Introduction
Accurate knowledge of spatial and temporal distribution patterns is fundamental to species management and conservation, and assessment of extinction risks (Gaston & Fuller, Reference Gaston and Fuller2009). For example, ≥ 47% of threatened species in groups that have been assessed for the IUCN Red List were categorized solely on the basis of range measures (Gaston & Fuller, Reference Gaston and Fuller2009). These measures include extent of occurrence (EOO) and area of occupancy (AOO). EOO is the geographical area bounded by the outermost known or projected contemporary species' records, and is often considered the species' range (IUCN, 2001; Hartley & Kunin, Reference Hartley and Kunin2003; Gaston & Fuller, Reference Gaston and Fuller2009). It is used in assessing the likelihood of simultaneous extinction in all areas occupied by the species, with the assumption that extinction risk is inversely related to range size (Gaston & Fuller, Reference Gaston and Fuller2009). AOO is a finer scale measure comprising specific locations within the EOO where species' occurrence has been recorded, generalized to an appropriate spatial resolution (IUCN, 2001; Hartley & Kunin, Reference Hartley and Kunin2003; Gaston & Fuller, Reference Gaston and Fuller2009). It reflects the rarity and fragmentation of occupied locations and thus the likely resilience of the distribution to threats from stochastic and directional processes (Hartley & Kunin, Reference Hartley and Kunin2003; Gaston & Fuller, Reference Gaston and Fuller2009). Occupancy is another measure that expresses the proportion of an area or collection of sampling sites that are occupied and is frequently used in monitoring programmes as a surrogate for abundance (MacKenzie et al., Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006).
Unfortunately, such information is often lacking for terrestrial carnivores because they are notoriously difficult and labour intensive to detect (Nowell & Jackson, Reference Nowell and Jackson1996; Sillero-Zubiri et al., Reference Sillero-Zubiri, Hoffmann and Macdonald2004). This data deficit was highlighted by a South African Conservation Assessment and Management Plan (Friedmann & Daly, Reference Friedmann and Daly2004) to update the evaluation conducted nearly 20 years previously (Smithers, Reference Smithers1986). As a result of this process 35% of South Africa’s terrestrial carnivores were categorized nationally as threatened, Near Threatened or Data Deficient (Friedmann & Daly, Reference Friedmann and Daly2004). This includes the brown hyaena Hyaena brunnea and serval Leptailurus serval, categorized as Near Threatened, and the cheetah Acinonyx jubatus, categorized as Vulnerable. However, insufficient data on trends and contemporary distributions hindered accurate assessment of many species (Friedmann & Daly, Reference Friedmann and Daly2004). Here we address this problem by mapping new data from large-scale surveys in northern South Africa. We compare current and historical distributions so as to extract spatial and temporal patterns that elucidate the present conservation status of the brown hyaena, serval and cheetah, and the black-backed jackal Canis mesomelas, caracal Caracal caracal and leopard Panthera pardus, categorized as Least Concern (i.e. not currently in danger of extinction) in South Africa (Friedmann & Daly, Reference Friedmann and Daly2004).
Study area
The study area is the North West province of South Africa and the adjoining Thabazimbi district of the Limpopo province, which border Botswana to the north. All six of the focal species occur within and outside protected areas, most of which have fences that are permeable to free-ranging animals. Historical and contemporary provincial boundaries relevant to the discussion are shown in Fig. 1.
Fig. 1 (a) Current provincial boundaries and location of the study area, which comprised the North West province and Thabazimbi district, an adjoining area of Limpopo province, and (b) historical provincial boundaries in South Africa.
Methods
We collected the data presented here during an intensive study of the Thabazimbi district in 2000 and a large-scale study of the North West province during 2006–2008. The cheetah was the focal species for the Thabazimbi study but we also collected data on the brown hyaena and leopard. The focal species for the study in the North West province were the brown hyaena, caracal, black-backed jackal, leopard, serval and cheetah. We carried out a number of systematic, random and opportunistic surveys throughout the study area. Sampling methods included socio-economic interviews using questionnaires, sign surveys and camera trapping. We also included records of verified complaints of problem animals lodged by landowners with the Nature Conservation Department during the same period. All observers were appropriately trained and experienced and any records with dubious reliability were excluded from analysis to minimize the likelihood of records of false presence (Karanth et al., Reference Karanth, Nichols, Hines, Karanth and Christensen2009).
