Relict trees are particularly interesting because they have survived and adapted to changing environmental conditions throughout millions of years and are the only representatives of previously widespread taxa (Kozlowski & Gratzfeld Reference Kozlowski and Gratzfeld2013; Grandcolas et al. Reference Grandcolas, Nattier and Trewick2014). Little research has yet been undertaken to make an inventory of the biodiversity linked specifically with relict trees. However, relict trees have been found to be of crucial importance in maintaining and giving shelter to widespread as well as rare, endemic or other relict taxa. This is the case, for example, with Zelkova sicula Di Pasq. et al. (Barbagallo Reference Barbagallo2002; Barbagallo et al. Reference Barbagallo, Cocuzza and Suma2009) or Dracaena cinnabari Balf.f. (Rejžek et al. Reference Rejžek, Svátek, Šebesta, Adolt, Maděra and Matula2016; Maděra et al. Reference Maděra, Habrová, Šenfeldr, Kholová, Lvončík, Ehrenbergerová, Roth, Nadezhdina, Němec and Rosenthal2019).
Furthermore, relict tree stands often contain very old trees (Fazan et al. Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012; Tang et al. Reference Tang, Peng, He, Ohsawa, Wang, Xie, Li, Li, Zhang and Li2013; Camarero et al. Reference Camarero, Sangüesa-Barreda, Montiel-Molina, Seijo and López-Sáez2018), and old trees are known to provide numerous microhabitats (Lindenmayer & Laurance Reference Lindenmayer and Laurance2017; Nordén et al. Reference Nordén, Jordal and Evju2018). These microhabitats (sometimes denominated in literature as ‘tree related microhabitats’; Kraus et al. Reference Kraus, Bütler, Krumm, Lachat, Larrieu, Mergner, Paillet, Rydkvist, Schuck and Winter2016; Larrieu et al. Reference Larrieu, Paillet, Winter, Bütler, Kraus, Krumm, Lachat, Michel, Regnery and Vandekerkhove2018; Bütler et al. Reference Bütler, Lachat, Krumm, Kraus and Larrieu2020) can also be formed by tree-associated taxa such as bryophytes or lichens. Some in turn foster a wide variety of other living organisms (e.g. invertebrates, plants, fungi, birds) and play a key role in maintaining or even increasing biodiversity (Paillet et al. Reference Paillet, Bergès, Hjältén, Ódor, Avon, Bernhardt-Römermann, Bijlsma, de Bruyn, Fuhr and Grandin2010).
The genus Zelkova (Ulmaceae) is a relict from the so-called Arctotertiary geoflora (Mai Reference Mai1991) whose members were important components of forests of the Northern Hemisphere during the Paleogene. Only six extant species of this genus are found today, and they show a remarkable disjunct distribution: Z. serrata (Thunb.) Makino, Z. schneideriana Hand.-Mazz. and Z. sinica Schneid. occur in eastern Asia, Z. carpinifolia (Pall.) Koch grows in the Transcaucasian region and Middle East, while Z. sicula and Z. abelicea (Lam.) Boiss. are endemic to the Mediterranean islands of Sicily (Italy) and Crete (Greece), respectively (Kozlowski & Gratzfeld Reference Kozlowski and Gratzfeld2013).
Zelkova abelicea grows in the mountainous regions of Crete above 900 m a.s.l., in rather cool and not too xeric sites such as north-facing slopes or around dolines (sinkholes), or at high elevations on south-facing slopes (Egli Reference Egli1997; Søndergaard & Egli Reference Søndergaard and Egli2006; Fazan et al. Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012; Goedecke & Bergmeier Reference Goedecke and Bergmeier2018). Due mainly to overbrowsing by goats, most individuals have a stunted dwarfed form, with multiple stems, a shrubby morphology and very slow growth (Fazan et al. Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012). Such individuals account for up to 95% of all populations, with some stands composed entirely of dwarfed plants (Kozlowski et al. Reference Kozlowski, Frey, Fazan, Egli, Bétrisey, Gratzfeld, Garfì and Pirintsos2014). Dwarfed individuals have been found to reach several hundred years in age and in some cases are older than arborescent trees (Fazan et al. Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012). Arborescent, 15–20 m high individuals are much rarer. Old arborescent Z. abelicea trees show signs of having been pollarded in the past, and their leaves were used as summer forage (Rackham & Moody Reference Rackham and Moody1996; Bauer & Bergmeier Reference Bauer and Bergmeier2011). These old trees are often found growing next to abandoned shepherd huts to which they probably provided shade. The recruitment of seedlings is difficult due to the almost permanent overbrowsing during the growing season and dry summer conditions influencing plant growth and seedling establishment (Egli Reference Egli1997; Søndergaard & Egli Reference Søndergaard and Egli2006; Fazan et al. Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012; Kozlowski et al. Reference Kozlowski, Frey, Fazan, Egli, Bétrisey, Gratzfeld, Garfì and Pirintsos2014, Reference Kozlowski, Bétrisey, Song, Fazan and Garfì2018).
