INTRODUCTION
Rabies is a zoonosis caused by a virus of the Rhabdoviridae family, responsible for more than 55000 human deaths every year, primarily in developing countries [Reference Knobel1], in which dogs represent the major source of infection for humans [Reference Cleaveland2].
Owing to the massive vaccination campaigns of dogs and foxes during recent decades, canine rabies is now considered to be eradicated from the European Union (EU) while sylvatic rabies remains sporadic [Reference Bourhy3–Reference Bourhy5]. Besides residual risk of transmission from foxes to domestic animals, the main risk for the EU resides in the introduction of dogs from endemic countries. This is a consequence of the permeability of borders and lack of awareness by travellers of the risk posed by taking their dogs abroad or from adopting animals from these areas. These importations threaten the rabies-free status of terrestrial animals in Western European countries and challenge the public health systems. Moreover, only a reduced number of rabies vaccines are licensed for use in Europe [Reference Bourhy5].
Between 2002 and 2008, 10 cases of rabies were reported in the EU arising from the importation of infected dogs from endemic countries. Most of these cases (n=6) came from Morocco [6]. Despite implementation of surveillance for animal rabies, vaccination of owned dogs and initiatives for controlling stray dogs, canine rabies is still prevalent in Morocco and rabies control strategies are currently being revised [Reference Dodet7]. Furthermore, large numbers of travellers (3 million annually) cross the relatively open EU–Morocco border [8]. In 2003, in order to prevent the introduction of rabies, the EU established animal health requirements applicable to the non-commercial movement of pets within the European Community and from third countries [9]. For pets arriving from third countries, the animal must be identified, vaccinated with an inactivated vaccine and have a positive result in a serological test for the detection of neutralizing rabies antibodies.
The main objectives of the present study were (i) to quantify the annual probability of rabies introduction into the EU through dogs coming from Morocco and (ii) to assess the effect of the application of stricter border control measures, by means of a probabilistic risk assessment model.
MATERIALS AND METHODS
Model pathways
Since dogs are considered the principal reservoir of canine rabies in Morocco, and there are few known movements of animals of other species, no other susceptible species such as cats or ferrets were considered. Four potential pathways (scenarios), to reflect different levels of risk were considered: (i) EU citizens that travelled to Morocco and returned with their dog, (ii) EU citizens that adopted a dog in Morocco (and later smuggled it into the EU), (iii) Moroccans that travelled to the EU with their dog, and (iv) Moroccans living in Europe that travelled to Morocco with their dog. These pathways were further divided depending on whether dogs were transported by ferry or by plane.
Seasonality
The likelihood of rabies introduction varies throughout the year. This seasonal component in the likelihood of rabies introduction might be attributed to two factors: (a) seasonal variations in the incidence of dog rabies, closely linked to the reproductive cycle of dogs [Reference Matter and King10], and (b) seasonal variations in the volume of travellers (and by extension dogs) entering the EU, with the highest number of entries occurring during the summer period [8]. This seasonality was taken into account in the model by estimating the likelihood of rabies introduction per month.
Risk assessment model
For each of the pathways (i) considered and each of the months of the year (j), the probability of one rabid dog entering the EU from Morocco (P eij) was estimated by taking into account (Fig. 1): (a) the probability of infection of a dog in Morocco in a given scenario (i) and a given month (j) (P Iij) and (b) the probability of no detection of rabies for scenario i: (1−P Di):
These individual probabilities (P eij) were applied to (c) the number of dogs at risk in each of the scenarios and each of the months of the year (N Rij) in order to estimate the probability of at least one rabid dog entering the EU from Morocco in a given scenario (i) and a given month (j) (P Eij):
By combining the probabilities for the different months of the year in a given scenario (i), we can estimate the annual probability of at least one rabid dog entering the EU from Morocco in a given scenario (i) (P Ei):
By combining the annual probabilities for the different scenarios, we can estimate the annual probability of at least one rabid dog entering the EU from Morocco by any of the four scenarios considered (P E):
The input parameters used in the model, their values and the sources of the data are shown in Table 1. To reflect the uncertainty and variability associated with some of the input parameters, different types of probability distributions were used.

Fig. 1. Pathway diagram for the estimation of the risk of rabies introduction into the EU through dogs coming from Morocco.