Questionnaire surveys involved semi-structured interviews with private landowners and managers. In the Thabazimbi district all contactable agricultural landowners were interviewed. In the North West province the primary sampling units were 25 randomly selected 415 km2 grid cells covering 16.9% of agricultural land in the province. We surveyed ≥ 2 landowners per sampling cell but, although the majority of interviews (64%) were conducted in the randomly selected survey cells, we also recruited additional participants at livestock auctions and farmers’ meetings.
We conducted preliminary sign surveys at ranches (n = 17) and reserves (n = 8) in areas deemed representative of climatic conditions (high, medium and low annual rainfall and mean daily maximum temperature), mammal community composition (with and without apex predators, game and domestic livestock), and densities of carnivore species (within and outside protected areas). We surveyed once in the wet season and again in the dry season. The mean area of the sites surveyed was 103 km2 and we scaled effort by carrying out ≥ 1 km of surveys per 5 km2 of site area. We conducted all sign surveys from a vehicle travelling along unpaved roads at 10–15 km h-1 (see Thorn et al., Reference Thorn, Green, Bateman, Cameron, Yarnell and Scott2010 for detailed methods). During sign surveys ≥ 2 trained observers searched for sign (tracks and scats) on and within a 2-m strip either side of the road. Sign was identified to species based on colour, dimensions, position and presence of accompanying signs (Stuart & Stuart, Reference Stuart and Stuart2000) but was not aged.
Later sign surveys were conducted in the 25 randomly selected 415 km2 grid cells where we had completed questionnaire surveys in the North West province. As in the preliminary surveys we detected sign more frequently in the dry season (although the difference was not significant), when sign persists for longer; these later surveys were conducted only in the dry season. We scaled effort according to the proportion of agricultural land in the cell (following Hines et al., Reference Hines, Nichols, Royle, MacKenzie, Gopalaswamy, Samba Kumar and Karanth2010), resulting in an effort of 11–25 km per cell. We used the same method as in the preliminary surveys except that the primary observer was seated in a spotter’s chair fixed above the front bumper whilst a secondary observer looked for sign from within the vehicle.
We carried out a preliminary camera-trap survey at 13 of the sites where we conducted preliminary sign surveys. At that time only four passive infrared 35 mm cameras were available to us, restricting effort to ≥ 5 camera days per site. Seasonal replicates were completed at nine of the 13 sites. We positioned single camera traps in locations where carnivore signs had been previously observed, to maximize detection probability (Karanth & Nichols, Reference Karanth and Nichols2002). They were secured to a suitable tree or bush at a height of c. 45 cm from the ground (Karanth & Nichols, Reference Karanth and Nichols2002) and baited daily with offal or animal carcasses. We set cameras to operate only at night, with time and date automatically imprinted on photographs.
Later camera-trap surveys used 35 mm passive infrared units set for 24 hour operation, fixed to trees, positioned to maximize detection probability, and set to imprint time and date, as in preliminary work. We carried out seven surveys in the randomly selected 415 km2 grid cells where we completed questionnaire and sign surveys in the North West province. As in initial sign surveys we chose sites that were representative of a range of climactic conditions and mammal community composition. We used 11–25 camera traps per grid cell (scaling effort in the same way as for sign surveys), with single camera traps c. 1 km apart along roads and trails. We baited with tinned fish and fermented eggs and left traps in place for ≥ 9 days.
