Suicide is a global public health concern, in that approximately 800 000 people die by suicide every year, accounting for 1.5% of all deaths.Reference Naghavi1 Non-fatal suicidal behaviour, such as suicidal ideation or attempt, is 10–20 times more common than fatal suicide.Reference Mann2 Identification of risk factors is imperative to monitor suicidal behaviour and intervene to reduce risk. Childhood abuse is a well-known risk factor for suicidal behaviour;Reference Bruffaerts, Demyttenaere, Borges, Haro, Chiu and Hwang3–Reference Ng, Yong, Ho, Lim and Yeo5 however, suicidal behaviour is likely to involve mediating or interacting effects among various precipitating and predisposing factors, making most explanatory models complex and difficult to interpret.Reference Fazel and Runeson6
Brain-derived neurotrophic factor for childhood abuse and suicidal behaviour
There has been indirect evidence that brain-derived neurotrophic factor (BDNF) may be involved in the association between childhood abuse and suicidal behaviour. BDNF is important for many neural processes, including neurogenesis, neuroplasticity and neurotransmission. Acute and chronic stresses have been found to be associated with decreased BDNF messenger RNA in the rat hippocampus,Reference Rasmusson, Shi and Duman7 and childhood maltreatment has been proposed to be associated with changed brain morphology via disrupted neuroprotective effect of BDNF in individuals with depression.Reference van Velzen, Schmaal, Jansen, Milaneschi, Opmeer and Elzinga8 Also, in post-mortem studies, significantly lower BDNF levels in the hippocampus and prefrontal cortex were found in individuals after death by suicide, compared with those who died of other causes.Reference Banerjee, Ghosh, Ghosh, Bhattacharyya and Mondal9 These findings suggest that pathological changes in BDNF levels resulting from stresses such as childhood abuse may be one of the causes of neurobiological deficits that impair an individual's ability to adapt to difficult situations and lead to suicidal behaviour.Reference Dwivedi10 Despite ample evidence for associations of BDNF with childhood abuse and suicidal behaviour, there has been little investigation of the interactions between BDNF and childhood abuse on suicidal behaviour. However, it was reported that the BDNF val66met polymorphism moderated the effect of childhood maltreatment on the violence of suicidal behaviour in those who attempted suicide.Reference Perroud, Courtet, Vincze, Jaussent, Jollant and Bellivier11
Blood biomarkers for suicidal behaviour
Peripheral blood biomarkers reflective of pathophysiological changes for suicidal behaviour may have several advantages in terms of convenience and cost-effectiveness. Serum BDNF levels have been found to be negatively associated with childhood trauma in psychotic patients.Reference Theleritis, Fisher, Shäfer, Winters, Stahl and Morgan12 However, studies comparing blood BDNF levels in individuals with and without a history of suicidal behaviour have been conflicting, in that some have reported significantly reduced BDNF levels in those who attempted suicide,Reference Kim, Lee, Won, Park, Lee and Lee13,Reference Grah, Mihanovic, Ruljancic, Restek-Petrovic, Molnar and Jelavic14 whereas others have found no significant associations.Reference Eisen, Perera, Bawor, Dennis, El-Sheikh and DeJesus15 This inconsistency may have arisen through not considering potential interactions with other risk factors; although, to our knowledge, there has been no empirical investigation of this.
Aims
The suicide rate in Korea was 24.6 per 100 000 in 2019 – the highest for countries in the Organization for Economic Cooperation and Development.16 Suicidal behaviour is strongly associated with depressive disorder. Using a prospective clinical data of Korean patients with depressive disorders, we investigated the individual and interactive effects of childhood abuse and serum BDNF levels on suicidal behaviour in patients with depressive disorders.
Method
Study overview
This study was conducted as a part of the MAKE Biomarker Discovery for Enhancing Antidepressant Treatment Effect and Response (MAKE BETTER) research. Study details have been published as a design paperReference Kang, Kim, Kim, Kim, Shin and Shin17 and the study was registered with the Clinical Research Information Service (cris.nih.go.kr; identifier KCT0001332). All data on sociodemographic and clinical characteristics at baseline, and treatment-related variables at follow-up examinations during the acute treatment phase (evaluated at 3, 6, 9 and 12 weeks) and the continuation treatment phase (evaluated at 6, 9 and 12 months), were acquired with a structured questionnaire conducted by research assistants blind to treatment modalities. These staff were trained in questionnaire administration and data acquisition methods by the research psychiatrists. Patient data were written on paper forms, registered on the online MAKE BETTER study (http://icreat.nih.go.kr/icreat/webapps/com/hismainweb/jsp/cdc_n2.live) site within 3 days, and checked by data control centre staff. The Chonnam National University Hospital Institutional Review Board (approval number CNUH 2012-014) approved this study.
