Mother-to-child transmission (MTCT) accounts for at least 90 % of HIV infections among children; today, an estimated one-half of new infections occur during breast-feeding( 1 ). The exclusive use of formula eliminates the transmission of the virus via breast milk. However, there is a risk of morbidity and mortality from other infections that is associated with formula feeding and other suboptimal infant feeding practices, even in high-income countries( Reference Black, Victora and Walker 2 , Reference Bartick and Reinhold 3 ). The health risks during early infancy are highest where ideal conditions do not exist and mixed feeding (breast milk plus other liquids or solids) is practiced( Reference Coutsoudis, Pillay and Kuhn 4 – Reference Iliff, Piwoz and Tavengwa 6 ). For infants living in poor communities with limited access to clean water, exclusive breast-feeding (EBF) for the first 6 months of life is recommended, regardless of their or their mother’s HIV status( 7 ).
Despite world-wide recommendations, the majority of mothers do not EBF for 6 months( Reference Black, Victora and Walker 2 , Reference Cai, Wardlaw and Brown 8 ). Reasons for not practicing EBF are diverse( 9 ) and may include, among others, (i) advice or social pressure to mix-feed( Reference Laar, Ampofo and Tuakli 10 – Reference Bland, Rollins and Coutsouis 12 ), (ii) perceptions of inadequate milk supply( Reference Kakute, Ngum and Mitchell 11 – Reference Sibeko, Dhansay and Charlton 14 ) or need for water supplementation( Reference Laar, Ampofo and Tuakli 10 ), (iii) societal feeding norms( Reference Fjeld, Siziya and Katepa-Bwalya 13 , Reference Sibeko, Dhansay and Charlton 14 ) and (iv) lack of adequate knowledge on breast-feeding and health( Reference Laar, Ampofo and Tuakli 10 , Reference Fjeld, Siziya and Katepa-Bwalya 13 ). HIV sero-positive women face additional difficulties related to infant feeding. In societies in which breast-feeding is the norm, mixed feeding rather than exclusive formula feeding may be selected because of fear of disclosing their HIV status and being the target of social stigma( Reference Laar, Ampofo and Tuakli 10 , Reference Doherty, Chopra and Nkonki 15 ), as well as because of conflicting information and lack of adequate support obtained from healthcare providers( Reference Bland, Rollins and Coutsouis 12 , Reference Fjeld, Siziya and Katepa-Bwalya 13 ). Recognising these challenges, the World Health Assembly endorsed the Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition, including a 2025 target to ‘increase the rate of EBF during the first 6 months of life to at least 50 %’( 16 ).
Compliance with both EBF recommendations and lifelong anti-retroviral therapy (ART) regimens for infected women reduces MTCT to a level such that elimination of child infections could be a near-term goal( 1 ). However, both optimal feeding and therapy are needed. The coverage of HIV testing and ART to prevent MTCT has increased markedly in Ghana. The estimated percentage of HIV-infected pregnant women who received ART increased from 38 % in 2008 to >76 % in 2013, although it dropped again in 2014 to 66 %( 17 ). In contrast, national surveys have shown the opposite trend for infant feeding; there was a decline in EBF between 2008 and 2013 (60–53 % for 2–3 months; 49–36 % for 4–5 months)( 18 , 19 ). To develop effective interventions that support EBF for HIV sero-positive and sero-negative mothers, it is important to understand in-depth the infant feeding practices and the factors that are associated with EBF for the recommended full 6 months.
The Research to Improve Infant Nutrition and Growth (RIING) study investigated the pathways by which maternal HIV status influenced infant nutrition and health. The aim of this analysis was to examine whether maternal HIV status was associated with suboptimal breast-feeding and early introduction of liquids and foods that could put the infant at increased risk of viral transmission and illness, as well as to determine other factors that were associated with infant feeding practices.
Methods
Study site
This study was conducted in the Yilo Krobo and Manya Krobo districts in the Eastern region of Ghana between 2003 and 2008. The first birth was in 2004. The HIV infection rate in this region in 2003 was 3·7 % (4·4 % of women), the highest in the country and considerably higher than the Ghanaian national prevalence of 2·2 % (2·7 % of women)( 20 ).
Study design, sample size and participant recruitment
Detailed descriptions of the methods have previously been published( Reference Okronipa, Marquis and Lartey 21 – Reference Garcia, Hromi-Fiedler and Mazur 23 ). The original RIING sample size was based on the outcome of interest requiring the largest sample size, morbidity (using a one-tailed test, a significance level of 0·05, power of 80 %, morbidity rate of 20 % and mean group difference of 15 %)( Reference Lartey, Marquis and Mazur 22 ). Assuming a loss to follow-up of 25 %, the sample size required was 189/group for HIV-positive (HIV-P), HIV-negative (HIV-N) and HIV-unknown (HIV-U) status. The sample available for the present analysis provided a statistical power of 93·1 % for the outcome: HIV group comparison of 6-month EBF rates.