We compiled the results of the field surveys and inferred species presence from signs and sightings. We then digitized the location of each occurrence (obtained with a global positioning system) in ArcView v. 3.3 (ESRI, Redlands, USA) and plotted our new records alongside those generated during the South African Conservation Assessment and Management Plan process. The Conservation Assessment and Management Plan distribution maps (Keith, Reference Keith2004) comprise a series of geographical information system shapefiles showing AOO at ¼ degree square (QDS) level and the minimum convex polygon (MCP) representing the most likely EOO of each species between 1990 and 2000. We based our comparisons on these maps rather than on the IUCN global distribution assessments because the former incorporate more detailed local AOO knowledge for 1990–2000 and therefore are a better reflection of fine-scale national distribution during that decade (Rodríguez et al., Reference Rodríguez, Ashenfelter, Rojas-Suárez, Fernández, Suárez and Dobson2000). The Conservation Assessment and Management Plan workshop that generated the maps was conducted under the auspices of the IUCN Conservation Breeding Specialist Group and the maps are consistent with IUCN criteria (IUCN, 2001). Our maps use the same QDS resolution but also show grid cells where surveys failed to detect the focal species (non-detections), and locations of breeding populations (inferred from sightings of offspring).
Results
Our sample included data from a total of 474 ranches and reserves (108 in the landscape-scale study of the North West province and 366 in the more intensive study of the Thabazimbi district). These properties were located in 71 of the 234 QDSs that fall wholly or partly within the study area. Complaints of problem animals added presence records of carnivores in a further eight QDSs that were not surveyed. We completed 298 questionnaire interviews, 55 sign surveys comprising a total of 1,540 km of effort, and 20 camera trap surveys comprising 1,571 days of trapping effort. Questionnaires were used in 62 QDSs with a mean effort of four questionnaires per QDS (range 2–39), sign surveys were used in 43 QDSs with a mean effort of 35.8 km per QDS (range 25–203) and camera trapping was used in 20 QDSs with a mean effort of 72 camera days per QDS (range 5–297; Fig. 2).
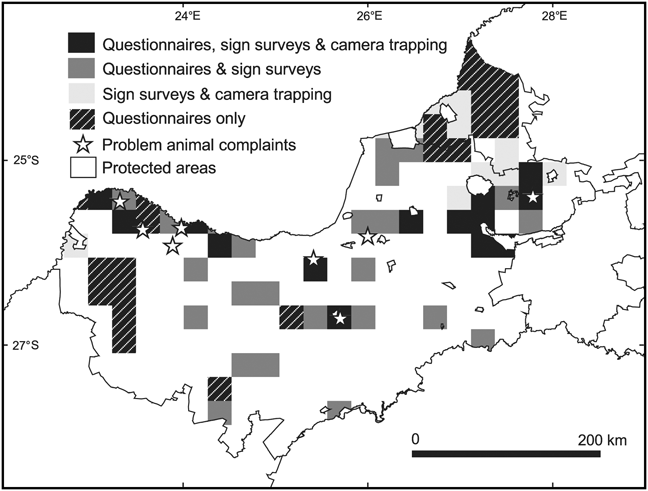
Fig. 2 Methods used to collect data in ¼ degree squares (QDS) in the North West province and Thabazimbi district (Fig. 1) between 2000 and 2008.
We estimated EOO and AOO of the six species in the study area (Table 1) from our revised distribution maps (Fig. 3). Trends were calculated as the percentage difference between our estimates and those calculated from Keith (Reference Keith2004). We defined EOO as extent of the study area falling within the South African MCP for the species. We calculated AOO as the summed area of all occupied QDSs (1 QDS ≈ 773 km2) falling wholly or partly within the study area. Naive occupancy was calculated as the percentage of surveyed QDSs within the species' EOO where the species was detected.

Fig. 3 Distribution maps showing the ¼ degree squares (QDS) in the North West province and Thabazimbi district (Fig. 1) believed occupied by each of the six carnivore species during 1990–2000 (Keith, Reference Keith2004) and the QDS where we measured occupancy during 2000–2008: (a) black-backed jackal Canis mesomelas, (b) caracal Caracal caracal, (c) serval Leptailurus serval, (d) brown hyaena Hyaena brunnea, (e) cheetah Acinonyx jubatus, (f) leopard Panthera pardus. Extent of occurrence (EOO) is the minimum convex polygon containing all South African records of the species, intersected with the study area.  QDS believed occupied during 1990–2000 (Keith, Reference Keith2004) but not re-sampled in our surveys.