Participants
From March 2012 to April 2017, patients with depressive disorders were consecutively recruited from those who had visited the Chonnam National University Hospital out-patient psychiatric department. All included cases were new treatment incidences regardless of whether depressive episodes were first-onset or recurrent. Inclusion criteria were as follows: aged >7 years; diagnosis of major depressive disorder (MDD), dysthymic disorder or depressive disorder not otherwise specified, as obtained with the Mini-International Neuropsychiatric Interview (MINI),Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller18 a diagnostic psychiatric interview based on the DSM-IV criteria;19 Hamilton Rating Scale for DepressionReference Hamilton20 score of >13; and capable of completing questionnaires, understanding the study objectives and signing the informed consent form. Exclusion criteria were as follows: unstable or uncontrolled medical status; unable to complete the psychiatric evaluation or comply with the pharmacologic treatment because of a severe physical disease; current or lifetime DSM-IV diagnosis of bipolar disorder, schizoaffective disorder, schizophrenia, schizophreniform disorder, psychotic disorder not otherwise specified or other psychotic disorder; history of organic psychosis or seizure disorder; history of anti-epileptic treatment; hospital admission resulting from a diagnosed psychiatric condition other than depressive disorder (e.g. alcohol or drug use disorder); received previous electroconvulsive therapy to treat the present depressive episode; or currently pregnancy or breastfeeding. Written informed consent was obtained by all participants. For those aged <16 years, informed consent was acquired from a parent or legal guardian, and written agreement was obtained from the participant.
Baseline characteristics
Childhood abuse
Childhood abuse was evaluated with the Nemesis Childhood Trauma Interview.Reference de Graaf, Bijl, Smit, Vollebergh and Spijker21 In this semi-structured interview, participants were asked whether they had ever experienced emotional/ psychological, physical and sexual abuse before the age of 16 years. Emotional abuse was evaluated by asking ‘Were you emotionally or psychologically abused, meaning being yelled at, falsely punished, subordinated to your siblings or being blackmailed?’; physical abuse was evaluated by asking ‘Were you physically abused, meaning being hit, kicked, beaten up or other types of physical abuse?’; and sexual abuse was evaluated by asking ‘Were you being abused sexually, meaning being touched or having to touch someone in a sexual way or pressured into sexual contact against your will?’. As these forms of abuse often occur together,Reference Ng, Yong, Ho, Lim and Yeo5,Reference Hovens, Wiersma, Giltay, van Oppen, Spinhoven and Penninx22 a broad definition of ‘childhood abuse’ (having at least one type of abuse) was used for the primary analysis.
Serum BDNF
Participants were instructed to fast from the previous night before morning blood sampling. They sat for 25–45 min quietly and relaxed before blood samples were acquired. Serum BDNF levels were determined using Quantikine ELISA Human BDNF Immunoassay (R&D Systems, Minneapolis, USA) at Global Clinical Central Lab (Yongin, Korea). Based on the median value of BDNF levels, participants were divided into higher and lower BDNF groups.
Covariates
Data on the following sociodemographic characteristics were obtained: age, gender, year of education, marital state (currently married or not), cohabitation status (living alone or not), religion (religious observance or not), occupational state (current employed or not) and monthly income degree (above or below $2000). Clinical characteristics assessed were composed of diagnoses of depressive disorders (MDD or other depressive disorders) with certain specifiers, including melancholic and atypical depressive features, age at onset and illness duration, number of previous depressive episodes, duration of present episode, family history of depression, number of concurrent physical disorders (obtained from a questionnaire asking for 15 disease categories on the main body systems) and smoking state (current smoking or not). Assessment scales for investigating symptoms were administered. Depressive and anxiety symptoms were evaluated with the Hospital Anxiety and Depression Scale depression subscale (HADS-D) and anxiety subscale (HADS-A),Reference Zigmond and Snaith23 respectively; and screening for alcohol-related problems was conducted with the Alcohol Use Disorders Identification Test (AUDIT).Reference Saunders, Aasland, Babor, de la Fuente and Grant24 Higher scores indicated more severe symptoms.
Pharmacologic treatment
The treatment details of this study have been published previously.Reference Kang, Kim, Kim, Kim, Shin and Shin17,Reference Kim, Stewart, Kang, Kim, Lee and Jhon25 Before treatment commencement, a comprehensive examination was conducted for patients’ clinical manifestations, illness severity, physical comorbidities and medication lists, and history of prior treatments. In the first step, patients received 3 weeks of antidepressant medication, taking into consideration the patient data and existing treatment guidelines.Reference Malhi, Bassett, Boyce, Bryant, Fitzgerald and Fritz26 General effectiveness and tolerability were evaluated before proceeding with the next step of measurement-based treatments. In cases of inadequate improvement or intolerable adverse events, patients were directed to choose whether they would prefer to stay in the present step or proceed to the next treatment step of switching antidepressants (S), augmenting with other drugs (A) or taking a combination of other antidepressants (C) (strategies: S + A, S + C, A + C and S + A + C). When determining treatment strategies, the patient's opinion was given priority for maximising drug adherence and treatment outcomes.