The overall number of lichen and bryophyte species known for Greece is small, despite significant recent progress in the study of both taxonomic groups. The first and only published lichen checklist of Greece (Abbott Reference Abbott2009) recorded almost 1300 species, while the most recent, online checklist (Arcadia Reference Arcadia2022) already includes c. 1500 taxa. This indicates there is ongoing dedication towards this group of organisms in the region, as supported by the studies in recent years of a dozen lichenologists occasionally working in Greece (Obermayer Reference Obermayer1997; Papp et al. Reference Papp, Lökös, Rajczy, Chatzinikolaki and Damanakis1999; Sipman & Raus Reference Sipman and Raus1999, Reference Sipman and Raus2002; Christensen Reference Christensen2000, Reference Christensen2007, Reference Christensen2014, Reference Christensen2018; Grube et al. Reference Grube, Lindblom and Mayrhofer2001; Spribille et al. Reference Spribille, Schultz, Breuss and Bergmeier2006; Christensen & Svane Reference Christensen and Svane2007; Vondrák et al. Reference Vondrák, Guttová and Mayrhofer2008). Furthermore, although Crete also offers a wide spectrum of habitats, only a small number of lichen species are known from this island (Vondrák et al. Reference Vondrák, Guttová and Mayrhofer2008), only 677 species according to Arcadia (Reference Arcadia2022).
Several authors have recorded bryophytes in Greece throughout the 20th century (e.g. Coppey Reference Coppey1907, Reference Coppey1909; Preston Reference Preston1981, Reference Preston1984; Düll Reference Düll1995), each time with an increasing number of species. In the 1980s, Preston (Reference Preston1984) reported 424 species, whereas more recent national inventories included 525 moss taxa (Sabovljević et al. Reference Sabovljević, Natcheva, Dihoru, Tsakiri, Dragićević, Erdağ and Papp2008) or 690 species when considering both mosses (536 spp.) and liverworts (154 spp.; Blockeel Reference Blockeel2013). Ros-Espin et al. (Reference Ros-Espin, Mazimpaka, Abou-Salama, Aleffi, Blockeel, Brugués, Cros, Dia, Dirkse and Draper2013) reported over 590 moss taxa from mainland Greece and slightly over 280 taxa from Crete, of which c. 25% have been recorded only once. This again shows how little these groups of organisms have been studied until recently and suggests a need for more detailed investigations of lichens and bryophytes on the island.
Studies including or focusing on the phorophyte species of Crete exist but are rare (e.g. Kleinig Reference Kleinig1966; Gradstein Reference Gradstein1971; Werner Reference Werner1998; Grube et al. Reference Grube, Lindblom and Mayrhofer2001; Spribille et al. Reference Spribille, Schultz, Breuss and Bergmeier2006; Christensen Reference Christensen2007, Reference Christensen2014; Vondrák et al. Reference Vondrák, Guttová and Mayrhofer2008), and none has addressed Z. abelicea specifically. The only record of epiphytes growing on Z. abelicea comes from Spribille et al. (Reference Spribille, Schultz, Breuss and Bergmeier2006), which mentions four macrolichen species that are common for Crete.
In this study, we focus on a so far neglected portion of the biodiversity linked with Z. abelicea, by investigating the lichens and bryophytes that use this species as a phorophyte. It is the first time that an attempt to list these groups of organisms has been made by targeting Z. abelicea and covering all mountain ranges where the tree species occurs. More specifically, we aimed to answer the following questions: 1) What is the diversity of epiphytic lichens and bryophytes on Z. abelicea? 2) Do Z. abelicea trees host specific epiphyte taxa that are found nowhere else? 3) What is their distribution on Z. abelicea individuals throughout Crete? 4) Which environmental factors might influence the observed epiphytic diversity and community composition? 5) Does one site or another stand out in terms of ecological indicator values?