QDS believed occupied during 1990–2000 (Keith, Reference Keith2004) but not re-sampled in our surveys.  QDS where we detected the species during 2000–2008 but Keith (Reference Keith2004) did not.
QDS where we detected the species during 2000–2008 but Keith (Reference Keith2004) did not. ![]() QDS where both our study and Keith (Reference Keith2004) reported the species.
QDS where both our study and Keith (Reference Keith2004) reported the species. ![]() QDS where we did not detect the species.
QDS where we did not detect the species. ![]() Revised EOO taking account of new data from this study.
Revised EOO taking account of new data from this study.  Areas falling outside of the EOO polygon or excluded in Keith (Reference Keith2004) as unsuitable habitat.
Areas falling outside of the EOO polygon or excluded in Keith (Reference Keith2004) as unsuitable habitat. ![]() Protected areas.
Protected areas.  Breeding records from this study.
Breeding records from this study.
Table 1 Estimates of minimum extent of occurrence (EOO; extent of the study area falling within the minimum convex polygon encompassing all records of the species in South Africa) and area of occurrence (AOO; the summed area of all occupied grid cells falling wholly or partly within the study area) in 2000 and 2010 for six carnivore species. Trends are expressed as the percentage difference between our estimates and those calculated from Keith (Reference Keith2004).
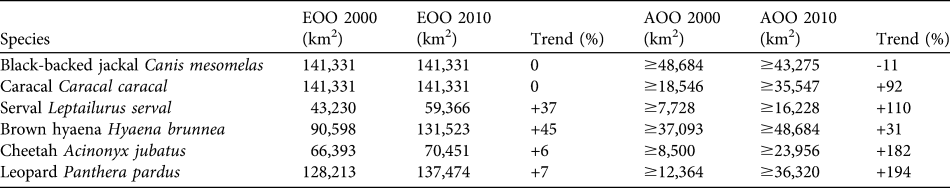
The EOO of the black-backed jackal remained unchanged from that reported for 1999–2000. Breeding records were numerous and distributed throughout the study area. As jackal occupancy was the highest (100%) of the focal species the negative trend in AOO probably reflects a lack of observations from our Thabazimbi survey (in which jackals were not a focal species) compared with the museum and personal observation records used in Keith (Reference Keith2004). If present jackal occupancy is the same in Thabazimbi as in the rest of the study area, the overall trend in AOO would be +8%. Caracal EOO remains unchanged and, at 82%, naive occupancy was high. The AOO showed a large increase and breeding reports were distributed widely throughout the study area. The serval had a naive occupancy of 81% and showed large increases in both EOO and AOO. Brown hyaenas had the highest AOO and largest increase in EOO of the focal species. Naive occupancy was 91% but breeding records were restricted to the northern half of the study area. The increasing trend in cheetah and leopard EOO resulted from the addition of a single outlying presence record in each case, marginally increasing the MCP area. Cheetahs had 60% naive occupancy and high AOO considering the species’ restricted range. The only breeding records were along the Botswana border. Leopard AOO showed the largest increase of the focal species and naive occupancy was 73%. Breeding records were restricted to the northern half of the study area, with the exception of one southern outlier.