Outcomes for suicidal behaviour
Suicidal behaviour was divided into four types: previous suicide attempt, baseline suicide severity, increase suicide severity and fatal/non-fatal suicide attempt. Previous suicide attempt was defined as self-reported information of committing intentional self-harm before the baseline evaluation, with at least some intention to die irrespective of the objective lethality.Reference Posner, Oquendo, Gould, Stanley and Davies27 Equivocal intention to die at that time of a self-harm act was also defined as a suicide attempt. However, self-injurious behaviours with no suicidal intention or unknown intention were not included in the definition. Baseline suicide severity was assessed with the Brief Psychiatric Rating Scale (BPRS)Reference Overall and Gorham28 suicidality item score. Participants were asked ‘Have you felt that life wasn't worth living? Have you thought about harming or killing yourself? Have you felt tired of living or as though you would be better off dead? Have you ever felt like ending it all?’. If participants reported suicidal ideation, further questions were asked: ‘How often have you thought about this? Do you have a specific plan?’. Participants’ self-report was recorded as a score from 1 to 7, and divided into lower (1 (not present) to 3 (mild)) versus higher (4 (moderate) to 7 (extremely severe)) suicide severity groups. To determine increased suicide severity, the BPRS suicidality item score was re-evaluated during the 1-year pharmacotherapy period at 3, 6, 9 and 12 weeks, and 6, 9 and 12 months. Any instance of an increase in the score during the follow-up period compared with the baseline score was defined as increased suicide severity. Fatal/non-fatal suicide attempt was defined as a suicide attempt (defined as above) or death by suicide during the 1-year pharmacotherapy period.
Statistical analysis
Sociodemographic and clinical characteristics, including assessment scales at baseline and treatment step during the 1-year pharmacotherapy period, were compared according to the presence of any childhood abuse, using t-tests or χ 2-tests, as appropriate. The same comparison was repeated for higher versus lower serum BDNF groups, presence of previous suicide attempt and lower versus higher baseline suicide severity groups. The variables that were associated at conventional levels of statistical significance (P < 0.05) in these analyses were considered as covariates in later adjusted analyses. The individual associations of any childhood abuse and serum BDNF groups with the four types of suicidal behaviour were analysed in logistic regression models before and after adjusting for potential covariates. Interactive effects of any childhood abuse and serum BDNF groups on the four types of suicidal behaviour were analysed with multinomial logistic regression tests, after adjustment for potential covariates. Additional analyses were carried out to investigate the effects of each childhood abuse type and age group on suicidal behaviour, according to serum BDNF group. All statistical tests were two-sided, with a significance level of P < 0.05. Statistical analyses were performed with SPSS software version 25.0 for Windows.
Results
Recruitment
