The prevalence of obesity and diabetes is increasing rapidly worldwide. There is an on-going debate concerning potential favourable effects of diets low in carbohydrates, but high in protein and fat, on weight control(Reference Larsen, Dalskov and van Baak1–Reference Hession, Rolland and Kulkarni3). How dietary macronutrient composition relates to long-term risk of obesity-related chronic disease, such as type 2 diabetes, is another clinically important question. Research and dietary guidelines have mainly involved the relative intakes of carbohydrates and fat, and, to a lesser extent, of protein intake. However, some protein-rich foods have, in several studies, been examined in relation to incidence of type 2 diabetes. Especially, consumption of processed meat(Reference Schulze, Manson and Willett4–Reference Pan, Sun and Bernstein8) and also consumption of red meat(Reference Schulze, Manson and Willett4, Reference Song, Manson and Buring5, Reference Aune, Ursin and Veierod7–Reference Fung, Schulze and Manson9) have been associated with increased risk, whereas legume consumption has been associated with decreased risk(Reference Villegas, Gao and Yang10). Protein intake per se has only been examined in relation to risk of incident type 2 diabetes in a few studies. High intakes of animal protein, but not vegetable protein, were associated with increased diabetes risk in the Dutch cohort of the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL)(Reference Sluijs, Beulens and van der11), as well as in the Women's Health Study(Reference Song, Manson and Buring5). Furthermore, replacement of protein with carbohydrates was associated with decreased risk of type 2 diabetes in the EPIC-Potsdam study(Reference Schulze, Schulz and Heidemann12). Total meat intake did not seem to explain the protein–diabetes association in the EPIC-NL cohort. Apart from this, it is uncertain whether the observations reflect the protein intake per se or intakes of specific protein-rich foods, such as processed meat.
Both red and processed meats contain compounds, including nitrates and Fe, which may potentially influence the development of diabetes(Reference Tong, Neusner and Longato13, Reference Forouhi, Harding and Allison14). Besides, it is theoretically plausible that the level of protein intake per se influences diabetes incidence, due to effects on insulin secretion as well as on insulin resistance(Reference Krebs15, Reference Tremblay, Lavigne and Jacques16).
In the present large population-based prospective study of men and women from the Malmö Diet and Cancer (MDC) cohort, we examined if the dietary intakes of protein and protein-rich foods are associated with incidence of type 2 diabetes. As changes in intakes of one macronutrient affect the dietary proportions of all macronutrients, we also examined intakes of fat, carbohydrates and fibre. In addition, we examined if increased consumption of one macronutrient at the expense of another is associated with incidence of type 2 diabetes(Reference Faerch, Lau and Tetens17).
Subjects and methods
Study population and data collection
The MDC study is a population-based prospective cohort study in Malmö, a city in the south of Sweden. Baseline examinations were conducted between 1991 and 1996. All women born between 1923 and 1950 and all men born between 1923 and 1945, living in the city of Malmö, were invited to participate. Details of the cohort and the recruitment procedures are described elsewhere(Reference Manjer, Carlsson and Elmstahl18). The participants filled out questionnaires covering socio-economic, lifestyle and dietary factors, registered meals and underwent a diet history interview. Anthropometric measurements were conducted by nurses. Weight was measured using a balance-beam scale, with subjects wearing light clothing and no shoes. Standing height was measured with a fixed stadiometer, calibrated in centimetres. Waist circumference was measured midway between the lowest rib margin and the iliac crest. Body composition was estimated with a bioelectrical impedance analyser (BIA 103, RJL systems, single-frequency analyser). Body fat percentage was calculated using an algorithm provided by the manufacturer. During the screening period, 28 098 participants (40 % of the eligible persons) completed all baseline examinations. The present study includes all participants without diabetes at baseline (n 27 140). Prevalent diabetes (n 958) was based on self-reported diabetes diagnosis, self-reported diabetes medication or register information indicating a date of diagnosis preceding baseline examination date. The ethical committee at the Lund University approved the study (LU 51-90) and the participants gave their written informed consent.