Discussion
Prior publications providing carnivore distribution records for our study area largely relied on collections data consisting of museum records (e.g. those contained in the South African Integrated Spatial Information System), literature sources, personal observations from taxon experts, and reports from provincial authorities (Skinner, Reference Skinner1976; Stuart et al., Reference Stuart, Macdonald and Mills1985; Skinner & Smithers, Reference Skinner and Smithers1990; Gelderblom et al., Reference Gelderblom, Bronner, Lombard and Taylor1995; Friedmann & Daly, Reference Friedmann and Daly2004). Such data are valuable but are often subject to quality problems such as bias towards sampling easier-to-study species or areas and omission of non-computerized records (Robertson & Barker, Reference Robertson and Barker2006). Furthermore, contemporaneous field surveys were mainly confined to protected areas and as non-detections were not displayed apparently unoccupied grid cells cannot be differentiated from those that were simply data deficient. Because of this, apparent changes in distribution may arise from greater direct search effort in areas that were previously ignored or poorly sampled (Dobson & Nowak, Reference Dobson and Nowak2010). This is a widespread issue that confounds analysis of range trends in many biodiversity studies (Dobson & Nowak, Reference Dobson and Nowak2010). Our data do not suffer from such problems and thus constitute an improvement over previously available baseline records. However, the variety of sampling designs, methods and timescales involved in our fieldwork precludes universal application of sophisticated analysis. For example, the time span of the data violates the closure assumption of occupancy models (MacKenzie et al., Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006) and therefore occupancy estimates cannot be adjusted to reflect imperfect detection (false absences). Similarly, temporal and spatial heterogeneity in detection probability cannot be estimated, confounding inference of patterns in relative abundance (Karanth & Nichols, Reference Karanth and Nichols2002). Although these analyses could be applied to a subset of the data, the results could not be generalized to reveal landscape-scale or long-term trends, which is our aim here.
Historical and present extent of occurrence
South African wildlife populations were depleted by colonial settlers and then almost extirpated by crop and livestock farmers in the early part of the 20th century (Pringle & Pringle, Reference Pringle and Pringle1979; Bothma et al., Reference Bothma, Suich, Spenceley, Suich, Child and Spenceley2009). Black-backed jackals began to appear in the highveld (east central South Africa) from 1953 (Walton & Joly, Reference Walton and Joly2003), suggesting range expansion or recolonization. Pringle & Pringle (Reference Pringle and Pringle1979) mentioned increasing numbers of caracals in eastern areas from c. 1970. Keith (Reference Keith2004) recorded that black-backed jackals and caracals were distributed throughout the study area by 2000. Our results provide no evidence of recent changes in EOO and thus we conclude that these species remain widespread.
In the latter half of the 20th century the serval was considered extinct in many areas of its historical range (Stuart, Reference Stuart1985; Smithers, Reference Smithers1986; Skinner & Smithers, Reference Skinner and Smithers1990; Hermann et al., Reference Hermann, Kalmer and Avenant2008) because of competitive exclusion by expanding caracal populations (Stuart, Reference Stuart1985). Brown hyaenas were widespread in the Transvaal (Smithers, Reference Smithers1986; Skinner & Smithers, Reference Skinner and Smithers1990) but thought to inhabit only 25% of their historical range in the Cape province (Stuart et al., Reference Stuart, Macdonald and Mills1985). We found a marked increase in EOO of both servals and brown hyaenas, indicating possible range expansion. This finding is corroborated by new serval occurrences in parts of the Free State and in southern areas of the North West province (Hermann et al., Reference Hermann, Kalmer and Avenant2008). The positive trend in brown hyaena EOO stemmed mainly from numerous detections in areas previously excluded from the species' range as obviously unsuitable habitat (e.g. extensive crop land). A breeding record and inactive den in these areas indicate that at least some individuals are resident, supporting inclusion of these areas in our map of the species’ EOO. The EOO of the serval and brown hyaena is considerably larger than previous estimates, substantially reducing the likelihood of comprehensive local extinction.
The historical distribution of cheetahs in South Africa is uncertain (Marnewick et al., Reference Marnewick, Beckhelling, Cilliers, Lane, Mills and Herring2007) but hunting and museum records indicate they certainly occurred as far south as the Western Cape province (Sclater, Reference Sclater, Skinner and Smithers1990). The 2004 assessment recorded cheetah presence only in a narrow strip along the northern border with Botswana (Friedmann & Daly, Reference Friedmann and Daly2004; Keith, Reference Keith2004). Current EOO in our study area is similar but distorted by the presence of one or two individuals in Pilanesberg National Park and an outlying presence record prior to 2000. Frequent non-detections and a lack of recent records of presence suggest that the south-eastern extremity of the MCP should be excluded from the species' range in the near future unless the population in Pilanesberg recovers substantially.