The recruitment process is summarised in Fig. 1. Of 1262 participants evaluated at baseline, 1094 (86.7%) provided a blood sample for measuring serum BDNF levels, comprising the baseline sample. All participants were aged >16 years. Of these, 884 (80.8%) completed the 12-week acute treatment and were followed up at least once between the 6- and 12-month continuation treatment phase, and consisted of the follow-up sample. No significant differences in baseline characteristics were found between those with or without a blood sample. However, follow-up loss at 12 months was significantly associated with unemployed state and melancholic features at baseline.
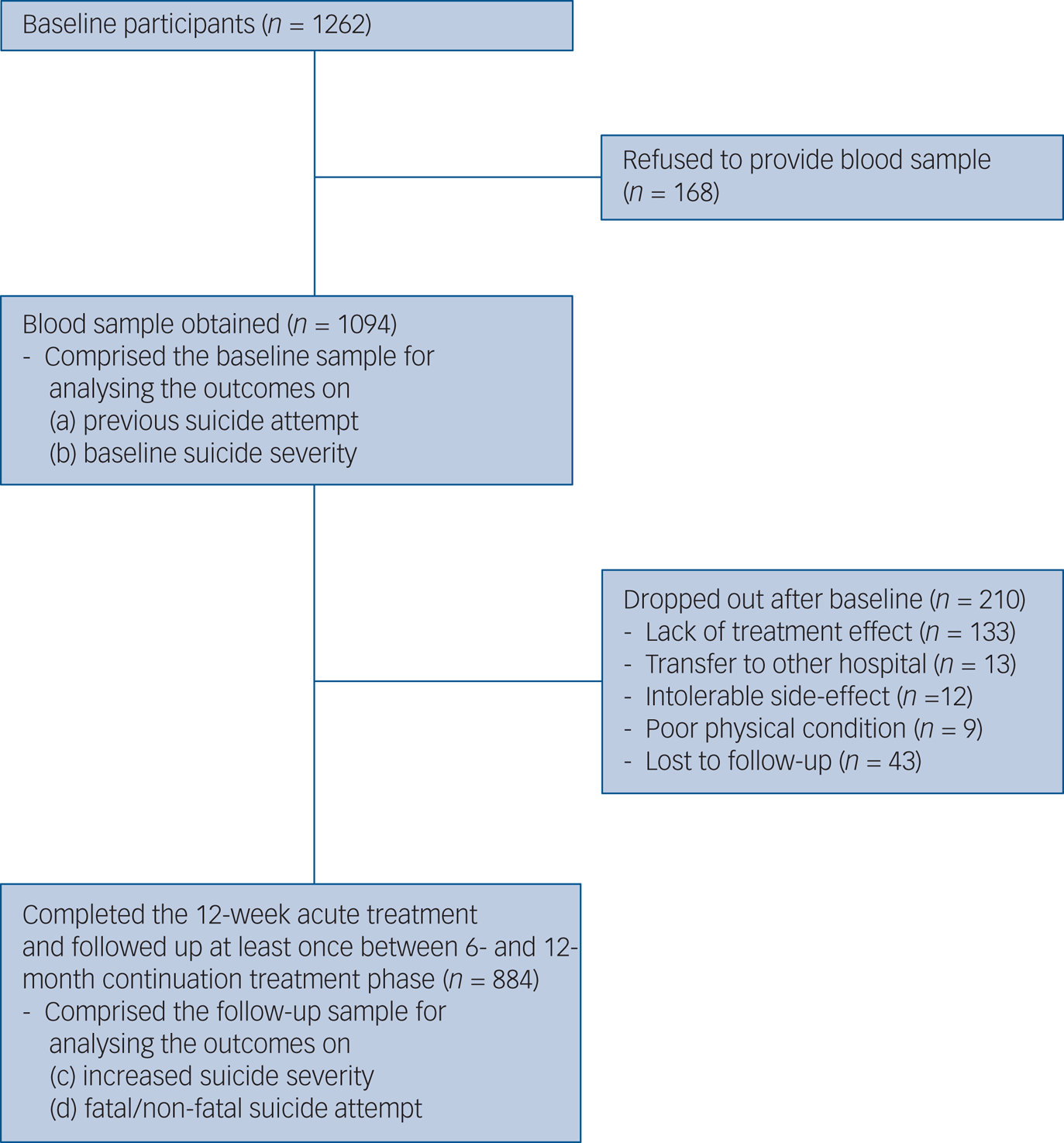
Fig. 1 Participant recruitment process.
Baseline main characteristics and outcomes
In the baseline sample (n = 1094), any childhood abuse was reported by 149 (13.6%) participants. Emotional, physical and sexual abuse was reported by 105 (9.6%), 98 (8.6%) and 30 (2.7%) participants, respectively. Median (interquartile range) and mean (s.d.) values of serum BDNF were 23.3 (8.6) and 23.8 (6.8) ng/mL, respectively. Previous suicide attempt and higher baseline suicide severity were present in 96 (8.8%) and 362 (33.1%) participants, respectively. Characteristics are compared according to reported childhood abuse in Table 1, which was significantly associated with younger age, male gender, higher education, unmarried status, no religion, higher monthly income, atypical depressive features, earlier age at onset, longer duration of illness, higher number of depressive episodes, longer duration of present episode, fewer physical disorders, lower serum BDNF levels and higher scores on the HADS-A and AUDIT. Characteristics are compared according to serum BDNF above versus below the median value (23.3 ng/mL) in Supplementary Table 1 available at https://doi.org/10.1192/bjp.2021.82. A lower serum BDNF level was significantly associated with female gender, living alone and higher number of physical disorders. Characteristics are compared according to previous suicide attempt and baseline suicide severity in Supplementary Tables 2 and 3, respectively. A previous suicide attempt history was significantly associated with younger age, male gender, higher education, unmarried status, no religion, higher monthly income, diagnosis of MDD, atypical depressive features, earlier age at onset, longer duration of illness, higher number of depressive episodes, current smoking status, higher scores on the HADS-A and AUDIT, and higher number of treatment steps over 1 year. A higher baseline suicide severity was significantly associated with younger age, unmarried status, living alone, no religion, unemployed status, diagnosis of MDD, atypical depressive features, earlier age at onset, longer duration of illness, higher number of depressive episodes, current smoking status, higher scores on the HADS-D and HADS-A, and higher number of treatment steps over 1 year. In addition, treatment step was significantly prospectively associated with suicidal behaviours (Supplementary Table 4). Considering these associations and collinearity between the variables, covariates for further adjusted analyses were selected as follows: age, gender, living alone, religious affiliation, monthly income, atypical depressive features, number of depressive episodes, number of physical disorders, smoking status, and scores on the HADS-A and AUDIT for all analyses for baseline outcomes analyses, plus treatment step for 1-year prospective outcomes.