Table 1. Input parameters: description of the parameter, notation, value and source of data
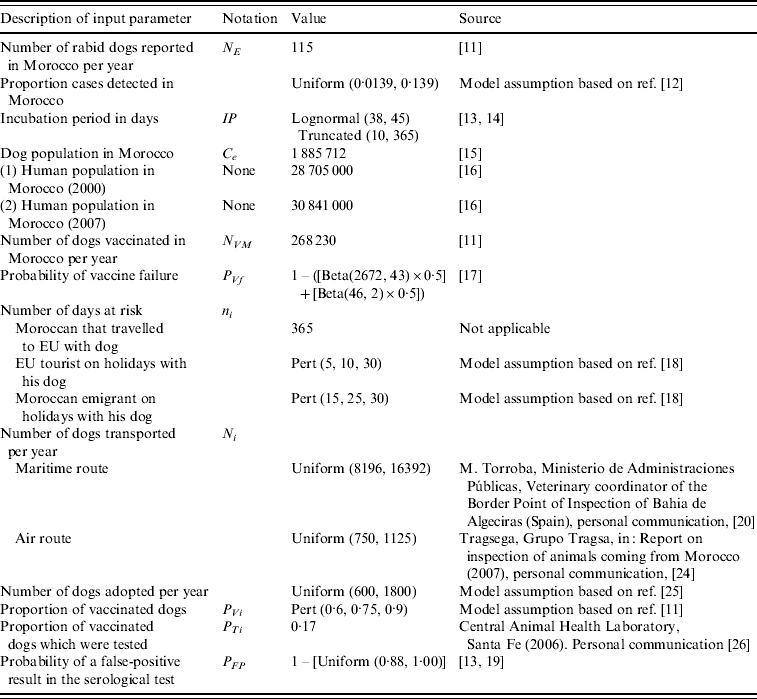
Probability of infection of a dog in Morocco in scenario i in month j (PIij)
The likelihood of rabies infection for a dog in Morocco in scenario i in month j (P Iij) was estimated taking into account: (a) the daily probability of rabies infection for a native Moroccan dog in scenario i in month j (P ij), and (b) the number of days a dog remained at risk in scenario i (n i):
Daily probability of rabies infection for a native Moroccan dog in scenario i in month j (Pij)
First, the cumulative incidence in month j (CI j) was calculated by dividing the number of native Moroccan dogs becoming infected in month j (N Ij) by the population of native Moroccan dogs at risk (N R):
Then, the daily probability of rabies infection in month j (P j) was obtained by dividing the cumulative incidence in month j (CI j) by the number of days in month j.
Finally, the daily probability of rabies infection for a native Moroccan dog in scenario i and month j (P ij) was calculated by multiplying the daily probability of rabies infection in month j (P j) by a factor that accounts for differences in the probability of exposure to the risk (i.e. contact with potentially infected dogs) among scenarios (E i):
Number of native Moroccan dogs infected in month j (NIj)
First, we estimated the number of native Moroccan dogs detected in month j (N Ej) by taking into account: (a) the annual number of rabid dogs reported to the International Organization of Epizooties (OIE) by Morocco in 2007 [11]: 115 dogs (N E), and (b) the proportion of dogs detected in month j (p j), based on data from several years [11] (January 2006 to June 2008) to account for variations between years:
Second, we estimated the number of native Moroccan diseased dogs in month j (N Dj). In Morocco, dog rabies cases are usually underreported due to the poor sensitivity of passive surveillance mechanisms [Reference Matter and King10]. The inefficiency of passive surveillance systems for rabies detection was demonstrated in a study performed in Kenya, where an active surveillance programme was able to detect >70-fold rabid dogs than the existing passive surveillance system [Reference Kitala12]. Assuming that in Morocco the surveillance system would be more efficient than in Kenya, the proportion of cases detected (underreporting factor) was modelled using a Uniform distribution in which the minimum was the proportion of cases detected in Kenya (0·0139), and the maximum was 10 times the minimum. The number of diseased dogs in Morocco in month j (N Dj) can be estimated by dividing the number of dogs detected in Morocco in month j (N Ej) by this underreporting factor.
However, a dog demonstrating rabies symptoms at a given moment was actually infected some time previously, and the length of this time is determined by the duration of the incubation period (IP). To account for this, a simulation model (independent of the main model) was developed. Each iteration, the incubation period: Lognormal (38, 45) (Table 1) was subtracted from the random day (RD) in month m that the animal developed clinical signs: Uniform (0, 30·4), where 30·4 represents the mean number of days in a month. If [Uniform (0, 30·4) – Lognormal (38, 45)]>0, then the dog was infected the same month in which it showed rabies symptoms. If not, and [(Uniform (0, 30·4) +30·4) – Lognormal (38, 45)] >0, then the dog was infected 1 month previously (m−1). The same reasoning was used for months m−2, …, m−11. By running a large number of iterations we estimated the probabilities that a dog which showed rabies symptoms in month m was actually infected in month m, month m−1, …, m−11, i.e. p m, p m−1, …, p m−11. Once estimated, we can convert the number of dogs diseased each month (N Dj) into the number of dogs becoming infected each month (N Ij), as:
For example, the number of dogs infected in May would be estimated as:
Number of dogs at risk in Morocco (NR)
The number of Moroccan dogs at risk was estimated by taking into account (Table 1) the dog population in Morocco (C e); the number of dogs vaccinated in Morocco (N VM) and the probability of vaccine failure (P Vf), as:
Based on the dog and human population in Morocco in 2000 (Table 1), we obtained the human/dog ratio in Morocco, and then, we applied it to the human population in Morocco in 2007 (Table 1), to obtain the dog population in Morocco in 2007.
