Small size at birth continues to be a problem even in populations with adequate energy and protein intakes. Many factors may account for small birth size, and it has been shown that low folate status or intake(Reference Neggers, Goldenberg and Tamura1–Reference Scholl, Leskiw and Chen4) and excess Pb burden(Reference González-Cossío, Peterson and Sanín5–Reference Jelliffe-Pawlowski, Miles and Courtney8) are independently associated with reduced birth weight and length. As discussed by Andrews et al. (Reference Andrews, Savitz and Hertz-Picciotto9) the magnitude of the association between Pb and size at birth varied among studies and could not be explained by study design or confounding. Similarly, in some studies, folate intake or status explained only a small proportion of variability in outcome(Reference Scholl, Leskiw and Chen4), suggesting that other factors may play a role.
Links between Pb exposure and folate intake have not been well studied, although there is evidence that folate metabolism may be related to Pb toxicity. In one study, serum folate levels were inversely associated with blood Pb concentrations among women of reproductive age(Reference Lee, Chun and Song10). A comparison of women who did and did not have fetuses with neural tube defects revealed higher Pb but lower folate levels in amniotic fluid at 15–20 weeks gestation(Reference Dawson, Evans and Harris11). In addition, Pb levels were inversely correlated with both folate and vitamin B12 concentrations. More recently, higher erythrocyte folate concentration appeared to attenuate the adverse relationship between Pb exposure and cognitive performance of children from the Philippines(Reference Solon, Riddell and Quimbo12). Further evidence of a connection is found in studies of plasma homocysteine. Both reduced folate intake and increased Pb exposure are associated with higher plasma homocysteine(13, Reference Chia, Ali and Lee14), which in turn is linked with adverse pregnancy outcomes(Reference Infante-Rivard, Rivard and Gauthier15, Reference Murphy, Scott and Arija16).
Variation in genes coding for folate-metabolising enzymes may be relevant because some polymorphisms of the methylenetetrahydrofolate reductase (MTHFR) gene, such as the C677T or A1298C, are common(Reference Gueant-Rodriguez, Gueant and Debard17) and produce enzymes with lower metabolic activity(Reference Weisberg, Tran and Christensen18). Other variants, such as the G1793A, are less frequent and their functional significance is unclear(Reference Xu, Shrubsole and Xiang19, Reference Bottiger, Hurtig-Wennlof and Sjostrom20). However, studies examining the effects of folate on fetal growth among women and infants with different MTHFR genotypes have not produced consistent results(Reference Nurk, Tell and Refsum21–Reference Engel, Olshan and Siega-Riz24).
Gene–environment and nutrient–environment interactions are receiving increased attention because the main effects of genetic or environmental factors often explain only a small proportion of variability in health outcomes. The study of nutrient–environment interactions in particular may identify ways of preventing or intervening where environmental exposures have occurred. We investigated whether excess Pb burden was associated with smaller size at birth in 474 apparently healthy women with uncomplicated pregnancies who delivered term infants in Mexico City, and whether this relationship was modified by maternal folate intake during pregnancy. Finally, we tested the hypothesis that the associations between folate and birth size, and Pb and birth size would be modified by MTHFR genotype. We examined maternal and infant genotypes separately. Both genotypes are likely to interact with Pb because it easily crosses the placenta when the mother is exposed and with folate because it is supplied by the mother. These interactions may differentially influence infant size at birth.
Experimental methods
Study sample and data collection
Study participants were identified from among pregnant women receiving antenatal care between January 1994 and June 1995 at three hospitals in Mexico City that serve low-to-middle-income populations. The women were invited before giving birth to participate in a randomised, placebo-controlled trial of Ca supplementation beginning at 1-month postpartum. Women were excluded if they lived outside the metropolitan area, had no intention to breast-feed, or had any of the following: premature delivery, multiple fetuses, pre-eclampsia, psychiatric, renal or cardiac disease, gestational diabetes, history of urinary tract infections, history (family or self) of kidney stones, seizure disorders, ingestion of corticoids, high blood pressure (>140 mmHg systolic, >90 mmHg diastolic). Sample selection and data collection methods are described in detail elsewhere(Reference González-Cossío, Peterson and Sanín5).