Dietary data
The MDC study used an interview-based, modified diet history method that combined (1) a 7-d menu book for registration of meals that varies from day to day (usually lunch and dinner meals), cold beverages and nutrient supplements and (2) a 168-item questionnaire for assessment of consumption frequencies and portion sizes of regularly eaten foods that were not covered by the menu book. Finally, (3) a 45-min interview completed the dietary assessment.
The diet analyses are adjusted for a variable called ‘method version’ because slightly altered coding routines of dietary data were introduced in September 1994 in order to shorten the interview time (from 1 h to 45 min). This resulted in two slightly different method versions (before or after September 1994), but did not have any major influence on the ranking of individuals(Reference Wirfalt, Mattisson and Johansson19). The relative validity of the MDC method was evaluated in the Malmö Food study 1984–1985(Reference Elmstahl, Riboli and Lindgarde20, Reference Riboli, Elmstahl and Saracci21). The Pearson correlation coefficients, adjusted for total energy, between the reference method and the MDC method were, respectively, 0·53/0·54 (for protein), 0·69/0·64 (fat), 0·70/0·66 (carbohydrates), 0·69/0·74 (fibre), 0·58/0·50 (bread), 0·73/0·74 (cereals), 0·24/0·35 (rice and pasta), 0·77/0·60 (fruits), 0·53/65 (vegetables), 0·51/0·43 (low fat meat), 0·80/0·40 (high fat meat), 0·70/0·35 (fish), 0·84/0·83 (milk) and 0·59/0·47 (cheese)(Reference Elmstahl, Riboli and Lindgarde20, Reference Riboli, Elmstahl and Saracci21), in women and men.
We used the following variables for nutrient intakes in the present study: total energy (MJ), non-alcohol energy (MJ), carbohydrates (percentage of energy (En%)), fat (En%), protein (En%), fibre (g/MJ), saturated fat (En%), monounsaturated fat (En%), polyunsaturated fat (En%), Fe (g/MJ) and Mg (g/MJ). Daily intakes of the following foods rich in protein were examined: legumes (g), fibre-rich cereal products (portions of fibre-rich bread and breakfast cereals), refined cereal products (portions of low fibre bread, refined breakfast cereals, pasta and rice), non-processed red meat (g), processed meat (sausage and cured meat) (g), poultry (g), fish/shellfish (g), dairy products (portions of milk, yoghurt, sour milk and cheese) and egg (g). In addition coffee (g) and soft drinks (g) were included as covariates in statistical models.
Portions, instead of grams, were used in order to analyse the sum of food items with different water content and usually consumed in different weights (e.g. cheese and milk). Standard portions sizes from the National Food Agency in Sweden were used(22): fibre-rich soft bread (50 g/portion), fibre-rich crisp bread (30 g/portion), breakfast cereals (25 g/portion), low-fibre soft bread (50 g/portion), low-fibre crisp bread (30 g/portion), pasta and rice (60 g dry product/portion), milk and yoghurt (200 g/portion) and cheese 20 g/portion. Nutrient densities were calculated by dividing total nutrient intakes by non-alcohol energy intake. Energy-adjusted food intakes were obtained by regressing food intakes on non-alcohol energy intake. Quintiles of nutrient densities and food residuals were used as exposure categories.
Diabetes case ascertainment
We identified 1709 incident cases of type 2 diabetes during 320 703 person-years of follow-up via at least one of three registers. The mean follow-up time was 12 years. The subjects contributed person-time from date of enrolment until date of diabetes diagnosis, death, migration from Sweden or end of follow-up (December 2006), whichever occurred first. We used information on the date of diagnosis from the registers prioritised in the following order: (1) the Regional Diabetes 2000 Register of Scania (information on date of diagnosis available for 562 of the 579 identified cases), (2) the Malmö HbA1c Register (date information for all the 835 additional cases that were identified by this register and for six of the cases without date information in Diabetes 2000)(Reference Enhorning, Wang and Nilsson23) and (3) the Swedish National Diabetes Register (date information for all the 295 additional cases that were identified by this register and for five of the cases without date information in Diabetes 2000)(Reference Cederholm, Eeg-Olofsson and Eliasson24). The remaining six cases without information on date of diagnosis in Diabetes 2000 were not identified in any of the other two registers. These cases were assigned the date of diagnosis equal to date of death, migration from Sweden or end of follow-up, whichever occurred first. The Diabetes 2000 register and the national register required a physician diagnosis according to established diagnosis criteria (fasting plasma glucose concentration ≥ 7·0 mmol/l or fasting whole blood concentration ≥ 6·1 mmol/l, measured at two different occasions). Individuals with at least two glycated Hb values above 6·0 % with the Swedish Mono-S standardisation system (corresponding to 7·0 % in the US National Glycohaemoglobin Standardization Program) were categorised as diabetes cases in the Malmö HbA1c Register.