Table 1 Characteristics compared according to reported childhood abuse
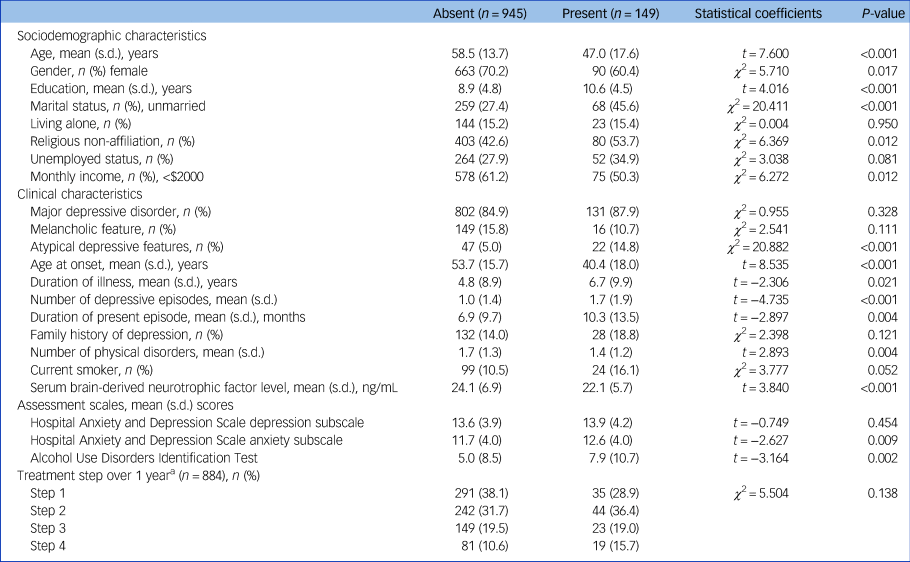
a. For patients with an insufficient response or uncomfortable side-effects, next treatment steps (1, 2, 3 and 4 or more) with alternative strategies (switching, augmentation, combination and mixtures of these approaches) were administered, taking into consideration measurements and patient preference, at 3, 6, 9 and 12 weeks and 6, 9 and 12 months.
Individual effects of childhood abuse and serum BDNF levels on suicidal behaviours
The individual associations of reported childhood abuse and serum BDNF group with previous suicide attempt and baseline suicide severity are described in Table 2. In the unadjusted analyses, reported childhood abuse and lower serum BDNF levels were significantly associated with previous suicide attempt and higher baseline suicide severity. The strengths of the associations reduced significantly after adjustment, in that only the association between reported childhood abuse and previous suicide attempt remained significant, whereas the associations between childhood abuse and baseline suicide severity, and those of serum BDNF levels with previous suicide attempt and baseline suicide severity, fell below statistical significance.
Table 2 Individual associations of reported childhood abuse and serum brain-derived neurotrophic factor (BDNF) levels with suicidal behaviour at baseline (n = 1094) and during 1-year follow-up (n = 884)

a. Brief Psychiatric Rating Scale suicidality item score of 4 (moderate) to 7 (extremely severe).
b. Increase in Brief Psychiatric Rating Scale suicidality item score during the follow-up period compared with the baseline.
c. Adjusted for age, gender, living alone, religious affiliation, monthly income, atypical depressive features, number of depressive episodes, number of physical disorders, smoking status and scores on the Hospital Anxiety and Depression Scale anxiety subscale and Alcohol Use Disorders Identification Test.
d. Adjusted for all covariates listed at baseline, plus treatment step.
*P < 0.05, **P < 0.01, ***P < 0.001.
In the follow-up sample (n = 884), increased suicide severity and fatal/non-fatal suicide attempt during the 1-year pharmacotherapy period were present in 155 (17.5%) and 38 (4.3%; 32 non-fatal, 6 fatal) participants, respectively. The individual associations of childhood abuse and serum BDNF group with follow-up suicidal behaviours are described in Table 2. In unadjusted analyses, the presence of any childhood abuse was significantly associated with fatal/non-fatal suicide attempt, and lower serum BDNF levels were significantly associated with increased suicide severity. However, after adjustment, no significant associations were found.
Interactive effects of childhood abuse and serum BDNF levels on suicidal behaviours
The interactive effects of any childhood abuse and serum BDNF levels on the four suicidal behaviour outcomes are displayed in Fig. 2. Prevalence/incidence of suicidal behaviours were all highest in the presence of both childhood abuse and lower serum BDNF levels. Reported childhood abuse was significantly associated with previous suicide attempt regardless of serum BDNF status, without a significant interaction term after adjustment. However, reported childhood abuse was significantly associated with higher baseline suicide severity, increased suicide severity and fatal/non-fatal suicide attempt during the 1-year pharmacotherapy period only in the presence of lower serum BDNF in the same adjustment model. Of these associations, interaction terms were statistically significant for higher baseline suicide severity and fatal/non-fatal suicide attempt as outcomes after adjustment.