Methods
Specimen and data collection
Specimens were collected in autumn 2018 and spring 2019 from eight study sites, covering the whole distribution range of Zelkova abelicea on Crete (Fig. 1). Three study sites were located in the Levka Ori (Omalos, Niato and Impros), one on Mt Kedros (Gerakari), one on Mt Psiloritis (Rouvas), two in the Dikti Mountains (Viannou and Katharo), and one in the Thripti Mountains (Thripti). In each site, two to seven individuals (dwarfed or arborescent; Fig. 2A & B) of Z. abelicea were sampled, giving a total of 36 individuals. In Niato and Thripti, sampling was carried out only on dwarfed trees as no arborescent individuals were present. On non-dwarfed trees, the top layer of bark (Fig. 2C) hosting epiphytes was cut off with a knife, without harming the vital, living parts of the tree trunk. In dwarfed individuals, whole twigs covered with epiphytes were cut off with a knife (Fig. 2D). The collected material was placed in paper bags and kept dry until identification.

Fig. 1. Location of the eight study sites (filled dots) distributed across all five Cretan mountain ranges that contain populations of Zelkova abelicea. Names in bold font indicate mountain ranges with stands of Z. abelicea; summits (m) are indicated with an ‘X’. The names of the study sites are given in italic font. In colour online.

Fig. 2. A, forest fragment with large Zelkova abelicea trees (Omalos). B, dwarfed, heavily browsed individuals (Thripti Mts). C, trunk of a large tree with exfoliating bark (Dikti Mts). D, branches of a heavily browsed individual (Mt Kedros). Examples of different lichen growth forms: E, Lecidella elaeochroma (crustose). F, Xanthoria parietina (foliose). G, Ramalina fraxinea (fruticose). Photographs: G. Kozlowski (A–C), H-R. Siegel (D), W. Fałtynowicz (E–G).
In each of the study sites, the following general information was also collected: geographical coordinates (latitude and longitude), altitude, topography (slope or doline floor) and browsing intensity (Table 1).
Table 1. Environmental characteristics of the study sites in Crete where epiphytic material of Zelkova abelicea was sampled. Temp. = mean annual temperature; Prec. = average sum of annual rainfall (gridded climatic data was extracted for the period 1970–2000 from WorldClim, www.worldclim.com/version2). Browsing intensity = + moderate, ++ strong.

Gridded climatic data (i.e. annual mean temperature and sum of annual precipitation) for the period 1970–2000 were extracted from WorldClim (www.worldclim.com/version2) at a 30 s (i.e. c. 1 km2) resolution (Fick & Hijmans Reference Fick and Hijmans2017) for each study site (Table 1).
Species identification
Epiphytic material was determined using standard stereoscopic and light microscopy. The taxonomic identity of the sampled material was assessed using identification keys. Lichens were determined using keys provided by Clauzade & Roux (Reference Clauzade and Roux1985), Smith et al. (Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) and Arcadia (Reference Arcadia2022). In a small number of necessary cases, thin-layer chromatography was used. Nomenclature of lichen species followed Index Fungorum (Index Fungorum Partnership 2022). Information on the morphological type of thallus (Cr – crustose, Fo – foliose, Fr – fruticose; Fig. 2E–G) was obtained from Arcadia (Reference Arcadia2022).
Bryophytes were determined using the keys provided by Nyholm (Reference Nyholm1965, Reference Nyholm1998), Smith (Reference Smith1978) and Ros-Espin et al. (Reference Ros-Espin, Mazimpaka, Abou-Salama, Aleffi, Blockeel, Brugués, Cros, Dia, Dirkse and Draper2013). Nomenclature of bryophytes followed Ros-Espin et al. (Reference Ros-Espin, Mazimpaka, Abou-Salama, Aleffi, Blockeel, Brugués, Cros, Dia, Dirkse and Draper2013). For each bryophyte taxon, the morphological type (P – pleurocarpous, A – acrocarpous) was obtained from Düll (Reference Düll1979) and Preston (Reference Preston1984).
Authorities for cited lichen and bryophyte species are given in Tables 2 & 3. The analyzed material was deposited in the collections of the University of Wrocław, Poland (lichens) and in the Natural History Collections of Adam Mickiewicz University in Poznań, Poland (bryophytes).
Table 2. List of the epiphytic lichens recorded on Zelkova abelicea in Crete showing the taxonomy, morphological type and occurrence in the study sites. The nomenclature follows Index Fungorum (Index Fungorum Partnership 2022) while the morphological type of thallus (Morph.) follows Arcadia (Reference Arcadia2022) (i.e. Cr – crustose, Fo – foliose, Fr – fruticose). Study sites (Site) are listed following a longitudinal gradient from the west to the east of Crete: O – Omalos, N – Niato, I – Impros, G – Gerakari, R – Rouvas, V – Viannou, K – Katharo, T – Thripti. Number of trees sampled per site (n) is also given. Species recorded for the first time in Greece are in bold, and those recorded for the first time in Crete are marked with *. (*) = species possibly recorded for the first time in Crete but treat with caution due to the uncertainty of their determination or the possible misidentification of previous Cretan records.