To examine the relationship between maternal HIV status and infant feeding outcomes, the study used a prospective cohort design to follow up caregivers and their infants for 12 months postpartum. Participant recruitment was carried out through the voluntary counselling and testing (VCT) programme of prenatal clinics in the three area hospitals. Women were counselled on the risk of HIV transmission, informed about the services available to them and, until 2005 through the ‘opt-in’ system, were invited to be tested for HIV( Reference Okronipa, Marquis and Lartey 21 ). After 2005, the national programme changed to an ‘opt-out’ structure, in which all women were tested unless they specifically requested otherwise. Once post-test counselling had occurred or mothers refused to be tested (HIV-U), the hospital VCT nurse informed the women of the study and identified those who were interested in participating. Project staff then obtained written informed consent from women for their participation and that of their infant.
Eligibility criteria included that the mother be pregnant at the time of enrolment, be at least 18 years of age, participate in VCT pre-test counselling, agree to have her HIV status released to the project supervisor if tested, plan to be available for the entire duration of the study and have no clinical or physical ailments that would limit her ability to care for her infant. In addition, mothers had to give birth to a live infant free of birth defects that might impair feeding. Only data from the second registered twin were included.
As part of the national protocol for the prevention of MTCT, nevirapine was provided by the hospitals to all women during labour and to the infant at birth( Reference Okronipa, Marquis and Lartey 21 ). Ethics approval for the RIING study was obtained from the Institutional Review Boards of the participating research institutions: University of Ghana, Iowa State University, University of Connecticut and McGill University.
Data collection
Maternal HIV status was determined by the hospital staff, using the Rapid Test Abbott Determine HIV-1/2 (Abbott Laboratories). Only survey variables used in the analyses are described here. These included the following: enrolment household socio-economic and demographic data and maternal obstetric history; household food security measured with an experiential-based eight-item survey that was adapted from the US Household Food Security Survey Model( Reference Garcia, Hromi-Fiedler and Mazur 23 , 24 ) and used at birth, and 3, 6, 9 and 12 months; maternal postnatal depression measured at birth and 6 and 12 months using the ten-item Edinburgh Postnatal Depression Scale( Reference Cox, Holden and Sagovsky 25 ); infant birth weight when the birth occurred in a health facility; and daily breast-feeding and complementary feeding practices evaluated based on World Health Organization definitions( 26 ). Mothers were visited at home twice weekly and were asked about their infant’s intake of breast milk, non-milk liquids, animal-based (non-human) milks, infant formula and solid and semi-solid foods, as well as symptoms of illness (≥3 liquid stools/d (diarrhoea), fever, cough and/or difficulty breathing (acute respiratory infection)) during the previous days since the last visit( Reference Okronipa, Marquis and Lartey 21 ). Weight to the nearest 100 g (Tanita Corporation of America Inc.) and length to the nearest 0·1 cm (Shorr Productions) were measured monthly( Reference Lartey, Marquis and Mazur 22 ). Results from data on morbidity and growth measurements have been previously published( Reference Okronipa, Marquis and Lartey 21 , Reference Lartey, Marquis and Mazur 22 ).
Data analysis
Analyses were performed using SAS version 9.3 (SAS Institute). A food insecurity score was constructed with seven of the eight items (one item was considered redundant), using Rasch modelling to determine the psychometric validity of the scale items( Reference Garcia, Hromi-Fiedler and Mazur 23 ). Households were initially categorised as food secure (0 affirmative responses), mild/moderately food insecure (1–4 affirmative responses) and severely food insecure (5–7 affirmative responses). Final models included households as either food secure (0) or food insecure (1–7). A household amenities score was created as a proxy for socio-economic status, and was developed using eighteen variables related to a household’s socio-economic status (household materials, household water, access to electricity and cooking fuel, appliance ownership)( Reference Lartey, Marquis and Mazur 22 ). Factor analysis with varimax rotation was used to calculate the final score for each household, with a lower value indicating a poorer household. A dichotomous variable was developed from the postnatal depression scale, with <13 categorised as not showing symptoms (no) and 13 and above as showing symptoms (yes)( Reference Okronipa, Marquis and Lartey 21 ). A birth-related variable was defined to reflect the opt-in/opt-out HIV testing system in the Ghana Health Services (2004/2005=opt-in, 2006 and beyond=opt-out). A dichotomous illness variable was calculated if the child had at least one symptom (diarrhoea, fever, acute respiratory infection) in the past 30 d. Weight was converted to weight-for-age Z-score (WAZ) using the WHO Child Growth Standards( 27 ). The World Health Organization( 28 ) guidelines for breast-feeding among HIV-infected women changed during the study. Birth date was also dichotomised as before (<2007) and after (≥2007) to capture the differences in policy.