Once N R is estimated, we can estimate the cumulative incidence in month j (CI j=N Ij/N R), and then estimate the daily probability of rabies infection for a native Moroccan dog in month j (P j) by dividing CI j by the number of days in month j. This probability (P j) represents the daily probability, for a non-protected dog in Morocco, of becoming infected by rabies (regardless of whether the dog is later introduced into the EU or not). However, the probability of exposure to the risk (contact with potentially infected dogs) is not likely to be the same for dogs coming from the EU as for dogs living in Morocco, which are free-roaming in many cases [Reference Matter and King10]. Dogs in pathways I and IV (EU citizens that travelled to Morocco with their dog and Moroccans living in Europe that travelled to Morocco with their dog, respectively) are more likely to be maintained indoors or kept on a leash, than dogs in pathways II and III (EU citizens that adopt a dog in Morocco and Moroccans that travelled to the EU with their dog, respectively). Therefore, a factor that accounts for differences in the probability of exposure to the risk (i.e. contact with potentially infected dogs) among scenarios (E i) was included in the model. It was assumed that dogs that remained in Morocco for a limited period of time, had between 25% and 75% of the daily probability of infection of dogs living permanently in Morocco.
Finally, the daily probability of rabies infection for a native Moroccan dog in scenario i and month j (P ij) was estimated by multiplying the daily probability of rabies infection in month j (P j) by E i.
Number of days the dog remained at risk in scenario i (ni)
The number of days a dog remains at risk is dependent on the scenario considered (Table 1). For Moroccan citizens that travelled to the EU with their dog, 365 days (maximum duration of the IP) were considered. For EU tourists and Moroccan emigrants travelling with their dog, ni was considered to be the duration of the stay in Morocco (Table 1). For travellers from the EU that adopted a Moroccan dog, the total time at risk was the sum of: (a) the age of the dog when adopted, and (b) the time from adoption until departure from Morocco. It was assumed that most of the adopted animals were puppies [Reference Jones17, Reference Have19].
The probability of infection of a dog in Morocco in scenario i in month j (P Iij) was dependent on: (a) the daily probability of rabies infection in scenario i in month j (P ij), and (b) the number of days at risk in scenario i (n i). However, as n i was longer than the duration of 1 month in some of the scenarios considered, it meant that a dog that was introduced into the EU in month j was subjected to a risk P ij for the n ij days in month j that the animal remained at risk; a risk P ij−1 for the n ij−1 days in month j−1 that the animal remained at risk, and so on. Therefore, in scenario i (given that n i=n ij+n ij−1+n ij−2+ ⋅⋅⋅ +n ij−11), the adjusted probability a dog introduced into the EU in month j became infected in Morocco was calculated as:
Probability of rabies detection (PDi)
It was assumed that a dog infected with rabies virus would be detected only if the animal developed clinical symptoms compatible with rabies before being transported to the EU. Therefore, the probability of detection was estimated by taking into account: (a) the number of days the animal remained at risk (n i); (b) the moment the animal became infected, assumed to occur between day 1 and day n i, and (c) the duration of the incubation period. If the number of days from infection to departure was greater than the incubation period, the animal would develop symptoms characteristic of rabies, the disease would be detected, and the animal would not be allowed to travel to the EU.
