. Therefore, DPI as food rich phytochemical score can be good indication of phytochemicals. Vincent et al. ( Reference Vincent, Bourguignon and Taylor 26 ) showed the DPI was positively correlated with the total carotenoid intake. In addition, this study showed that DPI decreased oxidative stress significantly. Phytochemicals were identified as antioxidant rich compounds. Therefore, decreasing oxidative stress could be a good reason that DPI evaluate the consumption of phytochemicals.
The value of the DPI can be judged from the range of interesting epidemiological studies that have shown that a high-DPI diet is associated with a number of chronic disease. For example, several studies showed that DPI is inversely related to obesity, oxidative stress, hypertriacylglycerolaemia, hypercholesterolaemia, insulin resistance, hypertension, prediabetes and breast cancer( Reference Bahadoran, Golzarand and Mirmiran 25 – Reference Abshirini, Mahaki and Bagheri 33 ). This research is of importance, as it shows that diets in which a high proportion of energy content are provided by whole, phytochemical-rich plant foods can protect health. Although DPI is similar to other diet scores such as the prudent diet score and Mediterranean scores( Reference Li, Lv and Wei 40 ) which were associated to mental health, these consist of a score inclusive of meats and fish or have some unhealthy components which gave reverse scores.
Depression is a highly prevalent mental health disorder, which imposes a substantial burden to both individuals and society. It is expected to be the leading cause of disease and disability by the year 2030( Reference Payne, Steck and George 41 ). In this study, we found a significant inverse association between DPI and lower prevalence of depressive symptoms among Iranian women. Consistent with our findings, a previous meta-analysis showed dietary patterns characterised by high intakes of fruits, vegetables, whole grains, olive oil, fish, low-fat dairy products and antioxidants, and low content of animal foods have been associated with a lower odds of depression( Reference Li, Lv and Wei 40 ). In addition, other studies showed that adherence to the Mediterranean diet (fruits, vegetables, cereals, nuts, olive oil, fish and wine) was associated with components of mental health( Reference Muros, Cofre-Bolados and Arriscado 42 ), particularly depression( Reference Sánchez-Villegas, Martínez-González and Estruch 43 ) and psychological resilience( Reference Bonaccio, Di Castelnuovo and Costanzo 44 ). The common characteristic of the aforementioned food groups is a wide range of phytochemicals with antioxidant and anti-inflammatory properties. A cohort study showed a significant decreased risk of depressive symptoms among women with higher intakes of flavonoids( Reference Chang, Cassidy and Willett 45 ). In addition, epidemiological studies suggest that dietary polyphenols, such as flavonoids, phenolic acids and lignans may confer protection against depression( Reference Sureda and Tejada 46 ). Moreover, one study found an inverse association between dietary polyphenol intake and depressive symptoms( Reference Godos, Castellano and Ray 47 ).
Anxiety are common disorders and are associated with elevated risk of CHD( Reference Kubzansky, Kawachi and Weiss 48 , Reference Albert, Chae and Rexrode 49 ). However, there has been less attention to the association between dietary patterns and anxiety. In the present study, we found that adherence to a higher DPI was associated with decreased anxiety. Previous studies have shown that vegetarian and traditional diets which included high phytochemical foods were associated to lower incidence of anxiety( Reference Jacka, Pasco and Mykletun 50 , Reference Yannakoulia, Panagiotakos and Pitsavos 51 ). Higher healthy diet scores (greater intake of vegetables, fruits, dairy products, nuts, olive oil and green olive, fish, legumes and whole grains) was associated with better mental health and lower anxiety( Reference Jacka, Mykletun and Berk 52 ).
Psychological distress can increase the risk of stroke, myocardial infarction, ulcers and mental illnesses such as depression( Reference Sapolsky 53 ). We found that a higher DPI was related to decreased risk of psychological distress in women. In line with our finding, one study showed that consuming a Mediterranean diet was associated with a lower prevalence of psychological distress( Reference Hodge, Almeida and English 54 ).
Several possible mechanisms may explain the inverse association between DPI and mental health. Oxidative stress which is the imbalance between the production of reactive oxygen species and antioxidant defenses( Reference Birben, Sahiner and Sackesen 55 ) may contribute to the incidence of depression. The consumption of foods high in phytochemicals may decrease neuronal damage caused by oxidative stress( Reference Gougeon 56 – Reference Popa and Ladea 58 ). Recent studies have shown some phytochemicals inhibit monoamine oxidase activity, mitochondrial enzymes that catalyse the oxidation of monoamines including serotonin, norepinephrine and dopamine have been associated with depression( Reference Grosso, Valentão and Ferreres 59 ). Phytochemicals could increase mRNA expression of pro-opiomelanocortin (a precursor of several peptides that seems to be dysregulated in depression). Phytochemicals have anti-inflammatory activity by decreasing biomarkers of inflammation, including TNF-α and IL-6, inhibiting neuronal growth especially in the hippocampus, and modification of gastrointestinal bacteria ratios( Reference Dash, Clarke and Berk 60 , Reference Rendeiro, Rhodes and Spencer 61 ). Some studies demonstrated that inflammation involved in pathogenesis of mental health disorders( Reference Howren, Lamkin and Suls 62 ). Phytochemicals increase extracellular signal-regulated kinase phosphorylation, a main component of the neurotrophic and specific signaling pathways participating in neuronal differentiation and plasticity( Reference Xiong, Jiang and Wu 63 ). Phytochemicals also have been shown to affect multiple neurotrophic and neuroprotective mechanisms( Reference Bahramsoltani, Farzaei and Farahani 64 ).
This study has some limitations. First, DPI was estimated by the amount of energy provided from foods rich in phytochemicals. However, some foods with high phytochemical content such as green and black tea were not considered because they did not contribute energy. Second, we did not take into account the type of phytochemicals consumed, therefore, the quality of dietary phytochemicals may have differed among people with the same DPI score resulting in a more varied effect of mental health. Third, the cross-sectional study design cannot show causal link between DPI and mental health. Fourth, our study assessed only symptoms of depression and anxiety, but not the diagnosis of these disorders. Fifth, although we attempted to control for known confounding factors, there may have been some residual confounding. Finally, the study was performed only in females aged 20–50 years, which affects the generalisability to the larger population.
In conclusion, we found that higher DPI was associated with a lower risk of depressive symptoms, anxiety and psychological distress. These initial findings need to be confirmed with prospective studies to confirm the relationship between DPI and mental health.
Acknowledgements
The study was financially supported by TUMS.
This study was extracted from a dissertation that was approved by TUMS (no. 9511468003).
M. D. M., F. S., B. G. and L. A. contributed to conception, design, data collection, statistical analyses, data interpretation, manuscript drafting and approval of the final version of the manuscript and agreed for all aspects of the work. N. B. revised the manuscript and contributed to data interpretation.
None of the authors had any personal or financial conflicts of interest.