Descriptive analyses compared characteristics at enrolment (or at birth where indicated) by maternal HIV status using ANOVA (with Bonferroni post hoc tests) or χ 2 tests as appropriate, and between mothers who EBF for 6 months or longer and those who breast-fed for <6 months using independent Student’s t tests or χ 2 tests, as appropriate. Kaplan–Meier curves were used to calculate and compare across HIV groups the median survival times in days for time to cessation of EBF, and time to introduction of non-milk liquids, animal-based (non-human) milks, and solids and semi-solids. In the case of formula, a median survival time could not be estimated; the 25th percentile was presented. Censoring was indicated if the mother was still EBF or had not yet introduced a liquid or food category on the last day of data collection. If data were missing for more than 30 d, the last day of valid data before the gap was counted and the mother’s data were then censored. The log-rank (Mantel–Cox) test of equality of survival distribution was used to determine significant differences between HIV groups for time to cessation of EBF and time of introduction of each liquid or food category. The Šidák correction was used to compare differences across HIV groups. The percentage of mothers who were breast-feeding at 6, 9 and 12 months was compared across HIV groups using χ 2 tests.
Bivariate survival analyses using Cox regression were performed between socio-demographic variables (at enrolment or at birth) and time to cessation of EBF, as well as time of introduction of formula, non-milk liquids, animal-based milks and solids and semi-solids. Food insecurity at birth, 3, 6, 9 and 12 months; symptoms of postnatal depression at birth, 6 and 12 months; and monthly morbidity and anthropometric indicators were included as time-dependent covariates.
Multi-variable survival analyses were conducted to determine predictors of time to cessation of EBF and time to introduction of each liquid and food category, using all available data between birth and 365 d. Independent variables were initially included in the models if P<0·25 in the bivariate analyses; after backward elimination was performed, variables with P<0·10 in at least one of the models were kept in all final models. A mother’s HIV status, age, ethnicity, marital status, household amenities, the number of children under 5 years of age, household food insecurity, opt-in/out-out system, morbidity and WAZ remained in the final models. Hazard ratios (HR) were calculated for each independent variable and reflect risk of outcome at any point in time from birth to 365 d. The models were rerun to estimate HR for time to introduction of each complementary liquid and food solely for the period 0–183 d, the period during which EBF is recommended. A similar 0–183-d model for EBF was not included, as all mothers had ended EBF by the 7th month and the results were essentially identical to that of the 0–365-d model included here.
A bivariate logistic regression was conducted to determine the factors associated with the dichotomous variable of EBF for 6 months or longer. In the multi-variable logistic regression, the same independent variables were initially included in the model if P<0·25 in bivariate analyses. Backward elimination was performed and variables with P<0·10 were kept in the final model. A mother’s HIV status, ethnicity and the number of children under 5 years of age remained in the final model.
Finally, to assess the possible influence of the change in the WHO guidelines on infant feeding recommendations, weaning prevalence was compared by birth date (<2007 v. ≥2007) and HIV status using the Cochran–Mantel–Haenszel test with the continuity correction, from 7 to 12 months. All statistically significant associations are reported with at least a P<0·05.
Results
Study population
A total of 552 women were enrolled in the study and 503 women had a live birth (including twelve sets of twins). Data from 482 infants and their mothers (150 HIV-P, 170 HIV-N and 162 HIV-U) were available for this analysis. During the 12 months of follow-up, fourteen moved out of the study area (five HIV-P, two HIV-N and seven HIV-U), forty-six withdrew from the study (twenty-five HIV-P, eleven HIV-N and ten HIV-U), ten mothers or children died (five HIV-P, one HIV-N and four HIV-U), five HIV-P mothers withdrew because of illness of mother or child and the field work finished before data collection was completed with eleven mothers and their children (seven HIV-P, two HIV-N and two HIV-U), giving a group difference in length of follow-up (P<0·001). All 482 participants were included in the study’s analysis up to the last day of available information; censoring was indicated, where necessary.
Maternal and household characteristics
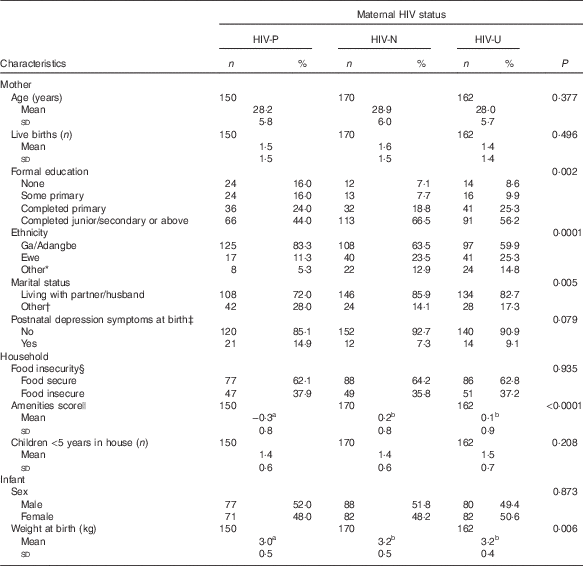
Maternal age ranged from 18 to 50 years with a mean age of 28·4 (sd 5·9) years. Approximately half of mothers (56·4 %, n 272) were traders or earned a salary or wages, one-third of the mothers (33·2 %, n 160) were artisans or students, 2·3 % (n 11) were farmers and 8·1 % (n 39) were unemployed. The majority of women (68·5 %, n 330) were of the Ga/Adangbe ethnic group; the proportion was highest among the HIV-P mothers (Table 1). Several socio-economic indicators suggested a more impoverished status among HIV-P mothers than those in the HIV-N and HIV-U groups, including having less education, fewer household amenities and less likely to live with a partner/husband.