Number of dogs at risk in scenario i in month j (NRij)
First, the number of dogs at risk for scenario i (N Ri) was obtained (Fig. 2), by taking into account the number of dogs transported from Morocco to Europe for each scenario (N i); the proportion of dogs which were vaccinated in scenario i (P Vi); the probability of vaccine failure (P Vf); the proportion of vaccinated dogs that were tested in scenario i (P Ti) and the probability of a false-positive result in the test (P FP). In order to do this, we first needed to estimate:
(a) The number of dogs not vaccinated (N 1i):

(b) The number of dogs vaccinated, not protected and not tested (N 2i):

(c) The number of dogs vaccinated, not protected, tested and giving a false-positive result (N 3i):

Then, the number of dogs at risk in scenario i (N Ri) can be estimated as:
Finally, the number of dogs at risk in scenario i in month j (N Rij) was estimated as the product of the number of dogs at risk in scenario i (N Ri) and the proportion of dogs transported in month j (P Mj):
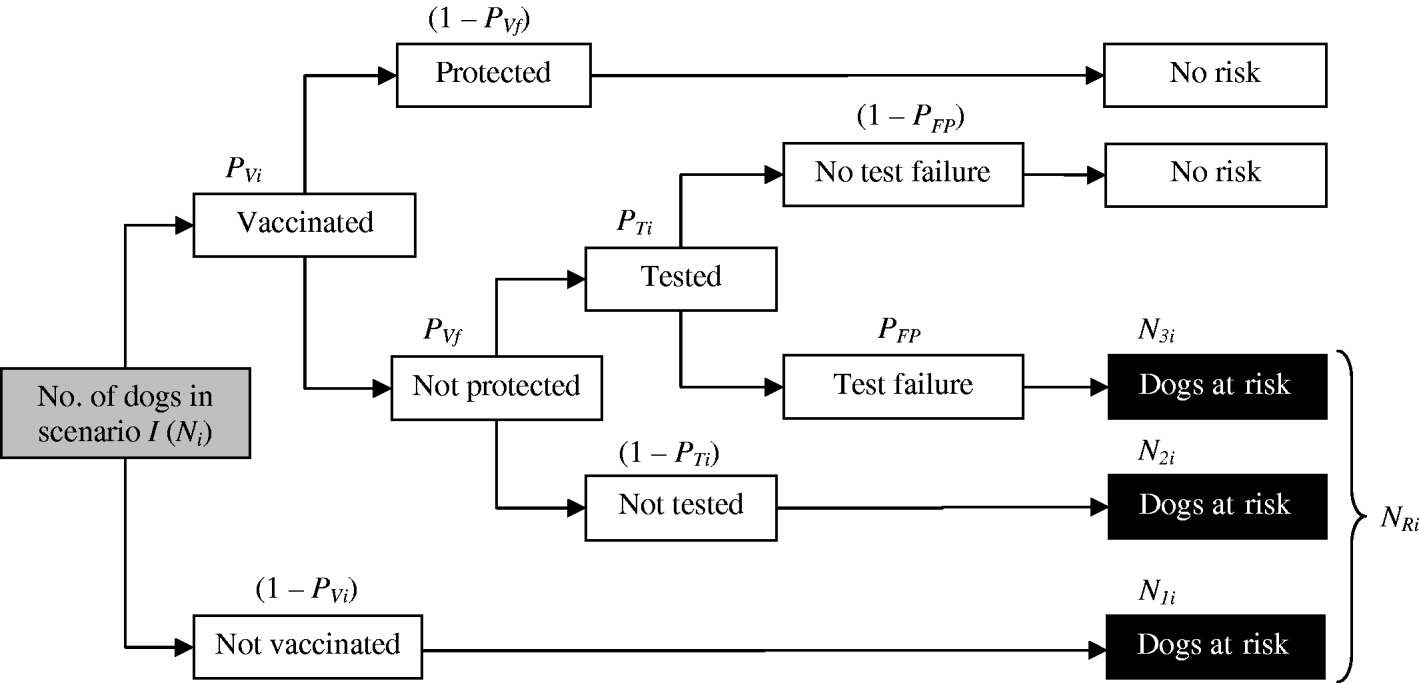
Fig. 2. Diagram for the estimation of the number of dogs at risk for the different scenarios (N Ri).
Number of dogs transported in scenario i (Ni)
In order to estimate the number of dogs transported from Morocco to the EU each year, the main maritime routes by regular ferry lines and air routes were considered.
Maritime routes
Between March and June 2001, the veterinary officers at the main Spanish seaports that connect with Morocco, detected 1422 dogs entering Spain from Morocco (Table 1). Then, given that 613993 passengers entered through these ports during the same period (Table 1), we estimated the proportion of travellers carrying a dog (one dog for every 432 passengers). This proportion was then applied to the monthly number of passengers that entered the EU in 2007 via the main Spanish seaports and via the Sête port, the main French port connecting with Morocco [21]. However this number (8196 dogs) is likely to be an underestimation as: (a) it is probable that not all dogs were detected, and (b) there is likely to have been an increase in the number of dogs transported since 2001, as shown by the number of dogs entering the UK, which increased from 23158 in 2001 to 89127 in 2007 [22]. This upward trend can not be attributed to regulatory changes, as the UK Pet Travel Scheme, which regulates the non-commercial movement of dogs, was launched in 2000 and has continued largely unchanged since then [22]. Consequently, it is more likely explained by an increase in the movement of companion animals due to tourism and immigration [Reference Marano, Arguin and Pappaioanou23]. As a result, the number of dogs introduced into the EU from Morocco by the maritime route was conservatively modelled using a Uniform distribution where the minimum number of dogs was set at 8196 and the maximum at twice this number of dogs.
