Introduction
Mucociliary health is essential for the protection of both the upper and lower respiratory tract. The cilia have an active and recovery phase that propels mucus upwards in the lower respiratory tract, backwards in the upper respiratory tract and downwards in the middle-ear cleft in an upright individual. This complex process involves the speed and coordination of cilia, the rheology of mucus influenced by water-soluble electrolytes, cells, proteins and mediators. Mucociliary clearance rate is similar in both the upper and lower respiratory tract.Reference Andersen and Proctor1
Respiratory diseases often affect mucociliary function, which is temporarily disordered in many cases. Excess secretions together with dead inflammatory and ciliated cells are shed over and above natural turnover before recovery. Children are prone to a catarrhal phase while their immunity to respiratory viruses develops, unless they develop allergies when it may continue. Such cases often presented to the paediatric otorhinolaryngologist.
Universal neonatal heal prick screening in the UK detects most patients with cystic fibrosis.Reference Murray, Cuckle, Taylor, Littelewood and Hewison2 Persistent infection, initially often bacterial with viral exacerbations, in the lower respiratory tract may result in permanent damage such as bronchiectasis. Fungal infection may become refractory as is sometimes the case in cystic fibrosis.Reference Leclair and Hogan3,Reference Pihet, Carrere, Cimon, Chabasse, Delhaes and Symoens4
A few children have syndromes where the cilia do not function adequately or at all, such as primary ciliary dyskinesia, a rare autosomal recessive condition.Reference Afzelius5 The condition of completely absent beats was previously called immotile cilia syndrome.Reference Kollberg, Mossberg, Afzelius, Philipson and Camner6,Reference Eliasson, Mossberg, Camner and Afzelius7 When this occurs, half the cases are associated with situs inversus.Reference Eliasson, Mossberg, Camner and Afzelius7 If this arises with bronchiectasis and sinusitis, it is called Kartagener syndrome.Reference Kartagener8,Reference Kartagener and Horlacher9 When less obvious, these chronic cases present diagnostic conundrums where it is difficult to determine cause or effect. As there is no definitive investigation, the study of ciliary action, tissue culture, ultrastructure and genetics may help in diagnosis and management.Reference Lucas, Burgess, Mitchison, Moya, Williamson and Hogg10,Reference Bush, Chodhari, Collins, Copeland, Hall and Harcourt11 In addition to these limitations, there are multiple sources of error in any test.
Nasal ciliated epithelium is easy to sample and biopsy by trained otorhinolaryngologists. Surface cells may be extracted by brushing. When transported in an appropriate medium, the cells may be examined under a phase-contrast microscope. If required, the ciliary beat frequency may be measured by collimating the light with a pinhole and recording the signal with a photodiode attached to a computer.Reference Rutland and Cole12 The ciliary beat frequency varies within a sample and in the respiratory tract but may be over 20 beats per second both in vivo and in vitro. It is above 5 beats per second in normal individuals.Reference Carson, Collier, Knowles, Boucher and Rose13 Nasal mucosa is continually renewed,Reference Hall and Levison14 and this may account for some of the variation seen within a sample. Biopsies may also be taken both for ultrastructural analysisReference Rutland, Dewar, Cox and Cole15 or for culture over several weeks to show whether newly generated epithelium has beating cilia.Reference Afzelius5 This may help differentiate primary and secondary conditions.
Techniques used to measure the ciliary beat frequency have evolved over the years.Reference Gray16–Reference Schipor, Palmer, Cohen and Cohen20 Computers display the measured ciliary beat frequency almost in real time. For analysis of the whole field of view under a microscope, a fast-frame digital camera may be used both to look at coordination and ciliary beat frequency. Such cameras will allow the pattern of ciliary movement to be evaluated by slowing the images.Reference Lucas, Burgess, Mitchison, Moya, Williamson and Hogg10,Reference Schipor, Palmer, Cohen and Cohen20,Reference Chilvers and O'Callaghan21
We reviewed the studies on the variation in temperature on ciliary beat frequency previously.Reference Clary-Meinesz, Cosson, Huitorel and Blaive22–Reference Green, Smallman, Logan and Drake-Lee24 The ambient air temperature at the anterior nares increases to around 31°C in the nasopharynx during inspiration.Reference Proctor, Andersen and Lundqvist25 During expiration, it drops from around 35°C at the nasopharynx. The surface temperature is higher than that of the air, but nasal cilia work at lower temperatures to those in the lower respiratory tract. In addition, the passage of air cools the surface by evaporation. A warmed stage on a microscope controls temperature, and the temperature should be noted when ciliary beat frequency is measured.