Table 1 Characteristics of Ghanaian mother–infant pairs at enrolment, by maternal HIV status (Numbers and percentages; mean values and standard deviations)
HIV-P, HIV-positive; HIV-N, HIV-negative; HIV-U, HIV-unknown status.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Akan, northern groups.
† Widowed, divorced or single.
‡ A dichotomous variable was developed from the Edinburgh Postnatal Depression Scale( Reference Cox, Holden and Sagovsky 25 ); <13 categorised as not showing symptoms (no) and ≥13 as showing symptoms (yes).
§ Food insecurity score was created from an eight-item household food security survey adapted from the US Household Food Security Survey Model( Reference Garcia, Hromi-Fiedler and Mazur 23 , 24 ); food secure (0 positive responses), food insecure (≥1 positive responses).
|| Household amenities score was created from a set of eighteen socio-economic variables (household building material, access to water, electricity and ownership of appliances) using factor analysis with varimax rotation; lower values reflect poorer status( Reference Lartey, Marquis and Mazur 22 ).
Infant characteristics and feeding practices
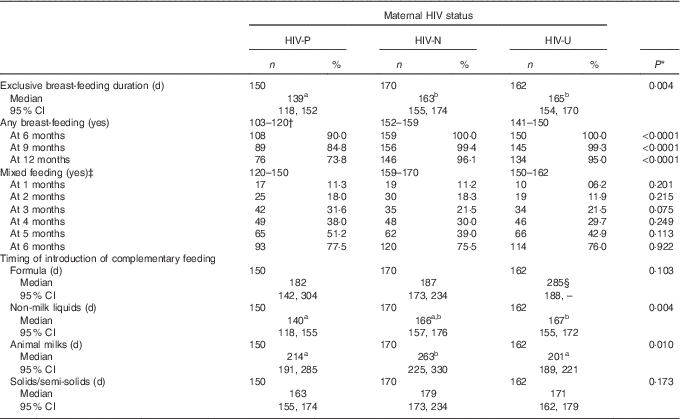
The mean infant birth weight was about 200 g lower among the HIV-P group compared with the other groups (P=0·006), with no difference in sex (Table 1). The median duration of EBF was 158 d (approximately 5·3 months), with the longest at 211 d. Being HIV-P was associated with a significantly shorter duration of EBF (P<0·01; Table 2, Fig. 1): a difference of 24 d compared with HIV-N mothers and 26 d compared with HIV-U mothers. A higher percentage of HIV-N (22·9 %, n 39) and HIV-U (22·2 %, n 36) compared with HIV-P (10·0 %, n 15) mothers EBF for at least 6 months (P=0·005). In addition, a higher percentage of mothers from the Ga/Adangbe (18·2 %, n 60/330) and Ewe (25·5 %, n 25/98) ethnic groups EBF for at least 6 months compared with mothers of the 'other' ethnic group (9·3 %, n 5/54; P=0·045). The number of children under 5 years of age tended to be higher in households in which mothers EBF for at least 6 months compared with mothers who did not do so (1·5 (sd 0·7) v. 1·4 (sd 0·6); P=0·08). Other variables were not associated with EBF for at least 6 months. The association between breast-feeding and HIV continued after EBF ended. Compared with HIV-P mothers, a higher percentage of HIV-N and HIV-U mothers were breast-feeding at 6, 9 and 12 months (all P<0·0001;Table 2).
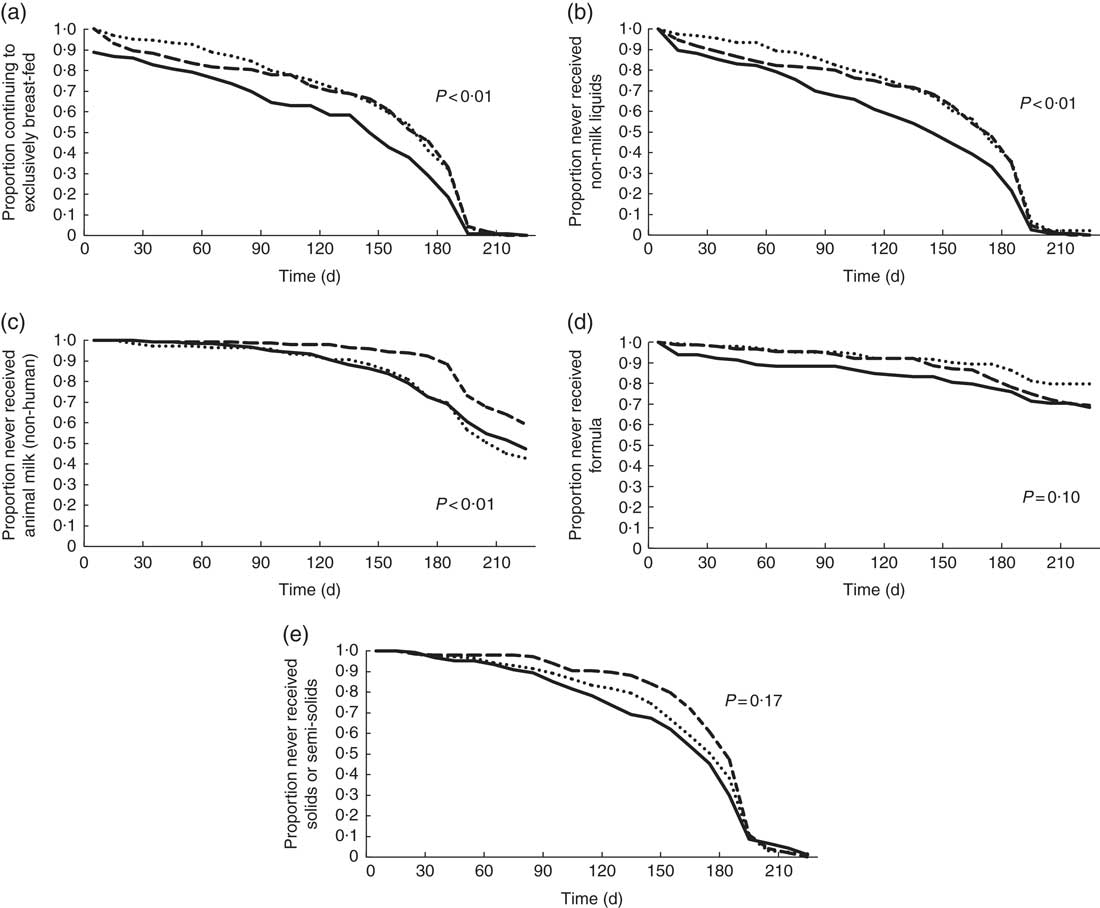
Fig. 1 Bivariate survival analysis results for 478 Ghanaian women: proportion continuing to exclusively breast-feed (a), and not introducing non-milk liquids (b), animal milks (non-human) (c), formula (d) and solids/semi-solids (e), by HIV status (![]() , positive;