Variables
Information on age was obtained from the personal identification number. Age was divided into 5-year categories. The BMI (kg/m2) was calculated from direct measurement of weight and height. The BMI was categorised into ≤ 25, 25–30 and >30 kg/m2 groups. Waist circumference and body fat percentage were divided into three sex-specific groups. Leisure-time physical activity was assessed by asking the participants to estimate the number of min/week they spent on seventeen different activities. The duration was multiplied with an activity-specific intensity coefficient and an overall leisure-time physical activity score was created. The score was divided into sex-specific tertiles and categorised as low, medium and high. The smoking status of the participants was defined as smokers (including irregular smokers), ex-smokers and never smokers. The total consumption of alcohol was defined by a four-category variable. Participants reporting zero consumption in the menu book, and indicating no consumption of any type of alcohol during the previous year, were categorised as zero reporters. The other category ranges were < 15 g/d for women and < 20 g/d for men (low); 15–30 g alcohol/d for women and 20–40 g/d for men (medium); >30 g of alcohol/d for women and >40 g/d for men (high). Participants were divided into four categories according to their highest level of education (<8 years, 9–10 years, 11–13 years or university degree). Dietary change in the past (yes/no) was based on the question ‘Have you substantially changed your eating habits because of illness or some other reasons?’. Participants reporting dietary change may have unstable food habits. Their reported dietary habits may reflect a short period of their lives and may therefore have less influence on the development of chronic disease.
Statistical methods
The SPSS statistical computer package (version 17.0; SPSS, Inc.) was used for all statistical analyses. All food variables were log transformed (e-log) to normalise the distribution before analysis. A very small amount (0·0001) was added before transformation to handle zero intakes.
We examined baseline status of established risk factors and potential confounders associated with type 2 diabetes using Cox proportional hazard regression. All models were adjusted for age. Pearson correlation coefficients between energy-adjusted intakes of nutrients and foods were computed. Baseline characteristics across quintiles of protein intake were examined with the general linear model for continuous variables adjusted for age (continuous) and with the χ2 test for categorical variables.
We used the Cox proportional hazards regression model to estimate hazard ratios (HR) of diabetes incidence associated with quintiles of nutrient densities and food intakes adjusted for energy intake using the residual method. The first quintile was used as the reference. Years of follow-up was used as the underlying time variable. We used covariates obtained from the baseline examinations. The basic model included adjustments for age (continuous), sex (when applicable), method version (categorical), season (categorical) and total energy intake (continuous). Our second model also included adjustments for the following categorical variables: leisure-time physical activity, smoking, alcohol intake and education. The full multivariate model also included BMI (continuous). The covariates were identified from the literature and indicated potential confounding in the MDC cohort due to associations with diabetes incidence and dietary intakes. Additional adjustments were made for intake quintiles of processed meat, fibre-rich bread/cereals, fruits/vegetables, coffee, soft drinks, saturated fat, monounsaturated fat, polyunsaturated fat, Fe or Mg. Tests for interactions between sex and nutrient/food intakes with regard to diabetes incidence were performed (sex × quintile of nutrients/foods (treated as a continuous variable)). We made all analyses for men and women separately. If no interaction between sex and nutrients or food groups was observed, the results from the full multivariate model are presented in the text for women and men together.
In order to reflect substitutions of one macronutrient with another, we excluded the replaced macronutrient in the statistical model, but included the macronutrient at increased intake as well as the one that was supposed to be kept constant(Reference Faerch, Lau and Tetens17). We estimated, for example, the risk of replacing carbohydrates with protein by including the variables for intakes of protein and fat, but not carbohydrates.