During our research of ciliary function in adults, we provided a diagnostic service at Birmingham Children's Hospital to help in the management of difficult respiratory cases. We present 92 children who had ciliary beat frequency measured, illustrating an important role for the paediatric otorhinolaryngologist in managing children with difficult airway problems.
Materials and methods
Patients
Ninety-two patients were referred to the service between 1996 and 2007. There were 29 females and 63 males (male to female ratio was just over 2:1). Their ages ranged from the neonatal period (5 patients) to 17 years. Fifty-two patients were under five years of age.
Provisional diagnoses
The provisional diagnoses were made by the referring clinicians following usual investigations. No patients with cystic fibrosis were referred. Although cases could be complex, we categorised the 92 patients into four main groups for analysis (Table 1): (1) refractory asthmatic patients; (2) bronchiectasis without dextrocardia; (3) dextrocardia (including patients with situs inversus and Kartagener syndrome); and (4) patients with recurrent or persistent respiratory infections.
Table 1. Diagnostic groups

The figures in brackets are the number of patients without a recordable ciliary beat frequency (CBF).
In the group of 46 patients with respiratory infections, 5 neonates were referred because of chest infections and failure to thrive, and 6 older children had unresolving upper respiratory tract infections. In those with lower respiratory infections, one male had a cardiac abnormality but not dextrocardia and had immotile cilia on testing. One child had laevocardia, a left liver and right stomach but had normal cilia on testing. A further child had transposition of the great vessels and normal cilia on testing. The remaining children had refractory lower respiratory tract infections.
Sampling
Except for the neonatal cases, sampling was performed under general anaesthesia, allowing a thorough examination of the nose and pain-free sampling after clearing nasal mucus. If a respiratory physician deemed it necessary to undertake a bronchoscopy, this could be coordinated and performed under the same general anaesthesia. On occasion, samples were taken for electron microscopy too.
No drugs were used on the nasal mucosa. A full nasal examination was performed in each case. Nasal polyps were identified in just one case in the respiratory infections group. Using a wetted 1.75 mm cytology brush, we sampled the nasal mucosa on both sides of the nose as described previously.Reference Green, Austin, Logan, Smallman and Drake-Lee19,Reference Robson, Smallman and Drake-Lee26,Reference Ahmad and Drake-Lee27 After collection, the brush was agitated in a modified Earle's salt solution initially and later in the study in viral transport medium.
Analysis
All specimens were examined by the first author. The cell suspension was transferred to the laboratory within six hours, having been kept at body temperature. A small drop was pipetted onto a welled microscope slide and a cover slip was applied. This was placed on a warmed stage, and the temperature was recorded when constant, initially between 35 and 37°C. Lower temperatures were used because of time constraints. This was repeated if the sample was inadequate.
Once aligned under a 0.05 mm pinhole, the specimen was examined using a phase-contrast microscope at ×400 magnification. Depending on the specimen, the ciliary beat frequency was measured on up to 25 groups of cells. The resulting interrupted beam was initially converted by a photomultiplier. When this failed, we replaced it with a photodiode. Both of these were connected to a microcomputer. The signal was analysed by software written in-house. Two versions of this software were written: the first was written in Borland Pascal (Borland, Austin, USA) software development system and the second, and later one, was written in LabView system software (National Instruments, Austin, USA). The results of the test were given to the referring clinician the next working day. When requested, we repeated the test.
Results
Our diagnostic groups are shown in Table 1. There were 29 female patients. When we looked for ciliated cells, they were absent in only one specimen, even though it was morphologically respiratory epithelium. This patient was previously diagnosed with primary ciliary dyskinesia and so was given a zero ciliary beat frequency for analysis. There were technical difficulties with the equipment for one patient, so the test was repeated. One patient was accidentally rereferred and the first ciliary beat frequency was used here (both were in the normal range).