Briefly, information on demographic characteristics, reproductive history and risk factors for Pb exposure were collected at baseline using a questionnaire. Newborn characteristics and estimated gestational age were extracted from medical records. Anthropometry, and maternal and umbilical blood were collected within 12 h of delivery. A semi-structured FFQ was administered and bone Pb measured at 1 month postpartum ( ± 5 d) at the American British Cowdray Hospital. Archived blood samples were used for DNA extraction and MTHFR genotyping, performed at the Harvard-Partners Center for Genetics and Genomics (Boston, MA, USA). Table 1 shows the characteristics of the women and infants included in the study and those excluded from the study.
Table 1 Characteristics of study participants and those excluded from the study
(Mean values and standard deviations or percentages)

* Value was significantly different from that of the women or infants included in the study (P < 0·05).
† Refers to values at the time women were approached about possible study participation.
‡ Measured at 1 month postpartum
§ Measured at 1 month postpartum; refers to habitual intakes in the previous year.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects or patients were approved by the ethics review boards at the National Institute of Public Health in Mexico, Harvard School of Public Health, and the participating hospitals. Written informed consent was obtained from all subjects and patients.
This is a cross-sectional analysis of data collected at baseline of a Ca supplementation trial, which ultimately enrolled 617 women. It expands on a previous report from our group on Pb exposure and infant birth weight, which included 315 mother–infant pairs from Mexico City(Reference González-Cossío, Peterson and Sanín5). Of the women available for the present study (n 617), those with a tibia Pb measurement, complete FFQ and plausible dietary intakes (2092–20 920 kJ/d (500–5000 kcal/d); < 5000 μg folate/d), at least two genotyped single nucleotide polymorphisms (SNP), information on gestational age and BMI, and a postpartum calf circumference measurement were eligible to participate. Three women did not have tibia Pb values, twenty-one had no reported or implausible dietary intakes, thirty-eight did not have at least two SNP, thirty-eight were missing gestational age, thirty-seven BMI values, and six postpartum calf circumference. The final sample included 474 women with complete data. For blood collection from neonates, we relied on nursing staff at the three hospitals and were able to obtain a convenience sample of umbilical cord bloods, with limited collection on weekends, at night and in the early morning. Due to these difficulties, we only included 321 newborns who had a blood sample for genotyping and a full set of covariates.
Dietary assessment
Maternal dietary intake was assessed by trained interviewers using a semi-quantitative FFQ with questions on usual food intakes in the previous year. The questionnaire was translated and validated for use in Spanish-speaking adult women(Reference Hernandez-Avila, Romieu and Parra25). The FFQ included 116 questions on the intake of eighty-two foods and beverages typical to urban Mexican diets. For each food listed, a portion size was specified and women were queried on the frequency of consumption (based on ten categories ranging from never, through monthly, weekly, to daily intakes). Nutrient intakes were calculated based on a nutrient database compiled by the National Institute of Nutrition in Mexico. Additional questions included: prenatal supplement use (type or brand, frequency, and duration), and whether women felt their diet had changed during the previous year, and why. Supplement use was included in the calculation of usual folate intakes.
Blood lead measurements
Blood Pb measurements were performed using graphite furnace atomic absorption spectrophotometry (model 3000; PerkinElmer, Wellesley, MA, USA) at the American British Cowdray Hospital Trace Metal Laboratory in Mexico City according to a previously described technique(Reference Miller, Paschal and Gunter26). The laboratory participates in the Centers for Disease Control and Prevention blood Pb proficiency testing program administered by the Wisconsin State Laboratory of Hygiene (Madison, WI, USA) which provided external quality-control specimens varying from 20 to 880 μg/l. The laboratory maintained acceptable precision and accuracy over the study period (correlation 0·98; mean difference 7·1 (sd 6·8) μg/l). The limit of detection for this technique was 8 μg/l, with no individual values falling below this limit.
Bone lead measurement
Bone Pb measurements were performed at the American British Cowdray Hospital in Mexico City with a spot-source 109Cd K X-ray fluorescence (KXRF) instrument constructed at Harvard University. Each woman underwent a 30 min in vivo measurement of the left mid-tibia shaft (cortical bone) and the left patella (trabecular bone). The instrument uses a Cd γ-ray source to provoke bone to emit fluorescent photons, which are detected and counted. The details of the procedure are provided elsewhere(Reference Aro, Todd and Amarasiriwardena27). Maternal tibia Pb, but not patella or blood Pb concentration, was previously associated with birth weight in this population(Reference González-Cossío, Peterson and Sanín5); thus only tibia Pb concentrations are considered in this analysis. In addition to the bone Pb concentration (μg Pb/g bone mineral), the instrument provides an estimate of uncertainty associated with each measurement that could be due to slight leg movements or adipose tissue. We excluded participants whose uncertainty estimates for tibia Pb exceeded 10 μg Pb/g bone mineral. According to our standard quality-control procedures, the instrument was calibrated once per week with a 10 parts per million (ppm) phantom measured twenty consecutive times. Means and standard deviations were calculated to identify changes in accuracy or precision. Once per month, a set of calibration phantoms (0, 5, 10, 15, 20, 30 and 50 ppm) was measured.