, positive; ![]() , negative;
, negative; ![]() , unknown).
, unknown).
Table 2 Infant feeding characteristics and time of introduction of complementary foods for Ghanaian infants, by maternal HIV status (Numbers and percentages; medians and 95 % confidence intervals)
HIV-P, HIV-positive; HIV-N, HIV-negative; HIV-U, HIV-unknown status.
a,b Median values within a row with unlike superscript letters were significantly different (P<0·05).
* HIV group difference tested with log-rank (Mantel–Cox) test of equality of survival distribution; Šidák correction was used to compare differences across groups.
† Range in n for the indicated age and HIV status.
‡ Mixed feeding: breast-feeding plus any type of non-human milk liquids and/or solids/semisolids.
§ Median could not be calculated for formula; values represent Q1 (lower 95 % CI only could be estimated).
Mixed feeding was common throughout the first 6 months, ranging from 10 % in the 1st month to 76 % at 6 months (Table 2). There was no statistically significant group difference in mixed feeding per se; however, groups did differ by what was given. In the bivariate analyses, non-milk liquids were introduced earlier among HIV-P mothers compared with HIV-N (median survival differences of 27 d; P=0·003; Fig. 1); the difference of 26 d compared with HIV-U did not reach significance (P=0·08). HIV-P mothers introduced animal milks 49 d earlier than HIV-N mothers (P=0·037), but timing was similar to that of HIV-U mothers. There was no significant difference by HIV status in the age of introduction of semi-solids/solids, with the median in the three groups between 5 and 6 months. Infant formula was uncommon and used by five HIV-P, eight HIV-N and seven HIV-U mothers in the 1st month; this increased to twenty-six HIV-P, twenty-four HIV-N and twenty HIV-U mothers, cumulatively, by 4 months of age. HIV-U mothers tended to introduce formula earlier compared with HIV-P mothers (P=0·080); no other differences were noted.
Survival analysis models
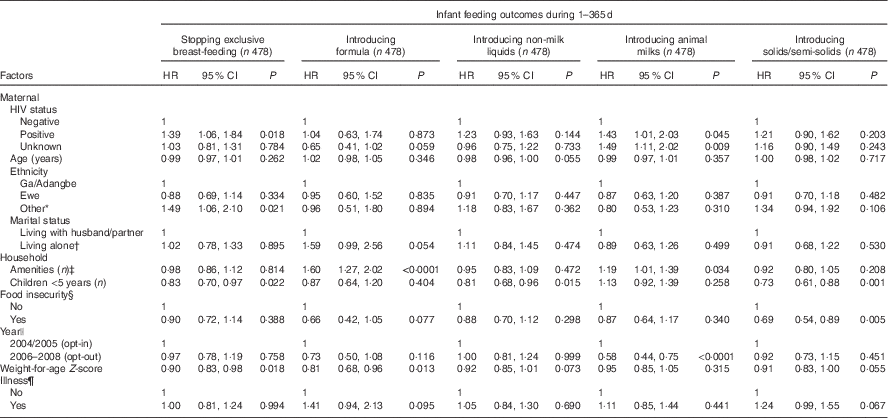
The initial multi-variable Cox regression analyses reflected risk of outcome at all time points throughout the 1st year and demonstrated that different factors were associated with the various infant feeding practices (Table 3). The risk of stopping EBF was higher among HIV-P and those of an ‘other’ ethnic group (about half were northern), and was lower in households with more young children and among infants with better WAZ. Compared with being HIV-N, an HIV-P status was associated with about a 40 % increased risk of stopping EBF at any point of time. In the bivariate survival analysis for EBF, the three ethnicity curves diverged at about 4 months of age.