In sensitivity analysis, we excluded individuals with reported dietary change in the past (24 % of the individuals). In a second sensitivity analysis, we excluded individuals with prevalent CVD at baseline (3 %). In order to obtain a more equal age distribution in women and men, we excluded individuals below 50 years of age. A test for interaction between quintile of protein intake (treated as a continuous variable) and BMI ( ≤ 25 or >25kg m2) was performed. All statistical tests were two-sided and statistical significance was assumed at P< 0·05.
Results
Baseline characteristics
In both men and women, BMI, body fat percentage and waist circumference were positively associated with incidence of type 2 diabetes. Leisure-time physical activity and education were inversely associated with type 2 diabetes. In addition, women reporting moderate alcohol intakes (i.e. 15–30 g/d) were at decreased risk compared with those with low intakes (i.e. 0–15 g/d). Among men, current smokers and ex-smokers were at increased risk compared with never smokers (data not shown).
Protein intake was positively associated with BMI, body fat percentage, waist, intakes of fibre, Fe, Mg and vitamin C, alcohol consumption and education, but inversely associated with age and with intakes of saturated fat, monounsaturated fat, polyunsaturated fat, total fat and carbohydrates (Table 1). Smoking frequency was not evenly distributed across quintiles of protein intake in women and high leisure-time physical activity was more frequent among men at low protein intakes.
Table 1 Baseline characteristic means across quintiles of protein intake among 16 590 women and 10 550 men from the Malmö Diet and Cancer cohort

En%, percentage of energy
* Calculated with the general linear model; adjusted for age (continuous) when appropriate.
† χ2 test.
Dietary intake and type 2 diabetes
Protein intake was positively associated with type 2 diabetes (Table 2; HR 1·27 for highest compared with lowest quintile; 95 % CI 1·08, 1·49; P for trend = 0·01 in men and women together). We saw a significant interaction between intake of carbohydrates and sex with regard to risk of type 2 diabetes (P= 0·02). In men, we saw a tendency of an inverse association between carbohydrate intake and type 2 diabetes (P for trend = 0·08); intake in the highest quintile was associated with lower risk of type 2 diabetes in men (HR in the highest quintile = 0·76; 95 % CI 0·62, 0·95). No such tendency was seen in women. Fat intake was not significantly associated with type 2 diabetes and no significant interaction was seen between fat intake and sex in the full multivariate model including BMI (P= 0·18). Increased consumption of 5 En% of protein at the expense of carbohydrates (HR 1·20; 95 % CI 1·09, 1·33) or fat (HR 1·20; 95 % CI 1·09, 1·33) was associated with increased risk (Fig. 1). Increased consumption of 5 En% of fat at the expense of carbohydrates was not significantly associated with type 2 diabetes in women or in men, but we saw a significant interaction with sex (P= 0·04) and observed non-significant tendencies in opposite directions in women (HR 0·95; 95 % CI 0·89, 1·01) and men (HR 1·04; 95 % CI 0·98, 1·11). Fibre intake was not significantly associated with type 2 diabetes when adjusting for other risk factors and potential confounders.
Table 2 Hazard ratio (HR) of type 2 diabetes associated with macronutrient intake in the Malmö Diet and Cancer cohort (Ranges, hazard ratios and 95 % confidence intervals)

En%, percentage of energy
* Adjusted for age (continuous), method version (before or after September 1994), season (winter, spring, summer, autumn) and total energy (continuous).
† Adjusted for age (continuous), method version (before or after September 1994), season (winter, spring, summer, autumn), total energy (continuous), education (<8 years, 9–10 years, 11–13 years or university degree), smoking (current, ex or never), alcohol intake (zero, < 15 g/d for women and < 20 g/d for men, 15–30 g/d for women and 20–40 g/d for men, >30 g/d for women and >40 g/d for men), leisure-time physical activity (tertiles of leisure-time physical activity score) and BMI (continuous).