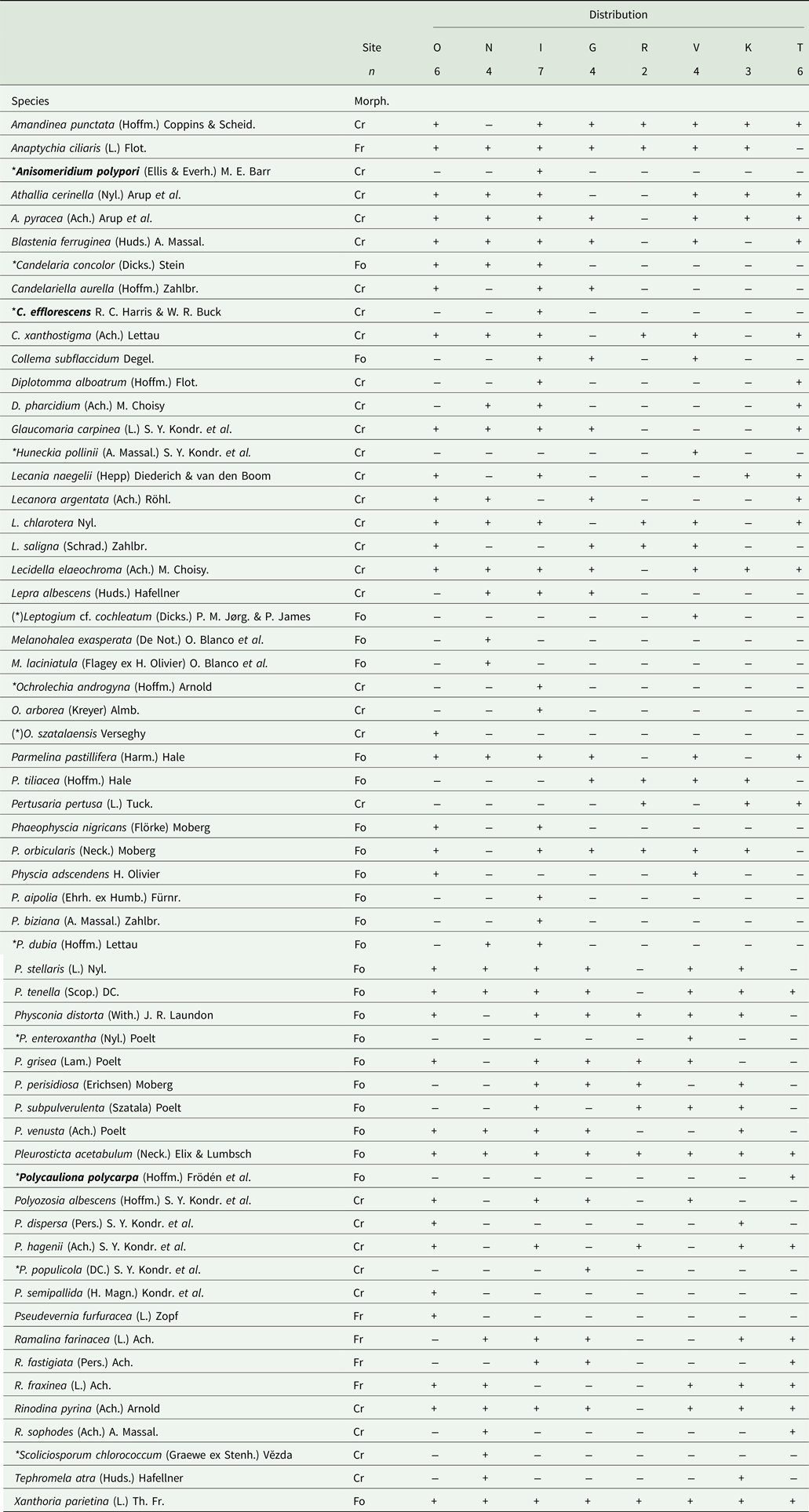
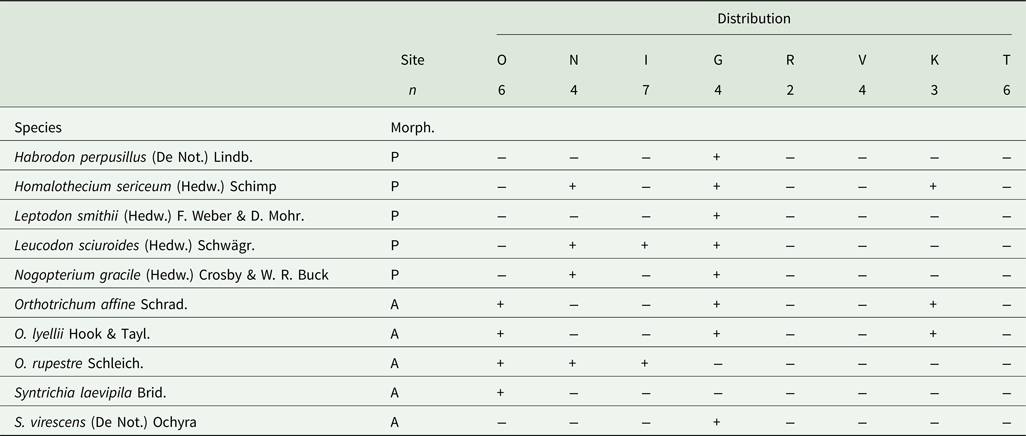
Table 3. List of epiphytic bryophyte species recorded on Zelkova abelicea with their distribution at sample sites in Crete. The nomenclature follows Ros-Espin et al. (Reference Ros-Espin, Mazimpaka, Abou-Salama, Aleffi, Blockeel, Brugués, Cros, Dia, Dirkse and Draper2013) and the morphology follows Preston (Reference Preston1984) and Düll (Reference Düll1979). Morph. = morphological type; P – pleurocarpous, A – acrocarpous. Study sites (Site) are listed following a longitudinal gradient from the west to the east of Crete: O – Omalos, N – Niato, I – Impros, G – Gerakari, R – Rouvas, V – Viannou, K – Katharo, T – Thripti. Number of trees sampled per site (n) is also given.
Statistical analyses and selection of environmental variables
Statistical analyses were performed using R (R Core Team 2020). A Kruskal-Wallis rank sum test (Hollander & Wolfe Reference Hollander and Wolfe1973) was carried out in order to determine if species numbers were significantly different between trees from different sites, as well as between mountain ranges.
The ordination method, distance-based redundancy analysis (db-RDA), was used to analyze and compare epiphytic communities (Legendre & Legendre Reference Legendre and Legendre2012; Oksanen Reference Oksanen2012, Reference Oksanen2015). This was performed in R with ‘capscale’ (package vegan; Legendre & Anderson Reference Legendre and Anderson1999; Anderson & Willis Reference Anderson and Willis2003). Since community data were of the type presence-absence (1 or 0), the Jaccard dissimilarity index (Real & Vargas Reference Real and Vargas1996) was selected to quantify the distance between communities. Constrained methods display the variation in the data of the environmental variables and are useful to test hypotheses and discover trends. In addition, permutation tests for the significance of constraints