By 2000 leopards remained widespread except in the south-east of the study area (Keith, Reference Keith2004). We recorded a highly fragmented AOO in the south, which is often associated with the outer edge of a species range (Brown, Reference Brown1984). This pattern increases the likelihood of range contraction if contiguous threats intensify. Frequent non-detection in the south suggests that this may already have occurred. However, a comprehensive survey of the south-eastern area is needed to demarcate definitively the range boundary and the EOO therefore remains unchanged pending confirmation of our findings.
Area of occupancy and naive occupancy
The home ranges of the black-backed jackal and serval are much smaller than a QDS. For example, mean home range for jackals in an ecologically similar study area in Botswana was 15.9 km2 (Kaunda, Reference Kaunda2001). If occupancy within QDSs is patchy, generalizing to the scale of QDSs may overestimate the area actually occupied. Absolute AOO values for these species should therefore be interpreted with caution. AOO for wide ranging species such as the brown hyaena, caracal, leopard and cheetah probably approximate better the land area they occupy, although positive bias is still possible. However, actual occupancy is frequently greater than naive occupancy because species that are present are not always detected. We did not sample comprehensively and thus it is also likely that at least some QDSs that were not sampled are occupied, especially those reportedly occupied in Keith (Reference Keith2004) and not re-sampled in our surveys. In view of this our AOO estimates are probably conservative, particularly for widespread species with large home ranges.
The black-backed jackal assessment in Friedmann & Daly (Reference Friedmann and Daly2004) considered the species abundant and common. We found 100% occupancy and our numerous, extensive breeding records also suggest high fecundity. In view of this and because AOO at fine spatial scales is often correlated with population size (Gaston & Fuller, Reference Gaston and Fuller2009), we conclude that the species remains abundant. Friedmann & Daly (Reference Friedmann and Daly2004) also considered the caracal common, and high occupancy, increasing AOO and extensive breeding records indicate that this is probably still the case. Both species seem to have recovered well from historical threats and appear resilient to contemporary pressures.
Our AOO data for leopards shows the highest increase of the focal species and indicates that they currently inhabit large areas of the northern half, and especially the north-east, of the study area. The distribution of breeding records and fragmented AOO pattern observed in the south implies residence mainly in the north. Cheetah AOO showed a marked increase and occupancy was relatively high given the restricted range of the species. Whether increased AOO reflects higher occupancy or search effort, current patterns for both the leopard and cheetah should afford a greater degree of resilience to threats than would have been predicted from previous records (Gaston & Fuller, Reference Gaston and Fuller2009). However, in both cases restricted and probably contracting ranges argue for precautionary monitoring of local status.
High occupancy for the serval, a large increase in AOO and apparent range expansion indicates continued recovery from historical threats. According to species' accounts, the serval is rarely found in arid areas and their distribution is restricted to high rainfall areas with riparian habitat (Nowell & Jackson, Reference Nowell and Jackson1996). However, serval records from sign and questionnaire surveys were evenly divided between sites with annual rainfall of ≤ 400 and ≥ 400 mm, indicating that the species is more tolerant of arid conditions than previously thought. Hermann et al. (Reference Hermann, Kalmer and Avenant2008) contended that this is mainly due to the creation of man-made water bodies, which provide suitable habitat for serval prey.
The brown hyaena was formerly considered rare in the Transvaal (Smithers, Reference Smithers1986; Skinner & Smithers, Reference Skinner and Smithers1990) but our high estimates of AOO and occupancy together with an increasing AOO indicate that this is no longer the case. Evidence of brown hyaena breeding was restricted to the northern half of the study area but the southern AOO pattern does not show the fragmentation usually associated with the edge of a species’ range. Future studies may therefore reveal breeding clans further south. High occupancy in extensive crop land demonstrates a greater than expected tolerance of degraded habitat, and current AOO, together with a larger estimate of EOO, gives cause for cautious optimism about the local status of this species.