Fig. 1 Replacement of one macronutrient with another in 16 590 women and 10 550 men from the Malmö Diet and Cancer cohort. Increased consumption of 5 % of energy (En%) of protein at the expense of carbohydrates (P= 0·05 in women and P= 0·001 in men) or fat (P= 0·01 in women and P= 0·01 in men) was associated with increased risk of type 2 diabetes. Increased consumption of 5 En% of fat at the expense of carbohydrates was not significantly associated with type 2 diabetes in women or in men, but the associations went in opposite directions and a significant interaction with sex was seen (P-interaction = 0·04). HR, hazard ratio.
In analysis of protein sources (Table 3), high intake of processed meat was associated with increased risk of type 2 diabetes (HR 1·16; 95 % C.I 1·00, 1·36; P for trend = 0·01; Fig. 2). High intakes of eggs was also associated with an increased risk (HR 1·21; 95 % CI 1·04, 1·41; P for trend = 0·02). In addition, poultry intake tended to be positively associated with type 2 diabetes (HR 1·15; 95 % CI 1·00, 1·32), although the trend across the intake quintiles was non-significant (P for trend = 0·07). In the sex-stratified analyses, we only observed the positive association between poultry intake and diabetes in women, but did not see any significant statistical interaction between poultry intake and sex (P= 0·25). No other food sources of animal protein were significantly associated with incidence of type 2 diabetes in the full multivariate model, including BMI; but, in the model without BMI, non-processed red meat was associated with increased risk (HR 1·17; 95 % CI 1·00, 1·37; P for trend = 0·02), and in men, high intake of dairy products was associated with increased risk (HR 1·39; 95 % CI 1·14, 1·70; P for trend = 0·01; P for interaction dairy intake × sex = 0·04). In a post hoc analyses of the animal sources of protein, but not including dairy products, we saw a positive association with type 2 diabetes (HR 1·24; 95 % CI 1·06, 1·44; P for trend = 0·001 (full multivariate model)), and intake of processed and non-processed meats, including poultry, was also associated with increased risk (HR 1·27; 95 % CI 1·09, 1·49; P for trend = 0·002 (full multivariate model)).
Table 3 Hazard ratio (HR) of type 2 diabetes associated with intakes of protein-rich foods in the Malmö Diet and Cancer cohort (Medians, hazard ratios and 95 % confidence intervals)

* Adjusted for age (continuous), method version (before or after September 1994), season (winter, spring, summer, autumn) and total energy (continuous).
† Adjusted for age (continuous), method version (before or after September 1994), season (winter, spring, summer, autumn), total energy (continuous), education (<8 years, 9–10 years, 11–13 years or university degree), smoking (current, ex or never), alcohol intake (zero, < 15 g/d for women and < 20 g/d for men, 15–30 g/d for women and 20–40 g/d for men, >30 g/d for women and >40 g/d for men), leisure-time physical activity (tertiles of leisure-time physical activity score) and BMI (continuous).
‡ Zero consumers, the higher categories are quartiles among the consumers.
§ Legume intake was not calculated for women (n 1220) and men (n 910) with base line examinations during the 1st year of the screening period, because the estimated intakes were not comparable to intakes from 1992 to 1996, due to changes in the handling of heat-treated and fresh food items.