Genotyping
DNA extraction and genotyping were performed in the Harvard-Partners Center for Genetics and Genomics (Boston, MA, USA). High-molecular-weight DNA was extracted from leucocytes with PureGene Kits (Gentra Systems, Minneapolis, MN, USA). Extraction yielded an average of 20–40 μg DNA/ml whole blood. After DNA quantification, samples were adjusted to Tris–EDTA (TE) buffer, partitioned into samples, and stored at − 80°C. A TaqMan platform was used to genotype the MTHFR C677T SNP rs1801133, A1298C rs1801131 and G1793A rs2274976. For quality assurance, a random sample of 5 % was re-analysed and checked for discordance. In the present study, 97 % of samples with adequate blood volume were successfully genotyped.
Statistical analysis
We used STATA 10 (StataCorp LP, College Station, TX, USA) for this analysis. We examined MTHFR allele distribution and tested allele frequencies using a χ2 statistic to confirm adherence to Hardy–Weinberg equilibrium principles. With this analysis we examined the following questions.
First, are folate intake and tibia Pb associated with infant size at birth? If so, does folate intake modify the negative effect of maternal Pb burden on newborn size? To address these questions, we modelled birth size (birth weight, length and head circumference) as a function of folate intake (model 1), tibia Pb (model 2) and their interaction. We considered an interaction to be significant at P < 0·1. Non-significant interaction terms were removed and regression models re-run testing the main effects of folate intake and tibia Pb (model 3). We did not adjust the significance levels for multiple comparisons. We used tibia Pb concentrations and folate intake levels both as continuous and categorical variables. For tibia Pb, we used the median of the distribution, 9·4 μg/g, as the cut-off. For folate, we used 400 μg/d, which is the recommended daily intake level. We adjusted all regression models for the same variables as in our previous report(Reference González-Cossío, Peterson and Sanín5): gestational age, postpartum calf circumference, parity, maternal education and smoking, and in addition adjusted for maternal age, marital status, pre-pregnancy BMI and the sex of the infant.
Second, we examined if MTHFR genotypes were associated with birth size or modified the association between folate intake and birth size. For this analysis we assumed dominant effects of SNP (i.e. the effects were due to having any copy of the variant gene). We also examined birth size by maternal and infant haplotypes but found no significant differences. Thus, we compared the following groups: 677CC v. 677CT/TT, 1298AA v. 1298AC/CC, and 1793GG v. 1793GA/AA.
Finally, we examined whether MTHFR genotype modified the association between folate intake, together with Pb exposure, and birth size. We performed all regression analyses stratified by genotype (677CC v. 677CT/TT, 1298AA v. 1298AC/CC, and 1793GG v. 1793GA/AA). Folate intake, tibia Pb levels and their interaction term (folate × Pb) were entered together in models predicting birth weight, height and head circumference. The folate × Pb interaction term was removed if P>0·1 and models were re-run without it. As a final step, we tested the three-way interaction: MTHFR genotype × folate intake × tibia Pb.
Regression diagnostics (residual v. fitted values, leverage v. residual squared, and augmented component-plus-residual plots, as well as checks for normality and homoskedasticity of the residuals) and tests of collinearity were performed. Regression diagnostics indicated acceptable model fits; regression assumptions were met.
Results
Participant characteristics
Women in the present study were aged 24·6 (sd 5·1) years at delivery. This sample was on average overweight and consisted of 40 % first-time mothers. Most women had lived in Mexico City since early childhood, potentially being exposed to Pb most of their lives. Approximately 40 % of the women reported ever smoking. Over 27 % of the women and 13·7 % of cord bloods were ≥ 100 μg Pb/l blood, with a correlation between the two of 0·8. Of the 44·8 % of women who felt their diet had changed the previous year, 18·5 % specifically mentioned pregnancy as the reason, and 6 % mentioned economic hardship. Other changes included dieting, eating more fruits, less salt, or eating more. The study sample differed from those excluded on years of education, percentage taking prenatal supplements for >2 months, mean (but not median) folate intake and birth weight (all P < 0·05). The difference in folate intake was due to the exclusion of implausible energy and folate values. The difference in birth weight was probably due to the exclusion of premature births.