Implications for future studies
Ideally, researchers and conservation practitioners should measure landscape-scale patterns in distribution, occupancy and relative abundance by conducting large-scale surveys using standardized methods that account for issues of spatial sampling and detectability. In practice, cost and logistical difficulties frequently restrict the extent and intensity of carnivore surveys (Thorn et al., Reference Thorn, Green, Bateman, Cameron, Yarnell and Scott2010), which is one of the main reasons landscape-scale distribution data are so scarce. Our records were gathered relatively quickly by a small team operating over a large area and demonstrate the value of synthesizing results from multiple smaller-scale surveys (Karanth et al., Reference Karanth, Nichols, Hines, Karanth and Christensen2009; Pettorelli et al., Reference Pettorelli, Lobora, Msuha, Foley and Durant2010). We constructed a fine-scale, contemporary snapshot of large-scale patterns that will facilitate monitoring of distributions (Dobson & Nowak, Reference Dobson and Nowak2010). This is fundamental to managing ecological challenges that have a spatial component, such as protected area network design and measuring the impact of changes in environmental variables such as climate or land use (Gelderblom et al., Reference Gelderblom, Bronner, Lombard and Taylor1995; Rodríguez et al., Reference Rodríguez, Ashenfelter, Rojas-Suárez, Fernández, Suárez and Dobson2000; Hartley & Kunin, Reference Hartley and Kunin2003; Karanth et al., Reference Karanth, Nichols, Hines, Karanth and Christensen2009; Pettorelli et al., Reference Pettorelli, Lobora, Msuha, Foley and Durant2010). Our results illustrate the utility of relatively simple detection/non-detection surveys in rapid assessment of carnivore distribution at large spatial scales. Such surveys can also be used to evaluate patterns of species richness and endemism (O’Brien, Reference O’Brien2008) and to extrapolate distribution patterns to areas that have not been surveyed (Karanth et al., Reference Karanth, Nichols, Hines, Karanth and Christensen2009). Such applications are of increasing importance in view of global population declines and accelerating anthropogenic impacts on biodiversity (Karanth et al., Reference Karanth, Nichols, Hines, Karanth and Christensen2009; Pettorelli et al., Reference Pettorelli, Lobora, Msuha, Foley and Durant2010).
Acknowledgements
We are indebted to the private landowners, the tribal authorities in Heuningvlei, Tshidilamololo and Ganyesa, and the North West Parks and Tourism Board, who allowed us to survey their land. We thank G. Gaboinewe and G.S. Molale, who helped administer questionnaires in Heuningvlei, R. Yarnell of Project Phiri, N. Ball for collecting data in Madikwe Game Reserve, V. Jacobs of the North West Department of Agriculture, Conservation and Environment for contributing problem animal records, and S. Uzzell for computer equipment. We are grateful to the University of Brighton, UK, the Leverhulme Trust UK Chester Zoo, UK, and the Earthwatch Institute for funding.
Biographical sketches
Michelle Thorn and Matthew Green’s interests include patterns and determinants of mammal distribution, the development of practical field protocols for conservation monitoring and the management of human–animal conflicts. Mark Keith focuses on the ecology and conservation of small- to medium-sized mammals, specifically to improve our understanding of lesser-known species. Kelly Marnewick is the manager of the Endangered Wildlife Trust’s Carnivore Conservation Programme. She is particularly interested in cheetahs outside protected areas and resolution of human–wildlife conflict. Bill Bateman’s research interests include African carnivores and urban and non-native carnivores in Australia. Elissa Cameron works on the behaviour and ecology of large mammals, particularly ungulates. Her focus is on sexual conflict and the resulting impacts on behaviour, ecology and social structure. Dawn Scott’s research specialisms are in mammalian conservation biology and landscape ecology and she is the principal investigator of the Brown Hyaena Research Project in South Africa.