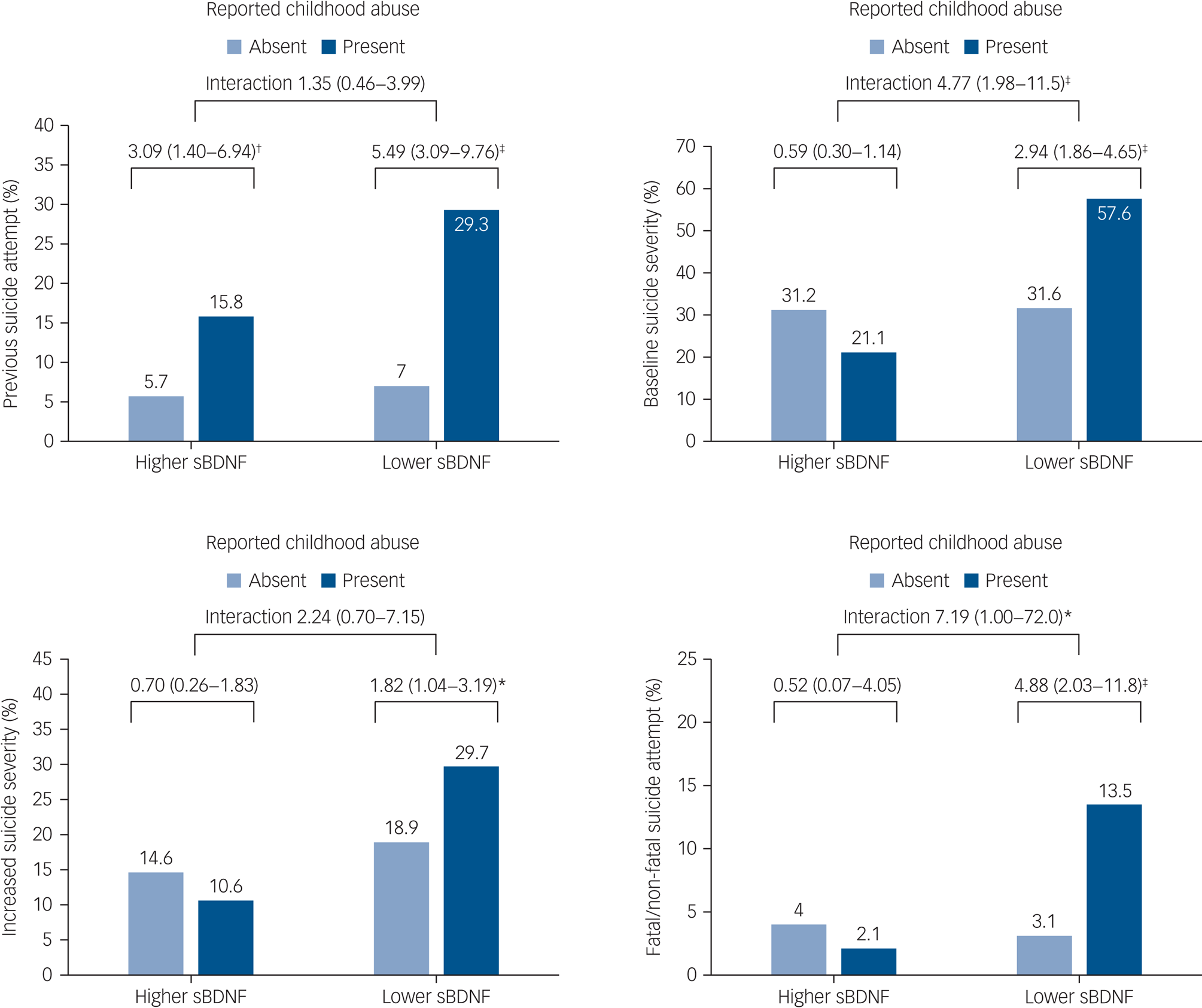
Fig. 2 Interactive effects of any childhood abuse and serum brain-derived neurotrophic factor (sBDNF) levels on suicidal behaviour at baseline (n = 1094) and at follow-up (n = 884). Odds ratios (95% confidence intervals) are given for baseline outcomes of previous suicide attempt and baseline suicide severity, and follow-up outcomes of increased suicide severity and fatal/non-fatal suicide attempt. Odds ratios were adjusted for age, gender, living alone, religious affiliation, monthly income, atypical depressive features, number of depressive episodes, number of physical disorders, smoking status and scores on the Hospital Anxiety and Depression Scale anxiety subscale and Alcohol Use Disorders Identification Test, with treatment step also included for follow-up outcomes. *P < 0.05, †P < 0.01, ‡P < 0.001.
Associations of childhood abuse type and age groups with suicidal behaviour
The individual associations between each childhood abuse type and suicidal behaviours are summarised in Supplementary Tables 5 and 6. All three childhood abuse types were significantly associated with previous suicide attempt before and after adjustment. The significant unadjusted associations between the three childhood abuse types and higher baseline suicide severity lost statistical significance after adjustment. No significant associations were found between the three childhood abuse types and increased suicide severity before or after adjustment. The significant unadjusted associations between the three childhood abuse types and fatal/non-fatal suicide attempt during the 1-year pharmacotherapy period lost significance for the emotional and physical abuse types, but remained significant for sexual abuse after adjustment.