The allele frequencies for maternal C677T, A1298C and G1793A were 0·59, 0·11 and 0·04, respectively. Infants' allele frequencies were similar, at 0·59 for C677T, 0·11 for A1298C and 0·03 for G1793A. Allele distribution was in Hardy–Weinberg equilibrium.
Main effects of folate intake and tibia lead on birth outcomes
Birth weight was 4 g higher for every 100 μg/d increase in folate intake (Table 2; model 1), and women whose daily folate consumption fell in the highest quartile (1080–4898 μg/d) had newborns who weighed 54·8 g more than women whose folate intake was in the lowest quartile ( < 279 μg/d) (P = 0·08 for trend). However, women who consumed dietary folate at levels below 400 μg/d did not differ on child birth weight from women consuming higher amounts (Table 2; model 1). In turn, each 1 μg/g difference in tibia Pb concentration was associated with a 4·9 g decrease in birth weight (Table 2; model 2). In a comparison among quartiles of tibia Pb concentrations, infants born to women with tibia Pb in the highest quartile (15·6–76·5 μg/g bone) weighed 140 g less than infants born to women with tibia Pb in the lowest quartile (below 4·1 μg/g bone) (P < 0·001). Compared with women with tibia Pb below 9·4 μg/g (median), those above 9·4 μg/g had infants who weighed, on average, 99 g less.
Table 2 Covariate-adjusted associations between maternal folate intake, tibia lead concentrations and infant size at birth†
(β Coefficients with their standard errors)
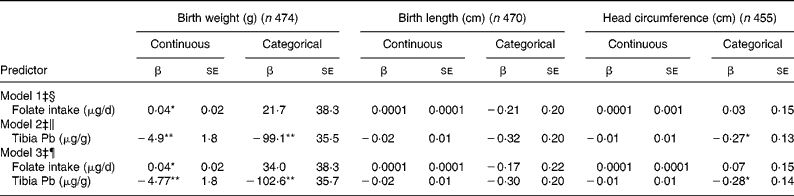
*P < 0·05, **P < 0·01.
† Folate intake and tibia Pb values model as continuous variables or categorical variables (folate < 400 μg/d, tibia Pb ≥ 9·4 μg/g).
‡ Adjusted for maternal age, pre-pregnancy BMI, height, total years of schooling, parity, marital status, ever smoking, postpartum calf circumference, infant gestational age and sex of infant.
§ Model 1 represents the covariate-adjusted main effect of folate intake on birth size.
∥ Model 2 represents the covariate-adjusted main effect of tibia Pb concentrations on birth size.
¶ Model 3 represents the covariate-adjusted relationship between folate intake and tibia Pb with birth size.
Maternal folate intake or maternal tibia Pb concentrations were not associated with birth length. Folate intake was not associated with head circumference, but women with tibia Pb >9·4 μg/g had newborns with smaller heads than women with lower Pb burdens (Table 2; model 2). When folate intake and tibia Pb were modelled together, they did not attenuate each other's association with birth size (Table 2; model 3). There were no significant interactions between folate intake and tibia Pb on any outcomes (data not shown).
Relationship between folate intake and methylenetetrahydrofolate reductase (MTHFR), and tibia lead and MTHFR
Neither maternal nor infant MTHFR genotype was associated with infant size at birth (data not shown). We also tested two-way interactions: folate intake × MTHFR genotype and tibia Pb × MTHFR genotype on birth weight (Table 3), birth length and head circumference (data not shown). The MTHFR genotype did not modify the effect of folate intake on any aspect of newborn size. We also found no significant interactions between MTHFR genotype and tibia Pb on newborn size.
Table 3 Interactions between methylenetetrahydrofolate reductase (MTHFR) genotype, folate and lead on birth weight†
(β Coefficients with their standard errors)

*P < 0·05, **P < 0·01.
† Adjusted for maternal age, pre-pregnancy BMI, height, total years of schooling, parity, marital status, ever smoking, postpartum calf circumference, infant gestational age and sex of infant.