Patients
The diagnostic groups and sex distribution are given in Table 1. Those without a recordable ciliary beat frequency are shown in brackets. This confirms the male prevalence seen in other studies. There was only one child with nasal polyps who had a ciliary beat frequency of 10 Hz at 28°C. This was reported as normal.
Twenty-six patients had no beating cilia, where the male to female ratio was 15:11 (1.4:1). This contrasted with the male to female ratio for beating cilia where it was 48:18 (2.7:1).
We retested patients when requested, and two examples of our reports when the ciliary beat frequency was zero are as follows. (1) There were large numbers of respiratory epithelial cells, which appeared dead, and there were no obviously beating cilia. The appearance was of a recent upper respiratory tract infection. Suggest a re-biopsy in a few weeks (the patient was not rereferred and is coded under respiratory infections). (2) This is a repeat biopsy and again there were no obviously beating cilia, and the epithelium contained large numbers of respiratory cells (the patient had situs inversus).
Although there is diagnostic doubt in children with asthma and respiratory infections, bronchiectasis and dextrocardia are much more certain.
Bronchiectasis
Only 1 of the 10 patients with bronchiectasis without dextrocardia had no beating cilia. The mean of the other nine patients was 9.9 Hz with a range of 7 to 12.7 Hz at mean temperature of 28°C.
Dextrocardia
Five of 18 patients had dextrocardia alone, and so situs inversus was present in 13. This included three patients diagnosed with Kartagener syndrome. One of the patients with dextrocardia had a 2q31 chromosome deletion and had no beating cilia. Five of the 18 children with dextrocardia had beating cilia. Two just had dextrocardia and three had situs inversus. In total, 13 of the 18 children had absent beating cilia, which is slightly higher than most series (see Discussion). Their mean ciliary beat frequency was 10.3 Hz with a range of 7.9 to 12.7 Hz at a mean temperature of 31°C.
Temperature
We evaluated the variation of ciliary beat frequency with changing temperature. A scatter plot (Figure 1) shows the distribution of ciliary beat frequency against temperature in the 66 children. Despite a constant temperature, there was a wide variation in ciliary beat frequency. Nineteen patients were recorded at 27°C, where the ciliary beat frequency was between 3.5 and 13.3 Hz with a mean of 8.2 Hz. Fifteen patients were recorded at 28°C, and the ciliary beat frequency ranged from 7 to 13.1 Hz with a mean of 9 Hz.
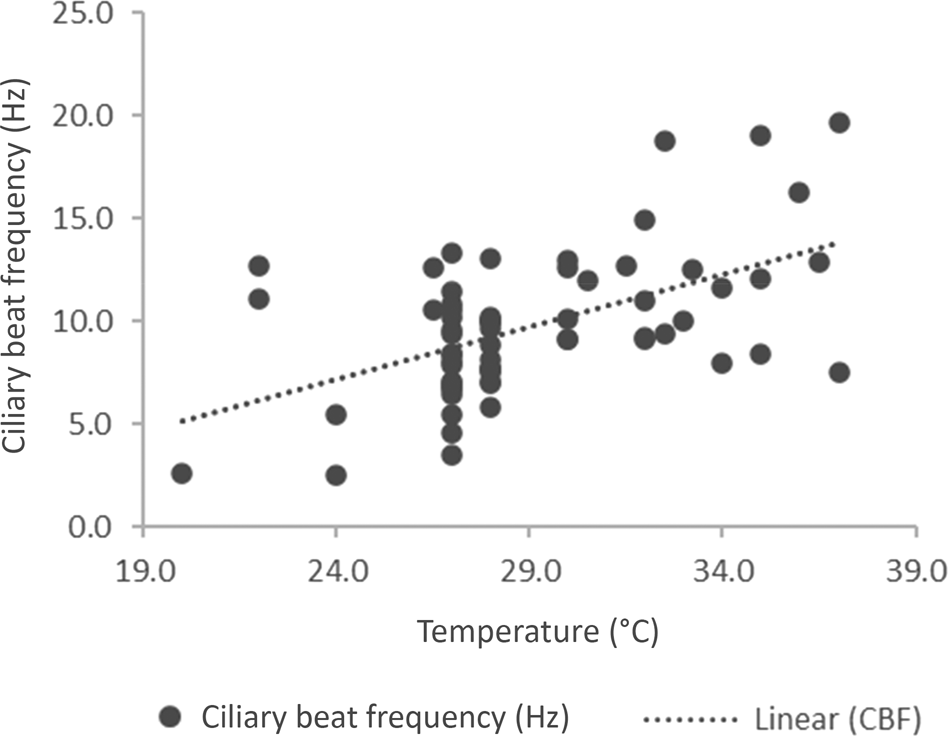
Fig. 1. A scatter plot of ciliary beat frequency against temperature for 66 patients (r = 0.282). CBF = ciliary beat frequency
From this, we evaluated the effect of temperature in two temperature ranges. Those with a temperature recorded at under 30°C and those between 30 and 37°C (Table 2). Even when the temperature was at least 30°C, 8 patients had a ciliary beat frequency below 10 Hz, and the child with the lowest ciliary beat frequency was measured at 37°C. The two samples were statistically different (p = 0.0003; unpaired, two tailed t-test for unequal sample sizes).