The interactive effects of each childhood abuse type and serum BDNF levels on suicidal behaviours are displayed in Supplementary Figs 1–3. Similar to the findings with any reported childhood abuse, emotional abuse showed significant interactions with serum BDNF levels on higher baseline suicide severity and fatal/non-fatal suicide attempt during the 1-year pharmacotherapy period after adjustment. Physical abuse showed significant statistical interaction with serum BDNF levels on higher baseline suicide severity and increased suicide severity after adjustment. No significant interactions were found with sexual abuse.
The effects of childhood abuse and serum BDNF levels with suicidal behaviour for three age groups (<31, 31–60 and >60 years) are summarised in Supplementary Table 7. Although the statistical significance of the associations was diminished in all age groups, the magnitude and direction of the odds ratios were similar to those in the full sample.
Discussion
In this study of people with depressive disorders receiving a stepwise pharmacotherapy intervention in a real-world clinical setting, individual associations of reported childhood abuse were significant only with the suicidal behaviour outcome of previous suicide attempt, as evaluated after adjustment for relevant covariates. Furthermore, no significant individual associations were found for serum BDNF with any suicidal behaviour outcome after adjustment. However, with respect to interactive effects, the presence of both childhood abuse and lower serum BDNF levels were associated with the highest prevalence and incidence of all four suicidal behaviours, and significant multiplicative interactions between childhood abuse and serum BDNF were found on baseline suicide severity and fatal/non-fatal suicide attempt during follow-up even after adjustment for relevant covariates. The findings of individual childhood abuse types and of age groups showed generally similar, but attenuated results.
Childhood abuse and suicidal behaviour
Childhood abuse has been shown to be a well-established risk factor for suicide attempt in a range of cross-sectional clinical and community samples,Reference Björkenstam, Kosidou and Björkenstam4,Reference Ng, Yong, Ho, Lim and Yeo5 as well as a recent meta-analytic review.Reference Fazel and Runeson6 However, in longitudinal studies with retrospective cohorts, associations of childhood abuse with incident suicidal behaviours have been controversial, with some studies reporting significant associations,Reference Ng, Yong, Ho, Lim and Yeo5,Reference Brezo, Paris, Vitaro, Hébert, Tremblay and Turecki29 and others finding no such associations.Reference Rabinovitch, Kerr, Leve and Chamberlain30 Our finding of a significant association between childhood abuse and previous suicide attempt was therefore consistent with previous reports. Our study is relatively rare in investigating worsening or incidence of suicidal behaviour with a prospective clinical cohort, although the findings of no associations between childhood abuse and prospective suicidal behaviours in adjusted analyses are in keeping with previous results from several retrospective cohorts.Reference Rabinovitch, Kerr, Leve and Chamberlain30 The significant association between childhood abuse and baseline suicide severity also disappeared after adjustment for various covariates, which included depression-related clinical characteristics; however, since suicidal ideation is one of the diagnostic criteria for depressive disorders,19 this may conceivably represent an overadjustment.
Serum BDNF and suicidal behaviour
Almost all previous studies of associations between blood BDNF levels and suicidal behaviours have been conducted with cross-sectional case–control designs,Reference Kim, Lee, Won, Park, Lee and Lee13–Reference Eisen, Perera, Bawor, Dennis, El-Sheikh and DeJesus15 comparing blood BDNF levels between patients with depressive disorders with and without histories of suicide attempts and healthy controls. The findings have been inconsistent, and the significant associations reported are usually from studies without adjusted analyses,Reference Kim, Lee, Won, Park, Lee and Lee13 whereas no associations are usually found in studies including adjustments.Reference Grah, Mihanovic, Ruljancic, Restek-Petrovic, Molnar and Jelavic14,Reference Eisen, Perera, Bawor, Dennis, El-Sheikh and DeJesus15 Our findings were therefore in line with these previous ones in that the significant unadjusted associations of lower serum BDNF levels with previous suicide attempt and baseline suicide severity no longer remained significant in adjusted models. As far as we are aware, there has been no study investigating prospective associations of blood BDNF levels with subsequent suicidal behaviour. In our study, associations of serum BDNF levels with subsequent increased suicide severity were significant in unadjusted analyses, but lost significance after adjustment, and those with incident suicide attempt/death were not significant before or after adjustment. Overall, the value of blood BDNF alone as a biomarker for suicidal behaviours is still unestablished.