were carried out using ‘anova.cca’ (package vegan; Legendre et al. Reference Legendre, Oksanen and ter Braak2011; Legendre & Legendre Reference Legendre and Legendre2012) with 9999 permutations. The environmental variables were standardized prior to performing the analyses. One tree from Gerakari was excluded from the final analysis because it was very different in terms of community composition to all other sampled trees since it had only four bryophyte species and no lichen species (probably due to a sampling bias) and influenced the analysis too strongly when included.
Spearman's rank correlation (Hollander & Wolfe Reference Hollander and Wolfe1973) was computed between environmental variables to check for codependent variables and exclude highly correlated (Spearman's rho > 0.7) variables that could negatively influence the reliability of the results of the distance-based redundancy analysis (Borcard et al. Reference Borcard, Gillet and Legendre2011). Correlation coefficients for all considered variables are found in Supplementary Material Table S1 (available online). Latitude was highly negatively correlated with longitude (P < 0.001, Spearman's rho = −0.93). Due to the fact that the latitudinal amplitude of the study sites was very low compared to their longitudinal amplitude (0.25° vs 1.97°), latitude was excluded from further analyses. Gridded temperature and precipitation were also excluded from further analyses due to their high correlation with longitude (P < 0.001, Spearman's rho = 0.88) and altitude (P < 0.001, Spearman's rho = 0.97), respectively, and the potential unsuitability and/or unreliability of gridded data in representing localized climatic events in the Cretan mountains due to the absence of weather stations from which to interpolate (Goedecke & Bergmeier Reference Goedecke and Bergmeier2018; Fazan et al. Reference Fazan, Remoundou, Dhosn, Nikoli, Pasta, Garfì and Kozlowski2022).