Table 3 Multivariable survival analysis for factors associated with time to stopping exclusive breast-feeding and time to introducing formula, non-milk liquids, animal milks and solids/semi-solids among Ghanaian mothers (Hazards ratios (HR) and 95 % confidence intervals)
* Akan, northern groups.
† Widowed, divorced or single.
‡ Household amenities score was created from a set of eighteen socio-economic variables (household building material, access to water, electricity and ownership of appliances) using factor analysis with varimax rotation. It is a continuous variable in which lower values reflect poorer status.
§ Food insecurity score was created from an eight-item household food security survey adapted from the US Household Food Security Survey Model( Reference Garcia, Hromi-Fiedler and Mazur 23 , 24 ); food secure (0 positive responses), food insecure (1–8 positive responses).
|| With the ‘opt-in’ system, women were invited to be tested for HIV; with the ‘opt-out’ system, all women were tested unless they specifically requested otherwise.
¶ Illness represented having reported at least one of the following symptoms over 30 d: ≥3 liquid stools/d, fever, cough or difficulty breathing.
Having a higher number of amenities and having a lower WAZ were associated with an increased risk of introducing formula. Risk of introducing formula tended to be lower in the HIV-U group (P=0·059) and among those who were food insecure (P=0·077). Risk tended to be higher among those living alone (P=0·055) and whose children were sick over the past 30 d (P=0·095). The difference by marital status was noted in the bivariate survival analysis from the 1st month of life. When the analysis was repeated separately for only the 0- to 6-month period (n 478), the risk was still reduced among the HIV-U group (HR 0·55; 95 % CI 0·31, 0·98; P=0·044).
The number of young children in the home, but not HIV status, was negatively associated in the full-year model with a risk of introduction of non-milk liquids. Maternal age (P=0·055) and WAZ (P=0·073) tended to be associated with a lower risk of use of non-milk liquids. However, when the analysis was done separately for only 0–6 months, HIV-P status tended to be associated with a higher risk of introduction of non-milk liquids (HR 1·34; 95 % CI 0·98, 1·83; P=0·068), and the association with WAZ became significant (HR 0·87; 95 % CI 0·78, 0·96; P=0·007).
The risk of introducing animal milks over the entire 12 months was higher among HIV-P and HIV-U compared with HIV-N mothers in addition to those households with more amenities. However, mothers who enrolled during the opt-out period were >40 % less likely to introduce animal milks compared with those who were enrolled earlier. Bivariate survival curves demonstrated a rapid introduction of animal milks in the 6th month among those who were enrolled when the opt-in system was in place; similar behaviour was not seen among participants who were part of the opt-out system (data not shown). Results from only the first 6 months showed an even stronger association with a more than 2-fold higher risk of introducing animal milks with HIV-P and HIV-U status (HR 2·37; 95 % CI 1·32, 4·24; P=0·004 and HR 2·11; 95 % CI 1·23, 3·64; P<0·007, respectively).
The risk of introduction of solids and semi-solids was reduced with having a larger number of children in the home and with household food insecurity. Higher WAZ tended to be associated with a lower risk, whereas illness was associated with a higher risk of introducing solids. When the model was rerun for 0–6 months only, all associations remained similar except for HIV, ethnicity and WAZ. During the first 6 months, HIV-P was strongly associated with an increased risk of introduction of solids (HR 1·56; 95 % CI 1·10, 2·22; P=0·011). The association of introduction of solids with HIV-U, being of an ‘other’ ethnicity, and a lower WAZ all tended towards significance (HR 1·31; 95 % CI 0·95, 1·81; P=0·098, HR 1·52; 95 % CI 1·00, 2·31; P=0·052 and HR 0·90; 95 % CI 81, 1·01; P=0·063, respectively).
Logistic regression
Logistic regression for the likelihood to EBF for 6 months demonstrated similar results as the survival analysis. Mothers with HIV-P status were less than half as likely to complete 6 months of EBF compared with HIV-N mothers (adjusted OR (aOR) 0·42; 95 % CI 0·22, 0·81; P=0·010). The ‘other’ ethnicity category had a lower odds of EBF (aOR 0·35; 95 % CI 0·13, 0·93; P=0·036) compared with being Ga/Adangbe, whereas having a higher number of children under 5 years of age tended to increase the odds of EBF for at least 6 months (aOR 1·35; 95 % CI 0·94, 1·94; P=0·101).