Relationship between folate intake, tibia lead and birth size, by genotype
We examined the interactions between folate intake and tibia Pb on birth size within each maternal and infant MTHFR genotype. We tested but found no significant three-way interactions between folate intake, tibia Pb, and each of the SNP. Furthermore, finding no significant folate intake × Pb interactions, we examined only the main effects of folate intake and tibia Pb within the SNP strata (Table 4). There were no consistent associations between folate intake and birth weight by maternal MTHFR genotype: only two of the six regression coefficients were significant and all were similar across strata (Table 4). For women with 677CC, 1298AA and 1793GG genotypes, the association between tibia Pb and birth weight appeared more adverse than for women with 677CT/TT, 1298AC/CC and 1793GA/AA genotypes. Analyses by the infants' genotype (Table 4) revealed no associations between maternal folate intake and birth weight in genotype strata. Tibia Pb was negatively associated with birth weight but there were no clear cross-strata differences in coefficients.
Table 4 Covariate-adjusted relationship between folate intake, tibia lead and birth weight, by maternal and infant methylenetetrahydrofolate reductase (MTHFR) genotype†
(β Coefficients with their standard errors)
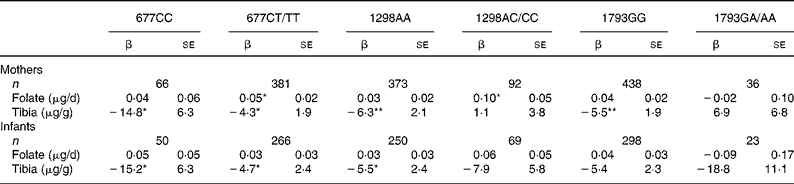
*P < 0·05, **P < 0·01.
† Adjusted for maternal age, pre-pregnancy BMI, height, total years of schooling, parity, marital status, ever smoking, postpartum calf circumference, gestational age and sex of infant.
The association between maternal folate intake and birth length was not significant and did not differ by maternal or infant SNP (data not shown). There were no consistent differences by genotype on the association between tibia Pb and birth length. The relationship between folate intake or tibia Pb and head circumference was not statistically significant within MTHFR strata (data not shown).
Discussion
The present study examined nutrient–environment, gene–nutrient and gene–environment interactions in determining newborn size. We confirmed that maternal folate intake is positively associated with birth weight and that maternal Pb exposure is associated with lower newborn size, particularly birth weight. In addition, the present study contributes to our understanding of how predictors of newborn size interact to affect these outcomes. We found that maternal folate intake does not modify the negative effects of Pb on birth size despite a reported interaction from a study on cognitive performance in children(Reference Solon, Riddell and Quimbo12). We also found that neither maternal nor infant MTHFR genotype alone was associated with newborn size and that these genotypes did not modify the effects of folate intake or Pb on newborn size; however, this may be due in part to limited power to detect an interaction.
In the present study, differences in newborn size in relation to maternal folate intake and Pb exposure were modest. Birth weight was higher by 54·8 g among women with high (1080–4898 μg/d) v. low ( < 279 μg/d) daily folate intake. In turn, infant birth weight was lower by 140 g among women with high tibia Pb levels (15·6–76·5 μg/g) compared with those with low tibia Pb ( < 4·1 μg/g). Even modest declines in newborn size, however, could affect later development and disease at the population level, particularly in an urban setting such as Mexico City, where pregnant women may have multiple environmental exposures and micronutrient deficiencies.
Numerous studies show positive associations between maternal folate intake and biomarkers, and fetal and newborn size(Reference Neggers, Goldenberg and Tamura1–Reference Sram, Binkova and Lnenickova3, Reference Scholl, Hediger and Schall28). Supplementation with folate also increases birth weight and prevents low birth weight and preterm birth(Reference Rolschau, Kristoffersen and Ulrich29, Reference Charles, Ness and Campbell30). The present results are consistent with those findings. Unlike others(Reference Nurk, Tell and Refsum21, Reference Relton, Pearce and Burn23, Reference Engel, Olshan and Siega-Riz24), however, we did not find effect modification by MTHFR genotype, possibly because we assessed folate intake rather than biomarkers of folate status or because folate intake was fairly high in this population. Of the women, 35·3 % had folate intakes below 400 μg/d, which is lower than the prevalence of inadequate intakes in low-income US women(Reference Scholl, Hediger and Schall28). Folate intake in our sample (median 520 μg/d) was also higher than previously reported for Mexico City women (median 187·7 μg/d)(Reference Baquera, Rivera and Espinosa-Montero31).