Ecological indicator values
For every lichen species, the following ecological indicator values (EIVs) were obtained from Nimis (Reference Nimis2016) and Nimis & Martellos (Reference Nimis and Martellos2021): pH of substratum, solar irradiation, aridity (i.e. air humidity), eutrophication and poleotolerance (i.e. tolerance to human disturbance). The same was achieved for mosses using the dataset of Düll (Reference Düll, Ellenberg, Weber, Düll, Wirth, Werner and Paulissen1991) for the following EIVs: light, temperature, continentality, humidity and pH of substratum.
EIVs help to provide useful insights into the ecological niche of single species and help to evaluate the habitat quality of species assemblages (Nimis & Martellos Reference Nimis and Martellos2001). For lichens, the values are based on ecological responses of lichens throughout their distributional range in Italy. For mosses, the EIVs focus on Central Europe and more precisely on some areas of Germany. We are aware that the ecological requirements of lichens as well as mosses occurring throughout Greece may differ. However, since no EIVs have yet been developed specifically for Greek lichens and mosses, the datasets of Düll (Reference Düll, Ellenberg, Weber, Düll, Wirth, Werner and Paulissen1991), Nimis (Reference Nimis2016) and Nimis & Martellos (Reference Nimis and Martellos2021) appear to be the best currently available. Furthermore, Christensen (Reference Christensen2014) argues that despite these shortcomings, the Italian dataset of ecological indicators for lichens can be applied to Greece. For lichen species that had more than one given value per indicator due to their broad ecological spectrum, the average value was computed. Values were then averaged by sampled tree and Kruskal-Wallis rank sum tests (Hollander & Wolfe Reference Hollander and Wolfe1973) were carried out for each EIV in order to see if there were significant differences between study sites.
Results
Epiphytic diversity and distribution
Overall, 70 epiphytic species were recorded on Zelkova abelicea: 60 lichen species belonging to 21 genera and 10 bryophyte species belonging to eight genera (Fig. 3, Tables 2 & 3). Four lichen taxa common in Greece and Europe were the most abundant: Pleurosticta acetabulum (found on 33 trees), Xanthoria parietina (30 trees), Physcia tenella (27 trees) and Lecidella elaeochroma (26 trees). All other species were recorded on 20 or fewer trees. Eighteen species occurred only once. For bryophytes, only moss species were found, and the most abundant mosses were Leucodon sciuroides (10 trees) and Orthotrichum affine (7 trees). The highest epiphytic diversity was found in Impros (42 spp.) while the lowest was found in Psiloritis (15 spp.; Fig. 4). Differences in epiphytic diversity per tree were non-significant among sites and mountains (χ2 = 7.1869, df = 7, P = 0.4097 and χ2 = 5.898, df = 4, P = 0.2069, respectively).

Fig. 3. Frequency of occurrence (%) of epiphytic lichens (dark grey) and bryophytes (white) growing on the investigated Zelkova abelicea trees on Crete.

Fig. 4. Diversity of epiphytic lichen (dark grey) and bryophyte (white) species of sampled Zelkova abelicea trees for each of the eight study sites on Crete. n = number of trees sampled per site.
Ten lichen species (i.e. 17%, namely Anisomeridium polypori, Candelaria concolor, Candelariella efflorescens, Huneckia pollinii, Ochrolechia androgyna, Physcia dubia, Physconia enteroxantha, Polycauliona polycarpa, Polyozosia populicola and Scoliciosporum chlorococcum were previously unrecorded for Crete. Three of these (i.e. 5%, namely Anisomeridium polypori, Candelariella efflorescens and Polycauliona polycarpa) were also previously unpublished for Greece and were found in our study on two different trees in Impros and on a single tree in Thripti (Table 2). All the sampled bryophyte species have been previously recorded for Crete or Greece.
All but one of the sampled trees hosted lichens, with variable species numbers (6–20 spp., with an average of 13 lichen spp. per tree; Fig. 5A). Trees with the highest diversity of lichen species were located in the three sites of the Levka Ori, as well as in Kedros. Trees from Psiloritis, Dikti and Thripti had lower species numbers, while one tree from Gerakari (Kedros) hosted no lichens. However, differences in number of lichens per tree between sites or mountain ranges were non-significant (χ2 = 4.4481, df = 7, P = 0.727 and χ2 = 3.6547, df = 4, P = 0.455, respectively). More lichen species were recorded in the Levka Ori sites compared to the other regions (Fig. 4). With 40 species, Impros had the highest lichen diversity, followed by Omalos (33 spp.), Niato, Kedros and Viannou (27 spp.), Thripti (24 spp.), Katharo (23 spp.) and Rouvas (15 spp.) (Table 4). Overall, 53 spp. of lichen were recorded in Levka Ori compared to 35 in Dikti, 27 in Kedros, 24 in Thripti and 15 in Psiloritis (Fig. 4, Table 2). However, differences in lichen diversity between sites or mountains were not significant (χ2 = 7, df = 7, P = 0.4289 and χ2 = 53958, df = 4, P = 0.249, respectively).