Table 2. Temperature: beating and non-beating cilia

Discussion
National Health Service reorganisation and the development of subspecialised clinical practice shaped the ENT service in Birmingham. Although the first author's adult practice was relatively constant, his paediatric service evolved through five hospitals culminating at the Birmingham Children's Hospital five years after appointment. A specialist adult rhinology clinic was set up at the Queen Elizabeth Hospital in 1987, and the investigation of the mucociliary system was part of this service.
The saccharin transit time was used as a screening tool throughout the service provision.Reference Andersen, Camner, Jensen, Philipson and Proctor28,Reference Proctor and Andersen29 Further developments included the use of a computer to measure ciliary beat frequency and radiolabelled technetium human serum albumin nasal transit. Although all measure aspects of mucociliary function, they each have a place. Saccharin transit continues to be an easy out-patient investigation, whereas a radiolabelled test requires a nuclear medicine department with spare γ-camera time. Similarly, the ciliary beat frequency is only one aspect of mucociliary transport and requires a phase-contrast microscope with a heated stage. Electron microscopy is an expensive technique.
After the service began, the saccharin transit time was compared to ciliary beat frequency in 44 adult patients.Reference Moriarty, Robson, Smallman and Drake-Lee30 There was no correlation between the saccharin transit time and ciliary beat frequency. This study found that ciliary beat frequency in 44 patients varied between 5 and 19 Hz with a mean of 11 Hz, and in the six controls, the ciliary beat frequency was between 13 and 16 Hz with a mean of 14 Hz. All measurements were performed at 37°C.
Radiolabelled transit was performed on some of those patients who failed screening. We reported our findings on 40 patients in 1999.Reference Prior, Schofield, Boivin, Anderson and Drake-Lee31 It was this research and clinical practice that led to the request for a diagnostic service at Birmingham Children's Hospital. The paediatric cases presented here are representative of those that are currently managed in a regional centre.
In children, recovery of the mucociliary system is essential for health of the respiratory tract.Reference Randell and Boucher32 Many young children have profuse secretions with anterior nasal discharge, and so both placing the saccharin and getting it to move backwards are problematic. Studies of ciliary activity, such as ciliary beat frequency, are more reliable, but again mucus and other cells may cloud the picture. Except for neonates, patients requiring investigation were listed for examination of the nose and ciliary brushing under anaesthesia after discussion with the referring clinician.
The otorhinolaryngologist's role in the management of these children included the nasal examination, sampling and the management of the associated secretory otitis media if present. The initial problem for many children is diagnostic uncertainty as shown here by the neonates who failed to thrive and had recurrent chest infection. All had normal beating cilia. It is also shown in those with refractory asthma or recurrent respiratory infections. For children with unresolving problems, once immunodeficiency and allergy are excluded, attention should focus on mucociliary clearance.