Air route
A total of 36 dogs entering from Morocco were detected at Barcelona and Madrid airports during 2007. Given that Barcelona and Madrid airports cover 86% of passengers arriving in Spain from Morocco, and Spain represents the entry point of 5·6% of the total air-passenger transit from Morocco to the EU, then 750 dogs were estimated to have been introduced from Morocco into the EU in 2007. In the case of the air route, as data were from 2007, any underestimation was likely to have been due only to the fact that all dogs were not detected. Therefore, the uncertainty in the number of dogs introduced by the air route was modelled using a Uniform distribution where the minimum number of dogs was set at 750 and the maximum at 1·5 times this number (Table 1).
In both the maritime and the air routes, it was assumed (based on expert opinion) that 90% of the dogs that entered the EU were associated with EU citizens that travelled to Morocco with their dog (pathway I), 5% were associated with Moroccan emigrants that travelled from the EU to Morocco with their dog (pathway III) and 5% associated with Moroccan citizens that travelled to the EU with their dog (pathway IV).
Although there was a high degree of uncertainty regarding the number of dogs adopted in Morocco and later smuggled into the EU, recent events demonstrate the importance of this pathway (5/10 rabid dogs introduced in the EU between 2002 and 2008 had been adopted in Morocco) [6, 11]. In an epidemiological survey performed in southern and central France during 2004, more than 300 pets (dogs and cats) were recorded as having been illegally introduced from Morocco, Algeria, Tunisia and Turkey (Table 1). These animals were not correctly identified and/or vaccinated against rabies. However, this number is likely to be an underestimate of the number of dogs adopted and illegally introduced into the EU from Morocco as the survey was restricted to France, and is unlikely to have detected all illegal entries. Therefore, dogs adopted in Morocco and later introduced into the EU were included in the model as an uncertain parameter and modelled using a Uniform distribution where the minimum value was assumed to be 600 and the maximum 1800. This allowed for the assessment of the importance of this parameter in the final risk of rabies introduction.
Proportion of vaccinated dogs in scenario i (PVi)
The information on the proportion of dogs vaccinated in different countries is scant, and in many cases not up to date. Based on the available data, and to reflect the uncertainty associated to this parameter, a Pert distribution was used (Table 1). This distribution was used for the different pathways, except for adopted dogs where none were considered to have been correctly vaccinated because: (a) they are not usually vaccinated, and (b) even if they are vaccinated by the new owner, the few days that most tourists spend in Morocco means that they are unlikely to be protected.
Probability of vaccine failure (PVF)
According to WHO and OIE, a serum antibody titre level of 0·5 IU/ml or higher is correlated with a very high probability of protection [Reference Have19], and therefore was used as indicative of protection.
Proportion of vaccinated dogs which were tested in scenario i (PTi)
In order to determine whether vaccination has elicited a protective response against rabies, dogs are required to be serologically tested at an approved rabies laboratory following vaccination. Since (a) Spanish tourists represented 5% of all EU tourists visiting Morocco (Table 1); and (b) 90% of the dogs travelling to Morocco were associated with European citizens (model assumption), then the number of dogs that travelled with Spanish tourists was estimated. Then, given that only 100 dogs were tested in 2006 at the Spanish rabies reference laboratory before travelling from Spain to Morocco (Table 1), we were able to estimate the proportion of Spanish dogs transported to Morocco that had been tested, and this proportion was assumed to be the same for all the dogs belonging to EU tourists.
Probability of a false-positive result in the test (PFP)
The probability of a false-positive in the test is calculated as 1 minus specificity, for a value of specificity between 0.88 and 1.00 (Table 1).
Proportion of dogs transported in month j (PMj)
The proportion of dogs transported in month j (P Mj) was assumed to be proportional to the number of passengers transported each month between Spain and Morocco during 2007.
Adjustment of the number of dogs transported in scenario i (N Ai)
Dogs that developed clinical signs of rabies before the date of departure were assumed not to be transported. These dogs were not accounted for in the number of dogs transported between Morocco and the EU (N i), as this number was derived from dogs that had already entered Spain. To account for this discrepancy, for each of the scenarios considered, the adjusted number of dogs (N Ai) was estimated as: [the number of dogs transported (N i+N NTi)/number of dogs not transported (infected+detected) (P NTi)], where:
Therefore
Sensitivity analysis
In order to identify those inputs which were more influential on the final output (annual risk of rabies introduction into the EU from Morocco), a sensitivity analysis was carried out. Rank order correlation method was chosen, as recommended by the OIE [20].
Effect of preventive measures
The risk reduction achieved by assuming 100% compliance in border controls was assessed. In this scenario, all dogs entering the EU were assumed to have been vaccinated against rabies with an inactivated vaccine and had a positive result in a serological test for the detection of neutralizing rabies antibodies. No adopted dogs were allowed through the border. In this case the risk would be only produced by dogs in which (a) the vaccine has failed and, (b) the serological test for the detection of neutralizing rabies antibodies has given a false-positive result.