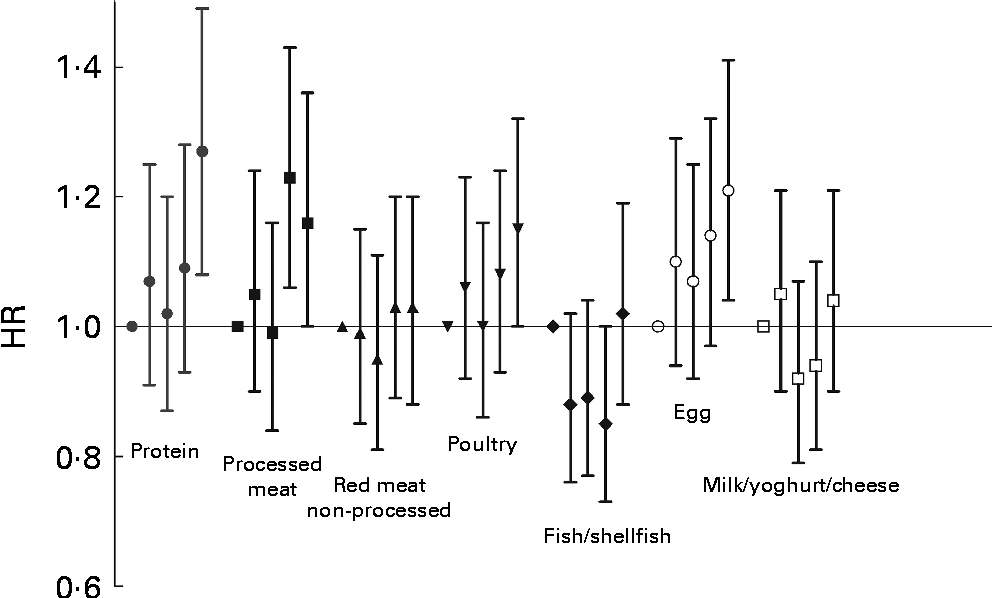
Fig. 2 Hazard ratios (HR) with 95 % CI of type 2 diabetes in intake quintiles of protein and animal protein sources among 27 140 individuals from the Malmö Diet and Cancer cohort. High intakes of protein (P for trend = 0·01), as well as high intakes of processed meat (P for trend = 0·01) and eggs (P for trend = 0·02) were significantly associated with increased risk of type 2 diabetes in analysis of women and men together. Similar tendencies were seen for high intakes of poultry (P for trend = 0·07). No significant associations were seen between intakes of non-processed red meat (P for trend = 0·55), fish/shellfish (P for trend = 0·85) or milk/yoghurt/cheese (P for trend = 0·94) and risk of type 2 diabetes.
A protective association was observed between intake of fibre-rich bread and cereals and type 2 diabetes (HR 0·84; 95 % CI 0·73, 0·98; P for trend = 0·004). No significant associations were seen between intakes of refined cereals or legumes and type 2 diabetes.
The Pearson correlation coefficients between intake quintiles of protein and processed meat, eggs and poultry were 0·08, 0·17 and 0·16, respectively (P< 0·001). Adjustments for intakes of processed meat, eggs or poultry did not change the positive association between protein intake and type 2 diabetes (P for trend = 0·01). Adjustment for intakes of fibre-rich bread and cereals, fruits and vegetables, coffee, soft drinks, saturated fat, monounsaturated fat, polyunsaturated fat, Fe or Mg did not substantially influence any of the observed significant associations. The association between protein intake and incidence of type 2 diabetes was not significantly different in normal weight and overweight subjects (BMI ≤ 25 or >25 kg/m2; P-interaction = 0·26).
Sensitivity analysis
After exclusion of individuals with reported dietary change in the past, the protective association between carbohydrates and diabetes in men was strengthened (P for trend = 0·003), whereas fat intake tended to be positively associated with diabetes in men (P for trend = 0·08). In addition, we observed a significant protective association between fibre intake and diabetes incidence in men (P for trend = 0·02), and the inverse association with fibre-rich bread and cereals was strengthened in men (P for trend = 0·002). In men, the previous borderline significant association between protein intake and type 2 diabetes reached significance in analysis excluding individuals with prevalent CVD at baseline (P for trend = 0·03). After exclusion of individuals below 50 years of age, high carbohydrate intake was still associated with lower incidence of type 2 diabetes in men (HR in the 5th quintile = 0·78; 95 % CI 0·63, 0·95), but not in women (P for interaction between intake of carbohydrates and sex = 0·06). All other reported results remained virtually unchanged in the sensitivity analyses.
Discussion
The present study suggests that high protein intake is associated with increased incidence of type 2 diabetes and that replacing carbohydrates or fat with protein is associated with increased risk. High intakes of processed meat and eggs were also associated with increased risk of type 2 diabetes, but did not alone explain the high risk associated with protein intake. A protective association was seen between intakes of fibre-rich bread and cereals and type 2 diabetes.