Some conditions, such as cystic fibrosis, affect mucus and ciliary activity is normal.Reference Rutland and Cole12 Ciliary dyskinesia may result in abnormal copious, thick and tenacious mucus with secondary infection with its typical cellular infiltrate.Reference Randell and Boucher32,Reference Bush, Payne, Pike, Jenkins, Henke and Rubin33 This is also a feature of cystic fibrosis. Although chronic infection is usually bacterial with acute viral exacerbations, fungi may infiltrate later, such as in cystic fibrosis.Reference Pihet, Carrere, Cimon, Chabasse, Delhaes and Symoens4
Patients with bronchiectasis without dextrocardia and situs inversus may be part of the spectrum of primary ciliary dyskinesia. Our small series of 10 patients had one patient with a zero ciliary beat frequency. This fits with the expected prevalence of around 13 per cent.Reference Verra, Escudier, Bignon, Pinchon, Boucherat and Bernaudin34 Similarly, we showed that the ciliary beat frequency was within the normal range in 5 of the 18 patients with dextrocardia.
Children with primary ciliary dyskinesia are predisposed to middle-ear effusions.Reference Sommer, Schäfer, Omran, Olbrich, Wallmeier and Blum35 When their hearing is impaired, ventilation tube insertion is often accompanied by persistent discharge.Reference Prulière-Escabasse, Coste, Chauvin, Fauroux, Tamalet and Garabedian36 Opinion differs on management with some advocating ventilation tubes.Reference Wolter, Dell, James and Campisi37 Most treat patients conservatively, and this is the current view in the UK.Reference Majithia, Fong, Hariri and Harcourt38 When treated by long-term ventilation tubes, this may result in perforation and continual discharge in half the cases.Reference Prulière-Escabasse, Coste, Chauvin, Fauroux, Tamalet and Garabedian36 As the problem is that of mucociliary dysfunction, the Eustachian tube functions normally and so retraction and cholesteatoma are no more common than expected.Reference Ghedia, Ahmed, Navaratnam and Harcourt39
After two years of age, the ethmoid sinuses are adequately developed for polyposis: a feature of children with cystic fibrosis.Reference Shwachman, Kulczycki, Mueller and Flake40,Reference Lee and Pitcher-Wilmott41 We observed none in the 26 patients without beating cilia. This is consistent with other studies.Reference Rollin, Seymour, Hariri and Harcourt42 They are more common in adults where a third of patients have them.Reference Bequignon, Dupuy, Zerah-Lancner, Bassinet, Honoré and Legendre43 Three of the 10 adult patients with refractory bronchitis or bronchiectasis reported in 1999 had polyps.Reference Prior, Schofield, Boivin, Anderson and Drake-Lee31 The sinuses continue to develop during childhood, and there is some evidence that they are not as well developed in adults.Reference Pappa, Sullivan, Lopez, Adams, Zanation and Ebert44
Nasal discharge may cause sampling difficulties. Adequate samples were taken for all the patients for analysis as the single problem was technical. Even after suction, there was often residual mucus on the sample, and so sometimes several groups of cells were inspected to get a good view of cilia. Sampling cilia both from the upper and lower respiratory tract is often described as an easy technique. The recent lateral flow test for severe acute respiratory syndrome coronavirus-2 has shown just how problematic this may be as it is uncomfortable to have a swab inserted into the nose. This highlights why general anaesthesia was usually used. Observation of extracted cells under a phase-contrast microscope will show the pattern of beating cilia and may be undertaken in any department with such a microscope.
In vitro measurements should be undertaken within nine hours as the ciliary beat frequency decreases after this.Reference Sommer, Gross, Hörmann and Stuck45 The study by Sommer et al. used a temperature of 22°C. All of our samples were analysed within six hours. Although it would have been better to use a standard temperature near that of the core for all samples, we decided to use a lower constant temperature.
As may be seen in Figure 1, there is a wide variation of ciliary beat frequency at different temperatures. The ciliary beat frequency at room temperature is around 8 Hz.Reference Clary-Meinesz, Cosson, Huitorel and Blaive22 These results show a similar mean ciliary beat frequency (8.4 Hz) at temperatures below 30°C. When it was measured nearer the core temperature, the mean ciliary beat frequency was 12 Hz. In normal patients, we found that the ciliary beat frequency was above 5 Hz at 20°C.Reference Green, Smallman, Logan and Drake-Lee24 A temperature above 40°C results in a reduction of ciliary beat frequency.Reference Green, Smallman, Logan and Drake-Lee24 Even when the ciliary beat frequency is zero, the test may need to be repeated. There were only four patients with a ciliary beat frequency between 0 and 5 Hz (2.5, 2.6, 3.5 and 4.6 Hz). None of these patients had dextrocardia or bronchiectasis. The ciliary beat frequency must be seen as part of the work up of such patients.