Software
The spreadsheet model was constructed in Microsoft Excel (Microsoft Office Professional Edition, 2003). The model was run for 50000 iterations (Latin Hypercube sampling) in @Risk version 4.5.5 (Palisade Corporation, USA). This allowed the convergence of all the probability distributions.
RESULTS
The distribution of the annual probability of rabies introduction into the EU through dogs coming from Morocco is shown in Figure 3. The mean value of this probability distribution was 0·21 (90% CI 0·02–0·65). The pathways that contributed the most to the likelihood (Fig. 4) were (a) EU citizens that adopted a dog in Morocco (59% of the total probability) and (b) EU citizens that travelled to Morocco with their dog by ferry (34% of the total probability).
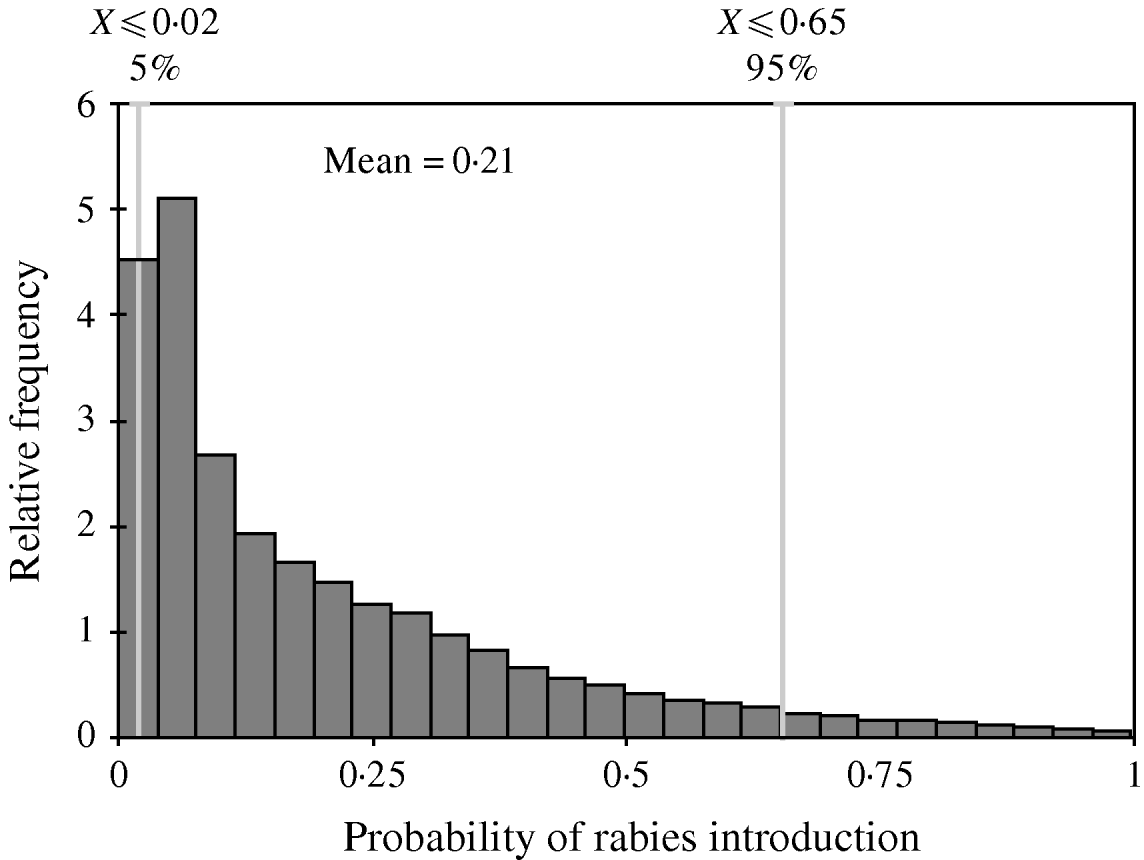
Fig. 3. Probability distribution of the annual risk of rabies introduction into the EU through dogs coming from Morocco.