Overfeeding may eventually lead to insulin resistance and is, together with physical inactivity, regarded as one of the most important lifestyle factors associated with the worldwide increased prevalence of type 2 diabetes. During the last few decades, the increasing intakes of energy-rich foods, high in fats and refined carbohydrates, have also been accompanied with an increased intake of animal products(Reference Popkin25, Reference Elmadfa, Meyer and Nowak26). High protein intake increases insulin secretion and may thereby counteract elevated postprandial plasma glucose concentrations(Reference Krebs15). However, the effects of long-term consumption of high protein diets on diabetes development are unknown. Some studies indicate that high protein intake promotes insulin resistance and that the amino acid composition of proteins can be crucial(Reference Tremblay, Lavigne and Jacques16). Processed meat contains preservatives, including nitrites, which react with amino compounds to form nitrosamines. Nitrosamines may cause insulin resistance by altering the expression and binding of insulin receptors(Reference Tong, Neusner and Longato13). In addition, they have been found to be β-cell toxic(Reference LeDoux, Woodley and Patton27).
High protein intake has recently been associated with type 2 diabetes in the EPIC-NL study, and replacing carbohydrates or fat with protein was associated with increased risk(Reference Sluijs, Beulens and van der11). Comparably, replacement of protein with carbohydrates was associated with decreased risk of type 2 diabetes in the EPIC-Potsdam study(Reference Schulze, Schulz and Heidemann12). In addition, a score representing a low-carbohydrate diet high in protein and fat was associated with increased risk of type 2 diabetes in men from the Health Professionals Follow-up Study(Reference de Koning, Fung and Liao28). A low-carbohydrate diet high in vegetable protein and vegetable fat was, however, not associated with type 2 diabetes, which is in agreement with findings for vegetable protein in the EPIC-NL study. In women from the Nurses' Health Study, a low-carbohydrate diet high in vegetable protein and vegetable fat was actually associated with a decreased risk(Reference Halton, Liu and Manson29). We concluded that the main score, which overlooks the origin of protein and fat, was not associated with type 2 diabetes. However, an association with elevated risk was observed before adjustment for BMI. We have also chosen to treat BMI as a potential confounder in the full multivariate model, but did not find the association between protein intake and incident type 2 diabetes to be modified by BMI. In the present study, high protein intake was associated with increased risk of type 2 diabetes, both with and without adjustment for BMI. However, the association was attenuated after inclusion of BMI in the statistical model, indicating that the association is partly mediated via obesity. In line with the present findings, indicating protective effects of fibre-rich bread and cereals, whole grain intakes have been inversely associated with diabetes in several studies(Reference de Munter, Hu and Spiegelman30).
The result obtained in the present study for processed meat is in agreement with meta-analyses in which processed meats were positively associated with risk of type 2 diabetes(Reference Micha, Wallace and Mozaffarian6, Reference Pan, Sun and Bernstein8). Egg consumption was also positively associated with risk of type 2 diabetes in the Physicians' Health Study and in the Women's Health Study, but few studies have examined this association and the results are not conclusive(Reference Djousse, Gaziano and Buring31–Reference Shi, Yuan and Zhang33). Adjustments for food sources that were associated with diabetes (intakes of processed meat, eggs, poultry or fibre-rich bread and cereals) did not affect our protein–diabetes association, indicating that the intake level of protein per se may also be of importance.
The present study has several strengths. Most importantly, it is a large study with long follow-up and with dietary data of high relative validity(Reference Elmstahl, Riboli and Lindgarde20, Reference Riboli, Elmstahl and Saracci21, Reference Willett34). As it is a population-based prospective study, we were able to minimise selection bias as well as reverse causation. In addition, we have extensive information on potential confounding factors, as well as the possibility to exclude individuals with reported dietary changes in the past. In contrast to other studies on protein intake and type 2 diabetes, we also examined intakes of several other protein-rich foods in addition to red and processed meat. However, our nutrient database does not contain information on intakes of different amino acids and we were not able to separate animal and plant proteins. It can therefore not be excluded that the intake of animal protein could explain the observed results concerning total protein intake and type 2 diabetes, although the adjustments for specific animal sources of protein did not change the results. We could see an increased risk at high aggregated intake of all protein-rich animal foods, except milk products. Legumes are valuable sources of vegetable protein, but the intake in Sweden may be too low for detection of associations between legume intakes and disease(Reference Halkjaer, Olsen and Bjerregaard35). It should also be addressed that the findings in females and males regarding intakes of protein, processed meat and eggs were, in most cases, only of marginal significance or only indicated a tendency. A probable reason for this is the lower power in the sex-stratified analyses. Finally, despite adjustments for known risk factors and potential confounders, we cannot exclude occurrence of residual confounding.