Digital camera technology has developed since this service began. Coherence of the beat is best demonstrated by high-speed digital imaging that may be slowed to allow the coordination of the cilia to be documented. It may be used to determine the ciliary beat frequency. Even if this is the best method, simple screening using a photodiode offers a much cheaper first step.
• Children with recurrent or persistent respiratory tract problems present diagnostic problems
• This series showed diagnosis is broad, including refractory asthma, bronchiectasis, dextrocardia and recurrent or persistent respiratory infections
• Nasal polyps were found in one child with persistent respiratory infection, but were not found in any children with dextrocardia or bronchiectasis
• Twenty-six children had no beating cilia, which was most common in patients with dextrocardia and then in the miscellaneous group of respiratory tract infections
• The results also confirmed the wide variation of ciliary beat frequency and the effect of increasing temperature in vitro on ciliary beat frequency
• Ciliary beat frequency alone is a helpful marker of ciliary function but needs to be incorporated into other test results
Electron microscopy of cilia requires considerable expertise both in sampling and in preparation as glutaraldehyde does not penetrate tissue easily. When samples are blocked, sectioned and stained, cilia are cut at different orientations and large numbers need to be studied to find the correct orientation. Even normal nasal respiratory tissue has cilia with abnormalities and duplicates (personal observation).Reference Drake-Lee and Price46,Reference Drake-Lee, Barker and Thurley47 These difficulties have been encountered by others.Reference Pifferi, Cangiotti, Ragazzo, Baldini, Cinti and Boner48 An extensive study of 1149 patients found that analysis was feasible in just over 70 per cent.Reference Papon, Coste, Roudot-Thoraval, Boucherat, Roger and Tamalet49 It also confirmed that normal patients have up to 10 per cent abnormal cilia. Cilia were reported as abnormal if more than 20 per cent had defects. Both adults and children were part of this study. The tissue was more suitable for analysis in older children. Adult biopsies were taken by an otorhinolaryngologist whereas the paediatric biopsies were bronchial and taken by a physician. The nasal biopsies were of better quality.
Conclusion
Practice has moved on from when these patients were investigated by the first author in terms of what happens once the diagnosis of primary ciliary dyskinesia is made. A European position paper in 2009 stressed the limited evidence for treatment, but it advocated a team approach including having an otolaryngologist to manage patients once the diagnosis of primary ciliary dyskinesia is made.Reference Barbato, Frischer, Kuehni, Snijders, Azevedo and Baktai50 This paper also noted that much of the treatment is based on patients with cystic fibrosis. A centre managing primary ciliary dyskinesia should develop the expertise to undertake ciliary beat frequency measurements, high-speed digital imaging, electron microscopy and, possibly, tissue culture of respiratory epithelium.
In the absence of dextrocardia, diagnosis of primary ciliary dyskinesia may not be considered as it is rare. For any patient with continuous chest and nasal disease that does not resolve during the summer, primary ciliary dyskinesia is a possibility and part of the differential diagnosis as cystic fibrosis may be missed too. If a neonate has unresolving chest and nasal discharge or neonatal respiratory distress, there should be a high index of suspicion. When children have persistent discharge from frequent insertions of ventilation tubes with both upper and lower respiratory tract symptoms, referral is advised. Occasionally, diagnosis is missed until adulthood. Unresolving upper and lower respiratory disease with sinus hypoplasia with or without nasal polyps may be because of primary ciliary dyskinesia. A history of male infertility would support this too. Older children and adults may be screened first with a saccharin transit time. If in doubt, it is always best to refer to a regional centre for a second opinion.
Acknowledgements
We received a grant from the Endowment Fund Medical Research Committee of the former United Birmingham Hospitals to develop the hardware and software to measure ciliary beat frequency.
Competing interests
None declared