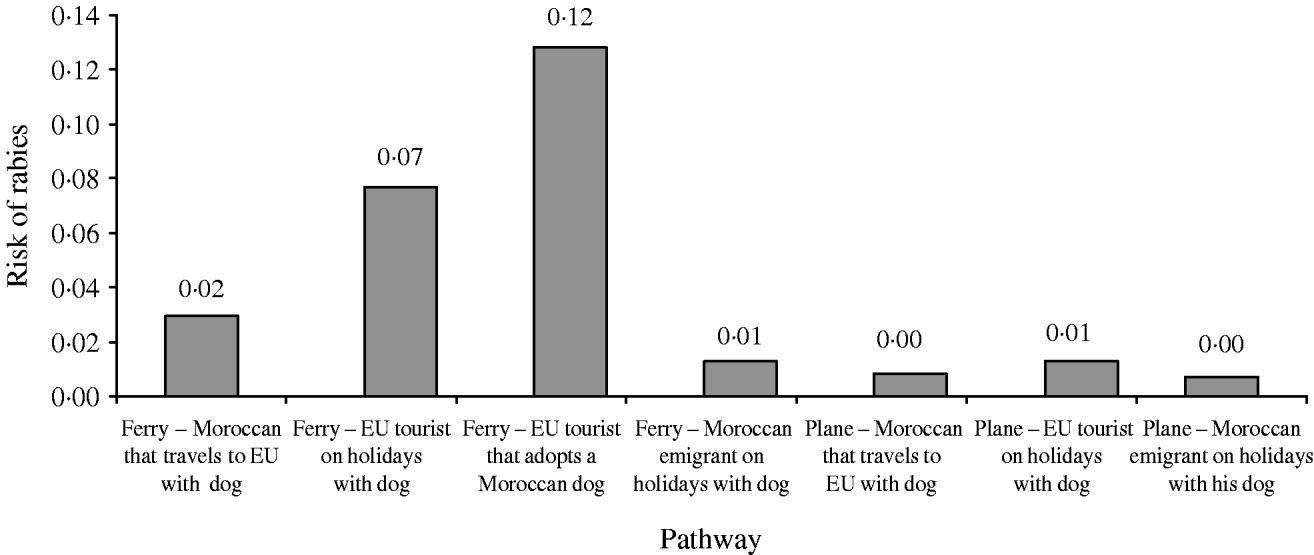
Fig. 4. Relative contribution of the different pathways to the annual risk of rabies introduction into the EU through dogs coming from Morocco.
The probability of rabies introduction into the EU from Morocco by month is shown in Figure 5. The months that contributed the most to the final risk were July (20% of the total probability) and August (19% of the total probability). The probabilities of (a) infection, and (b) detection, per pathway unit (one dog) for the different pathways are shown in Table 2.

Fig. 5. Monthly probability of rabies introduction into the EU through dogs coming from Morocco.
Table 2. Probabilities of infection and detection per pathway unit (one dog) for the different pathways

* An animal is assumed to be detected when it develops clinical signs before entering the EU.
Sensitivity analysis
The most influential parameters on the final model output (probability of rabies introduction into the EU through dogs coming from Morocco) were: the incubation period (ρ=0·44), the proportion of cases detected in Morocco (ρ=−0·44), the moment the adopted dogs became infected (ρ=0·30), the reduced probability of exposure to rabies in European dogs (ρ=0·16) and the number of dogs transported by sea (ρ=0·11). The chart of the rank-order correlation sensitivity analysis of the risk of rabies introduction is shown in Figure 6.

Fig. 6. Tornado chart of a rank-order correlation sensitivity analysis of the risk of rabies introduction into the EU through dogs coming from Morocco.
Effect of preventive measures
In the event of the application of stricter border controls (100% compliance) the estimated mean risk of rabies introduction into the EU from Morocco would be 0·00075 (a risk reduction greater than 270-fold).
DISCUSSION
Enforced movement restrictions placed on animals was probably more influential than vaccination policy for the eradication of canine rabies, from the EU [Reference Bourhy3]. However, canine rabies remains endemic in some countries bordering the EU, and the introduction of rabid animals constitutes a risk not only for animals but also for humans [Reference Bourhy5]. Owing to trends in tourism and immigration, globalization of companion animal movements have increased the potential for the translocation of diseases [Reference Marano, Arguin and Pappaioanou23]. In the case of the Spain–Morocco border, the number of passengers in 2007 was tenfold greater than in 1968 [20].
Several risk assessment models have been undertaken to estimate the risk of rabies introduction from endemic countries [Reference Jones13, Reference MacDiarmid, Corrin and Elms14, Reference Have19]. However, none of these models took into account the fact that the time a dog remained at risk was not the same in all cases (local vs. foreign dogs), nor did they consider the importance of seasonality.
The annual probability of rabies introduction from Morocco into the EU estimated by the model (0·21) can be considered high, although smaller than what appears to have actually occurred in recent years. The probabilities of rabies introduction from Morocco into the EU for a single dog (between 0·03% and 0·18% depending on the scenario) were higher than the risk considered acceptable in the study conducted by EFSA (10−6) for importing a rabies-infected pet into the UK, Ireland, Sweden and Malta [Reference Have19]. Jones et al. [Reference Jones13] estimated that the median annual probability of rabies entering Great Britain from North America was between 4·9×10−5 and 9·8×10−7, depending on the level of compliance with the control measures applied. In another study, the annual probability of introducing rabies into Norway from several European countries was estimated to be 0·00052%, 0·0052% and 0·051% for the importation of 100, 1000 or 10 000 unvaccinated young pets [Reference Høgåsen28].