We observed an interaction between sex and carbohydrate intakes, as well as different tendencies in women and men when carbohydrates were replaced with fat. We have previously seen an interaction between the genetic predisposition for obesity and macronutrient intake in the MDC cohort(Reference Sonestedt, Roos and Gullberg36). The optimal distribution of macronutrients with regard to obesity-related diseases may also vary between individuals and be different in women and men. Fat intake may, for example, influence circulating sex hormone levels(Reference Aubertin-Leheudre, Gorbach and Woods37). Endogenous sex hormones may have a role in the development of type 2 diabetes, but their importance seems to differ in women and men(Reference Ding, Song and Malik38). However, differences in intake levels may also explain sex-dependent associations. We did not see any major differences in the distribution of macronutrient intakes between women and men, but previous observations within the MDC cohort indicate that their food patterns differ(Reference Wirfalt, Hedblad and Gullberg39), and that vegetable and fruit intakes are higher in women(Reference Wallstrom, Wirfalt and Janzon40). Other studies indicate that food choices and accuracy of diet assessment varies with sex(Reference Margetts and Nelson41, Reference O'Doherty Jensen and Holm42). We cannot exclude that these factors could have contributed to the diverse tendencies in women and men. Differences in age distribution may also explain the results, because diabetes appears later in life in women(43), but the exclusion of individuals below 50 years of age (with a higher proportion of women) did not substantially change the results of the present study.
Among the study participants who reported a change of dietary habits in the past, a large fraction (83 %) indicated health as the important reason for the change(Reference Sonestedt, Wirfalt and Gullberg44). During the baseline examinations in the MDC study (1991–1996), dietary recommendations in Sweden were emphasising the benefits of low-fat and high-carbohydrate/fibre intakes. Associations with variables related to dietary fat–carbohydrate composition may therefore be especially influenced after exclusion of individuals reporting dietary change, and may explain the strengthened associations observed for those variables in men.
Type 2 diabetes affects the life quality of many individuals and the high prevalence has public health implications. In addition, type 2 diabetes has been associated with premature death from CVD and several types of cancer(Reference Seshasai, Kaptoge and Thompson45). It is therefore of great concern to identify modifiable environmental factors, such as diet, that may affect the escalating incidence of the disease.
In conclusion, the results of the present study indicate that diets with high protein content may be associated with increased risk of incident type 2 diabetes, and that high consumption of fibre-rich bread and cereals is associated with decreased risk. Low-carbohydrate, high-protein diets have been suggested as an alternative for weight reduction, but the findings of the present study suggest that long-term consumption of such diets could be a health hazard.
Of the 28 098 participants in the MDC cohort, 1758 incident diabetes cases and 1758 controls are included in the EPIC InterAct Consortium for the study of genetic factors and gene–lifestyle interactions with regard to incident diabetes. Being a large cohort study, the MDC represents a different study design, compared to the case–control study design of EPIC InterAct. The dietary data used within EPIC InterAct is harmonised between several study centres, and many details found in the MDC dietary data, used in the present study, are lacking in these harmonised data. That is, different study design, different study size, extensive information on confounding variables, the possibility to exclude individuals with reported dietary change, as well as uniform dietary data of high relative validity ensure the uniqueness of the present study v. the pooled analyses that may be performed within the EPIC InterAct.
Acknowledgements
The Swedish Medical Research Council, the Region Skåne, the Skåne University Hospital, the Albert Påhlsson Research Foundation and Novo Nordic Foundation funded the present study. The authors' responsibilities were as follows: U. E. wrote the manuscript, performed the data analysis and contributed to the study design and the interpretation of results; E. S. contributed to the interpretation of results and the revision of the manuscript; B. G. gave statistical advice and contributed to the study design and revision of the manuscript; S. H. contributed to the revision of the manuscript; G. H. contributed to the revision of the manuscript; E. W. contributed to the study design, the interpretation of results and revision of the manuscript; and M. O.-M. contributed to the study design, the interpretation of results and revision of the manuscript. None of the authors had any conflicts of interest.