Fig. 5. Boxplots of the number of lichen (A) and bryophyte (B) species found on Zelkova abelicea trees in each of the eight study sites on Crete. n = number of trees per site. Grey dots represent individual trees. The midlines of the boxplots show the median, the boxes show the 1st and 3rd quartiles and the whiskers extend up to 1.5 times the interquartile range.
Table 4. Proportion in percentage of lichens and bryophytes recorded on Cretan Zelkova abelicea trees per site. The number of epiphytes per site is given in brackets. Thallus morph. = proportion of lichens based on the morphological form of their thallus (Cr – crustose, Fo – foliose, Fr – fruticose). Morph. = proportion of bryophytes based on their morphology (P – pleurocarpous, A – acrocarpous).

Bryophytes were found only on 16 out of 36 (i.e. 44%) sampled trees, and the number of species per tree varied from 1–4 with an average of 2.25 spp. (Fig. 5B). The number of bryophytes per tree was significantly different among sites, as well as among mountain ranges (χ2 = 16.166, df = 7, P = 0.02364 and χ2 = 13.99, df = 4, P = 0.007328, respectively). Bryophytes were most abundant at Gerakari on Mt Kedros, where 8 spp. were counted, followed by Omalos and Niato (both 4 spp.), Katharo (3 spp.) and Impros (2 spp.); no bryophytes at all were recorded on trees at Rouvas, Viannou and Thripti (Fig. 4, Table 4). Total bryophyte diversity was not significantly different among sites or mountain ranges (χ2 = 7, df = 7, P = 0.4289 and χ2 = 5.4641, df = 4, P = 0.2429, respectively).
The most frequently recorded lichen thallus morphology (i.e. 52%, 31 spp.) was crustose, while 40% (24 spp.) of lichens had a foliose and only 8% (5 spp.) a fruticose thallus morphology (Table 4). Half of the 10 most abundant lichen species were foliose, one was fruticose while the remaining four were crustose. Crustose lichens dominated in Omalos, Niato, Impros and Thripti, foliose lichens dominated in Rouvas and Viannou, and both co-occurred in Gerakari and Katharo. Fruticose lichens were always in the minority (Table 4). Half of the recorded bryophyte species were acrocarpous, the other half were pleurocarpous but with local disparities. A majority of pleurocarpous species were found in Niato and Gerakari, while acrocarpous species dominated in Katharo and no pleurocarpous species were found in Omalos. In Impros, both co-occurred (Table 4).
Influence of environmental variables on the epiphytic communities of Zelkova abelicea
The permutation tests for the distance-based redundancy analysis (see Supplementary Material Table S2, available online) showed that among the selected environmental variables, longitude, topography and browsing intensity were significant (P < 0.05) with regard to epiphytic composition, while altitude was not significant (P = 0.21) and was thus excluded from further analyses. Figure 6 shows the results of the distance-based redundancy analysis of epiphytic lichen and bryophyte communities on Z. abelicea. Several clusters of trees stand out. A first group includes all trees from Thripti. A second group includes all trees from Niato. A third group consists of five trees from Impros and one tree from Gerakari. A fourth group is composed of all individuals from Rouvas and one tree each from Viannou and Katharo. The remaining trees, and all those from Omalos, are grouped between these four clusters.

Fig. 6. Ordination plot of the distance-based redundancy analysis of epiphytic lichen and bryophyte communities on Zelkova abelicea trees on Crete. Each symbol represents the community found on a single Z. abelicea tree. Each mountain range is represented by a different shape (![]() Levka Ori,
Levka Ori, ![]() Mt Kedros,
Mt Kedros, ![]() Mt Psiloritis,
Mt Psiloritis, ![]() Dikti Mts,
Dikti Mts, ![]() Thripti Mts) and each study site by a different colour. Significant environmental variables are fitted (represented by arrows). Arrow lengths are proportional to the significance of the variables in the permutation test.
Thripti Mts) and each study site by a different colour. Significant environmental variables are fitted (represented by arrows). Arrow lengths are proportional to the significance of the variables in the permutation test.