Of particular interest in the present study was the examination of folate intake × Pb interactions in affecting newborn size because such interactions would have important implications for prenatal care of Pb-exposed women. Two studies have found inverse correlations between folate status and Pb levels in women who were of reproductive age(Reference Lee, Chun and Song10) or pregnant(Reference Dawson, Evans and Harris11). Another showed that folate status attenuated the adverse effect of Pb on children's cognitive performance(Reference Solon, Riddell and Quimbo12). In the present study, folate intake and maternal Pb burden were not correlated and we found that folate intake did not modify the association between tibia Pb and newborn size. It is unclear whether the lack of effect modification in the present study was due to how we measured folate status (intake v. serum or blood indicators), to the range of folate intakes and Pb exposures among the study women, or to birth size being a less sensitive outcome than cognitive performance. Also, the processes by which Pb produces neurotoxicity probably differ from those exerted on fetal growth, and as such the present study does not contradict prior reports of folate × Pb interactions on neurodevelopmental outcomes.
In terms of Pb effects on newborn size, our group has previously shown that long-term Pb exposure is associated with lower birth weight(Reference González-Cossío, Peterson and Sanín5) and length(Reference Hernández-Avila, Peterson and González-Cossío6). Our birth-weight findings are consistent with other studies(Reference Berkowitz, Price-Green and Bove7, Reference Jelliffe-Pawlowski, Miles and Courtney8) although different measures of Pb exposure were used, and investigations of birth length and head circumference in Pb-exposed populations are limited. Pb exposure levels in the present study were higher than in US populations(Reference Harville, Hertz-Picciotto and Schramm32, Reference Schell, Denham and Stark33). Very few studies measure bone Pb, so comparisons with other populations are difficult. It is unclear whether similar findings on newborn size would be present in populations with lower Pb exposures.
Despite finding no statistically significant interactions between tibia Pb and MTHFR genotypes, we believe that the differing regression coefficients for the Pb–birth weight relationship in maternal genotype strata deserve further study with larger samples. The possible protective effect of MTHFR polymorphisms on birth weight in prenatal Pb exposure should be investigated in studies that include biomarkers of both Pb and folate status. Pb × MTHFR interactions are potentially important in populations characterised by a high prevalence of variant genotypes, particularly the 677CT/TT. The allele frequency for this variant in our population was 0·59, in line with other studies of Mexican populations, but much higher than among Northern Europeans and Africans(Reference Gueant-Rodriguez, Gueant and Debard17). The allele frequency for 1298AC/CC was also in line with other studies(Reference Gueant-Rodriguez, Gueant and Debard17).
A potential limitation of the present study was the use of a 12-month recall period to estimate folate intakes in pregnant women. The consumption of foods changed between the first and second trimesters for about 50 % of Boston women(Reference Rifas-Shiman, Rich-Edwards and Willett34) and between pre- to mid-pregnancy in women from rural New York(Reference Olson35). In the present study, 44·8 % of women reported changes in diet in the previous year, but this was unrelated to newborn size. The concern with an FFQ that has a longer recall period is that it may inadequately reflect intakes over that period and that important effects may be missed when diet is not assessed more frequently. The recall period in the present study may be another reason why we did not find an effect modification of folate intake by MTHFR genotype, particularly if intakes in one trimester of pregnancy are more relevant to this relationship than in another.
In summary, we found that higher maternal folate intake was positively, but higher bone Pb concentrations were negatively, associated with newborn size. Although the associations between maternal nutrition and Pb burden and child size at birth were modest, they underscore the importance of environmental exposures to child health, because birth outcomes such as weight predict postnatal growth and development of the child, and disease risk in adulthood.
Acknowledgements
The present study was supported by the US National Institute of Environmental Health Sciences (NIEHS; grant nos. P42-ES05947, P30-ES 00002, K23-ES000381, RO1-ES014930, RO1-ES013744 and RO1-ES007821), Consejo Nacional de Ciencia y Tecnología (CONACyT) grant 4150M9405 and CONSERVA, Department of Federal District, Mexico. We thank the American British Cowdray Hospital (Mexico City) for use of their research facilities.
K. K. analysed the data and drafted the manuscript. A. S. E. assisted with data collection and interpretation, and manuscript editing. H. L.-F. and M. M. T.-R. assisted with data interpretation and manuscript editing. M. H.-A. and H. H. obtained funding for the birth cohort. R. O. W. obtained funding for genotyping.
The authors declare no competing financial interests.