The fact that the main contributors to the total likelihood of rabies introduction into the EU from Morocco were EU citizens that adopted a dog in Morocco (59% of the total probability) is in agreement with what actually occurred [6, 11]. On the other hand, EU citizens that travelled to Morocco with their dog by ferry were responsible for 34% of the total probability, while the contribution of the rest of the pathways was much more limited.
The model showed a marked seasonality in the risk of rabies with almost 40% of the annual risk concentrated in July and August, because of the concurrence of the peak of dogs transported (July–August) and the peak of rabies infections (June–July).
The probabilities of (a) infection and (b) no detection, per pathway unit (one dog) for the different pathways depend on the length of time the dog was exposed to the risk. The longer a dog remained at risk (in Morocco), the higher the probability of infection, but also the higher the probability of detection.
The most influential parameter on the probability of rabies introduction into the EU was the incubation period. The incubation period has both a variable component, which depends on factors such as viral dose, strain or site of inoculation, and an uncertain component, which is due to the limited data available on incubation periods after natural infection [Reference Jones17]. In our model, the incubation period was based on the combination of both natural infections and experimental infections. With regard to natural infection, the information available is very limited, and therefore the collection of data on the distribution of incubation periods following natural infection was one of the recommendations by EFSA [Reference Have19].
The second most influential parameter on the probability of rabies introduction into the EU was the proportion of cases detected in Morocco (P=−0·44). In most of the developing world, passive surveillance is ineffective and thus rabies is underreported. Maintaining a comprehensive surveillance system for rabies is too expensive for most countries. The passive-reporting systems used in these countries suffer from two fundamental problems [Reference Mansfield29]. First, the difficulty in diagnosing rabies rapidly, which is exacerbated by the fact that there are no pathognomonic signs of rabies [Reference Marano, Arguin and Pappaioanou23]. Second, because there is no strong incentive for reporting suspected rabies cases, veterinary services are unable to track rabies outbreaks. In Morocco, the fact that reported rabies cases in herbivores exceed those in carnivores was considered as indicative of the underreporting of dog rabies [Reference Mansfield29]. The situation persists, as rabies cases in herbivores constituted 66% of the total rabies cases recorded in Morocco and reported to the OIE between January 2007 and June 2008 [11]. Given that the proportion of cases detected in Morocco is the most influential (uncertain) parameter, the most likely explanation for the model's underestimation of the overall annual risk would be that it underestimated the level of underreporting.
The third most influential parameter was the moment the adopted dog became infected, which is a variable parameter, indicating that the effect of chance plays an important role in the risk of rabies introduction. The closer to the date of travel to the EU that the dogs became infected, the lower the probability they would be detected prior to travel, and therefore, the higher the risk of rabies introduction.
The fourth most influential parameter was the reduced probability of exposure to rabies in European dogs. The extent to what these dogs are protected from potentially infected dogs (e.g. being maintained indoors or kept on a leash), has an important influence on the model's results.
The fifth most determinant parameter was the number of dogs transported by sea. Given that the number of dogs introduced into the EU from Morocco by the maritime route was conservatively modelled, this may have also contributed to a certain underestimation of the risk of rabies.
The application of stricter border controls (100% compliance) would result in a reduction of the annual risk of rabies introduction into the EU by >270-fold, and therefore would be effective in reducing the likelihood of rabies introduction into the EU.
One of the limitations of the model is the fact that it includes both variable and uncertain parameters, which makes it impossible to assess the relative contribution of the variability and the uncertainty on likelihood of rabies introduction. Uncertainty and variability may be separated by developing a second-order model [Reference Murray27], but in our case it was not feasible given the large number of uncertain input parameters. Other models have dealt with this problem by using point estimates for the uncertain parameters [Reference de Vos31]. However, we agree with Wooldridge and colleagues [Reference Wooldridge32] who assert that this ignores the need for uncertainties, when present, to be taken into account of in any decision-making process. Further, the approach adopted allowed us to assess the importance of the uncertain parameter on the final risk of rabies introduction.
Another limitation of the work presented is the fact that, for some of the parameters used in the model, assumptions were based on limited available data. If more reliable data in relation to these parameters becomes available, it will allow readjustment of the model outputs.
ACKNOWLEDGEMENTS
The authors thank Lucía Pitarch (Central Laboratory of Animal Health of Santa Fe) and Ángeles Gonzales (Tragsa) for providing useful data.
DECLARATION OF INTEREST
None