Risk of weaning and WHO policy change
By the end of the 1st year, a 5–6-fold higher percentage of infants were weaned in the HIV-P group compared with HIV-N and HIV-U (26 v. 4 and 5 %, respectively; P<0·0001; Table 2). The prevalence of weaning between 7 and 12 months was similar for infants born before or after the release of the WHO breast-feeding guidelines when analysed across the different HIV groups (all months P>0·10). However, when analysed among only the HIV-P group, which had substantial weaning, there tended to be a difference; infants born before 2007 were twice as likely to be weaned at 12 months than those who were born in 2007 (22·7 v. 46·7 %; P=0·051).
Discussion
This study provided a unique opportunity to examine EBF and the introduction of liquids and solids/semi-solids into infants’ diets in an HIV-affected region of Ghana. The association of infant feeding with HIV status and other predictors varied depending on the specific behaviour that was being carried out, suggesting that multiple intervention approaches may be needed to fully promote optimal feeding.
In our study site, HIV infection was linked to being less likely to follow the international recommendation to EBF for 6 months; the duration difference associated with HIV was almost 1 month. Some studies, but not all, concur with our results. The feeding practices of a random selection of 727 mothers (HIV-U) participating in a 2003 district-wide survey were compared with those of 235 HIV-infected mothers attending outreach clinics in 2005 in the same area of Uganda( Reference Fadnes, Engebretsen and Wamani 29 ). Among those with children under 6 months of age, only 24 % of HIV-infected mothers reported EBF compared with 45 % of the general population (P<0·05). The prevalence of EBF in both groups may have been inflated, as it was estimated using a 24-h dietary recall. Infants experience brief interruptions in an EBF pattern( Reference Marquis, Díaz and Bartolini 30 , Reference Zohoori, Popkin and Fernandez 31 ) that can be easily not reported in cross-sectional surveys. Twice-weekly home visits allowed our project field staff to establish rapport with the mothers and to identify precisely any changes in feeding practices. In addition, mothers’ willingness to respond truthfully to questions about breast-feeding may vary by HIV status, leading to reporting bias. Increased trust between mothers and field staff established by frequent visits can be expected to improve data quality.
In contrast to our results and those in Uganda, a longitudinal study in 2001 that followed a cohort of 177 HIV-infected and 177 HIV-uninfected Zambian mothers from pregnancy to 4 months postpartum found no difference in EBF duration( Reference Chisenga, Kasonka and Makasa 32 ). The 4-month EBF rate for both groups was the same as that of our HIV-P mothers (37 %); however, uninfected women in our setting had a higher rate of 51 %. Our overall EBF median survival time of 5·3 months was higher than national rates reported in 2003 (2·3 months)( 20 ) or 2008 (3·3 months)( 18 ). These differences may be partly because of selection bias. All participants were recruited through hospital VCT clinics where mothers were exposed to infant feeding education. In addition, the project referred mothers to an independent breast-feeding counsellor if they requested assistance. Aidam et al.( Reference Aidam, Pérez-Escamilla and Lartey 33 ) demonstrated the potential for high-quality counselling to improve EBF duration. In their study carried out close to our site, almost all mothers with prenatal and postnatal lactation counselling chose to EBF for 6 months compared with 48 % of those mothers who received standard-of-care health services (P=0·008). Laar et al.( Reference Laar, Ampofo and Tuakli 10 ) reported that mothers living in districts adjacent to our study site were discouraged from using formula by their HIV nurse counsellors. Healthcare providers reported that even if the appropriate circumstances were present formula was not promoted because the hospitals were signatories of the Baby Friendly Health Initiative. Their commitment to breast-feeding supports the higher EBF duration results from our population; however, the almost 1-month difference in EBF associated with HIV status suggests that there remained an inadequacy in HIV-specific feeding education and support. The dangers of mixed feeding should be emphasised to all mothers; however, the additional risk of MTCT of HIV( Reference Iliff, Piwoz and Tavengwa 6 ) demonstrates the need for specific ‘how-to’ messages and counselling for HIV-P mothers.
In addition to the influence of health services, mothers’ infant feeding decisions may have been influenced by the child’s health and nutritional status. Better nutrition was associated with a decreased risk of stopping EBF and introducing formula, and a tendency to not introduce non-milk liquids and solids. In other settings, weaning age was inversely related to the nutritional status and health of the child( Reference Marquis, Habicht and Lanata 34 , Reference Simondon, Simondon and Costes 35 ).
There are inconsistent results regarding the association between HIV and the introduction of non-breast milk items. In Lusaka, Zambia, HIV-infected mothers introduced liquids to their infants below 2 months of age more frequently than uninfected mothers (28 v. 11 %, respectively; P=0·03) and only HIV-infected mothers introduced semi-solids before 2 months (P=0·002)( Reference Omari, Luo and Kankasa 36 ). Women reported introducing foods early because of a perception of milk insufficiency, a common belief in our study district( Reference Otoo, Lartey and Pérez-Escamilla 37 ). In the previously mentioned Ugandan study, HIV-infected mothers compared with women in the general population also reported early (before 6 months) introduction of water (54 v. 23 %; P<0·001), animal-based milks (54 v. 35 %; P<0·05) and solids (49 v. 14 %; P<0·001)( Reference Fadnes, Engebretsen and Wamani 29 ). In contrast, Ghanaian HIV-P mothers were less likely to give formula and non-human milks than other liquids.