Interaction of childhood abuse and serum BDNF on suicidal behaviour
The presence of both childhood abuse and lower serum BDNF levels was associated with the highest prevalence/incidence of suicidal behaviour outcomes in our study. These synergistic effects are plausible for several reasons. First, early childhood abuse has been associated with reduced BDNF expression in animal and human studies.Reference van Velzen, Schmaal, Jansen, Milaneschi, Opmeer and Elzinga8 Similarly, serum BDNF levels were significantly lower in patients reporting childhood abuse than in other participants in our study (Table 1). The pathological alteration of BDNF related to childhood abuse may lead to reduced neural plasticity, followed by impaired ability to adapt to crisis situations.Reference Dwivedi10 Second, there is considerable evidence for associations of BDNF with serotonin, norepinephrine and other neurotransmitters, which are also major biochemical markers of suicidal behaviour.Reference Sudol and Mann31 Third, both childhood traumaReference Williams, Debattista, Duchemin, Schatzberg and Nemeroff32 and lower blood BDNFReference Shi, Luan, Song and Zhang33 predict worse antidepressant treatment responses. Since suicidal behaviours are closely related to depression severity, the present interactive effects of childhood abuse and serum BDNF on prospective suicidal behaviours could reflect previous reports on depression treatment outcomes.
The findings of each childhood abuse type and the three age groups showed generally similar results to those of any childhood abuse type and of all age groups, respectively, reflecting lower statistical power rather than effect modification. The present findings supported the previous observations that indicators of childhood adversity tend to occur in clusters, rather than as single events.Reference Björkenstam, Kosidou and Björkenstam4,Reference Hovens, Wiersma, Giltay, van Oppen, Spinhoven and Penninx22
Limitations and strengths
Several limitations should be considered for interpreting the study findings. First, peripheral rather than central BDNF levels were measured. However, BDNF can cross the blood–brain barrier and blood BDNF has showed similar changes to those in the brain, suggesting parallel changes between the blood and brain levels.Reference Karege, Schwald and Cisse34 Second, serum BDNF levels were measured at only one point at baseline, and so it was not possible to investigate the association between potential treatment-related alterations of serum BDNFReference Shi, Luan, Song and Zhang33 and prospective suicidal behaviour. Third, because of the naturalistic study design, pharmacologic treatment was determined by patient preference with a psychiatrist's instruction rather than via a predetermined protocol; thus, inter-therapist variability could affect the outcomes. However, since psychiatrists made treatment decisions without knowledge about childhood abuse or serum BDNF levels, it is not likely that inter-therapist variability affected the associations of interest. Fourth, there was significant sample attrition during the 1-year treatment period. Because of poor prognostic characteristics of patients lost to follow-up, such as unemployed state and melancholic features, these are most likely to have attenuated rather than exaggerated the observed findings. However, childhood abuse and serum BDNF levels did not differ by follow-up status. Fifth, the important childhood adversities of bullying and victimisation were not considered in this study. Sixth, this study was carried out in a single centre, which might limit the representativeness of the findings, although the sample size was relatively large.
This study has several strengths, including its novelty on both retrospective and prospective designs for evaluating suicidal behaviours. The sample size was large, and patients were assessed with a structured questionnaire and widely known and standardised scales. A range of covariates were considered, which could obviously affect the study findings.
Implications
In conclusion, we found synergistic interactive rather than individual effects of child abuse and serum BDNF levels on suicidal behaviours before and after pharmacological treatment in patients with a depressive disorder. The value of serum BDNF levels as biomarkers for suicidal behaviour might be significantly improved by being considered in combination with the presence of childhood abuse. Furthermore, our findings may provide an explanation for hitherto controversial associations of blood BDNF levels with suicidal behaviour. The observed suicide rate during the 1-year follow-up was 678 per 100 000 (6 out of 884 participants), approximately 27 times higher than that of all Korea in 2019 (24.6 per 100 000).16 Our findings may have clinical utility in screening and identifying depression associated with high suicidality. Future studies are needed to replicate these findings in other clinical and high-risk populations.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1192/bjp.2021.82
Data availability
The data that support the findings of this study are available from the corresponding author, J.-M.K., upon reasonable request.
Author contributions
J.-M.K., I.-S.S. and R.S. were responsible for study conceptualisation, design, data curation and formal analysis. J.-W.K., H.-J.K., J.-Y.L., S.-W.K. and B.-J.C. formulated the specific research question and methodology. All authors contributed to writing and/or editing of the manuscript and approved the final version.
Funding
The study was funded by the National Research Foundation of Korea (grants NRF- 2019M3C7A1031345 and NRF-2020M3E5D9080733 to J.-M.K.). R.S. is part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, and the NIHR Applied Research Collaboration South London at King's College Hospital NHS Foundation Trust (Grant number is not specified due to sufficient achievements by R.S.). R.S. is also an NIHR Senior Investigator.
Declaration of interest
J.-M.K. declares research support from Janssen and Lundbeck in the past 5 years. S.-W.K. declares research support from Janssen, Boehringer Ingelheim, Allergan and Otsuka in the past 5 years. R.S. declares research support from Roche, Janssen, GlaxoSmithKline and Takeda in the past 5 years. All other authors report no biomedical financial interests or potential conflicts of interest.








eLetters
No eLetters have been published for this article.