Even with its low use, HIV status was associated with an increased risk of early introduction of animal-based milks. Women in other poor settings reported the decision to not wean specifically because of their inability to purchase milk, which they considered to be the acceptable replacement for breast milk( Reference Marquis, Habicht and Lanata 34 ). It is likely that similar decisions were made by our Ghanaian study population; a higher amenities score (more wealth) was associated with an increased risk of introducing milk. These results are consistent with those of Laar et al.( Reference Laar, Ampofo and Tuakli 10 ) who reported that the perceived high cost of replacement feeding was associated with an increased likelihood of EBF in the first 3 months of life in Ghana (aOR 4·60; 95 % CI 1·40, 15·14). Economic considerations need to be part of nutrition counselling.
In our study, being of an ‘other’ ethnic group (half of whom were northerners) was associated with the increased risk of stopping EBF. The Ga/Adangbe was the predominant ethnic group. Mothers from the local groups may have had more social networks to provide emotional and physical support, which has been found to help mothers properly bond with a new infant, use health services more successfully and ultimately to engage in better feeding practices( Reference Webb-Girard, Cherobon and Mbugua 38 ). Further understanding of the diverse cultural practices and beliefs in Ghana is needed.
The HIV-U category is primarily a reflection of the ‘opt-in’ HIV testing policy. When clinics changed to an ‘opt-out’ HIV testing policy, fewer women refused testing. Although the distribution of HIV-P and HIV-N mothers in this group is unknown, the HIV-U results here and in previous publications( Reference Lartey, Marquis and Mazur 22 ) suggest that the group is a combination of infected and uninfected women.
Over the time of the project, the WHO changed its infant feeding guidelines related to HIV. In 2003, WHO guidelines stated ‘… exclusive breastfeeding is recommended during the first months of life and should then be discontinued as soon as feasible’( 39 ). By mid-project in late 2006, new guidelines were developed that recommended stopping ‘once a nutritionally adequate and safe diet without breast milk can be provided’( 28 ). One expected to see a higher prevalence of weaning among the early study participants. However, weaning prevalence among infants in the HIV-P group tended to be higher at 12 months among those born after the new guidelines were released. Either other factors related to time were relevant or the new policy had the unintended result of increased weaning among a population that most likely did not meet the suggested conditions.
The study would have been enriched by having a qualitative component to look in-depth at unmeasured maternal and household characteristics, maternal and stakeholders’ knowledge and beliefs regarding feeding practices, and social dynamics in the home, health setting and community. Furthermore, the relatively small sample size and the large number of censored cases for some foods may have diminished the ability to identify meaningful associations. Although recall bias may have existed because of mothers’ self-reporting of feeding practices, this was minimised by collecting information twice weekly.
The associations between HIV and infant feeding practices reported in this paper are likely to be as relevant today as they were 10 years ago. National-level economic and health improvements over the past 10 years have not occurred homogeneously. Although the country met the Millennium Development Goal of halving extreme poverty, the Eastern Region experienced an increase in poverty incidence between 2006 and 2013( 40 ). As the nation experienced a 64 % decrease in the prevalence of HIV between 2007 and 2013, the Eastern region’s rate increased and remains today the highest in the country at 3·7 %, the same as 2003( 41 ). Experiences of stigma in health services( Reference Dako-Gyeke, Dako-Gyeke and Asampong 42 ) and an unstable supply of antiretroviral drugs remain problematic. Although general breast-feeding promotion is well established in the Ghana Health Services, interactive education is needed to provide practical solutions to problems that mothers have in practicing EBF and complementary feeding. Recent research has documented little improvement in the quality of the nutrition education in rural communities of the Eastern region( Reference Laar, Ampofo and Tuakli 10 ).
This study investigated the association between HIV status and infant feeding practices in Ghana. EBF is the most effective and feasible feeding option for mothers in low-resource settings; increased and better interventions are needed to reach the WHO’s 2025 goal and to ensure that all women receive the support needed. New strategies will require inter-sector and multi-level approaches that include strong coordination between facility and community-based infant feeding promotion, protection and support systems.
Acknowledgements
The authors would like to thank Boateng Bannerman and Rula Soueida for their work on data management and Dr Roger Cue for assistance with the statistical analyses. The authors are indebted to the research and health services teams that worked with us and the families that let us into their lives.
This work was supported by the National Institute of Child Health and Development (NICHD)/National Institutes of Health (grant no. HD 43260). The National Institutes of Health had no role in the design, analysis or writing of this article. The contents are solely the responsibility of the authors and do not necessarily represent the official views of NICHD.
G. S. M., A. L., R. P.-E., R. E. M. and L. B. developed the research questions, designed the study and participated in the field work; K. A. B. carried out the statistical analysis; G. S. M. and K. A. B. drafted the paper; A. L., R. P.-E., R. E. M. and L. B. contributed to the writing of the paper; all authors contributed to the interpretation of results.
There are no conflicts of interest.