Ecological indicator values
EIVs of lichens for the eight study sites are shown in Fig. 7 and Supplementary Material Table S3 (available online). Significant, or close to significant (P < 0.1), differences among study sites exist for the following indicators: pH of substratum (χ2 = 18.971, df = 7, P = 0.008), aridity (χ2 = 12.7, df = 7, P = 0.08) and eutrophication (χ2 = 20.743, df = 7, P = 0.004).

Fig. 7. Ecological indicator values for lichens recorded on Zelkova abelicea trees at different study sites on Crete following Nimis (Reference Nimis2016) and Nimis & Martellos (Reference Nimis and Martellos2021). Detailed information is found in these publications and Supplementary Material Table S3 (available online). Only the observed values are described here. A, pH of substratum; 2 = acid substrata, 3 = subacid to subneutral substrata, 4 = slightly basic substrata. B, solar irradiation; 3 = in sites with plenty of diffuse light but scarce direct solar irradiation, 4 = in sun-exposed sites without extreme solar irradiation, 5 = in sites with very high direct solar irradiation. C, aridity (air humidity); 2 = rather hygrophytic, intermediate between 1 and 3, 3 = mesophytic, 4 = xerophytic but absent from extremely arid stands. D, eutrophication (including deposition of dust and nitrogen compounds); 2 = resistant to very weak eutrophication, 3 = resistant to weak eutrophication, 4 = occurring in rather highly eutrophicated situations. E, poleotolerance (i.e. tolerance to human disturbance); 1 = species occurring in natural or semi-natural habitats, 2 = species occurring in moderately disturbed areas (e.g. agricultural areas, small settlements, etc.). The midlines of the boxplots show the median, the boxes show the 1st and 3rd quartiles and the whiskers extend up to 1.5 times the interquartile range while values exceeding this threshold are plotted as open circles.
The lichen biota living on Z. abelicea showed a wide range of bark pH preferences (Fig. 7A), with species tolerating very acid substrata (value 1) to species preferring basic substrata (value 5), although the most frequently distributed lichen biota showed preferences for acid to slightly basic bark conditions (values 2–4). No species linked to very shaded conditions (value 1) were found, and only one species restricted exclusively to shaded sites (value 2; Anisomeridium polypori) was found on one tree from Impros (Fig. 7B). All other species are light demanding species and occur in sites with diffuse light (value 3), sun exposed sites (value 4) or with very high direct solar irradiation (value 5). The majority and most frequent species are mesophytic to xerophytic species in terms of air humidity (Fig. 7C). Only two species are indicators of relatively hygrophytic conditions (value 1; Ramalina farinacea and Tephromela atra), while three species are tolerant to very arid conditions (values 4 or 5 only; Diplotomma alboatrum, Diplotomma pharcidium and Polyozosia dispersa). The most frequently occurring lichens were adapted to weak to high eutrophication (values 2–4; Fig. 7D). Only two species are strict indicators of no eutrophication (value 1; Ochrolechia androgyna and Ochrolechia szatalaensis), found only on three trees from Impros and one from Omalos, while six species are strict indicators of high eutrophication (values 4 or 5 only; Candelariella efflorescens, Phaeophyscia nigricans, P. orbicularis, Physcia dubia, Physconia grisea and Polyozosia semipallida). The most frequently recorded lichens have a wide poleotolerance scale (values 1–3) and tolerate anthropogenic disturbance (Fig. 7E). Species indicating low or null human disturbance were often found only on single trees. Only one species found on one tree in Viannou is indicative of old trees growing in ancient, undisturbed forest stands (value 0; Leptogium cf. cochleatum), while 10 species are strict indicators of natural or semi-natural habitats with low disturbance (value 1; Anisomeridium polypore, Candelariella efflorescens, Huneckia pollinii, Melanohalea laciniatula, Ochrolechia androgyna, O. szatalaensis, Physconia venusta, Polyozosia populicola, P. semipallida and Ramalina fraxinea).
EIVs for mosses for the five study sites in which mosses are present are shown in Fig. 8 and Supplementary Material Table S4 (available online). None of the differences among study sites were significant for the different EIVs. The moss species present on Z. abelicea are mainly light-tolerant species (value 8), although one half-shade species (value 5; Nogopterium gracile) and two intermediate (values 6 & 7; Habrodon perpusillus and Orthotrichum lyellii) were also present. Both Niato and Gerakari have, in addition to light-tolerant mosses, species that prefer more shaded conditions (values 5 & 6) and which were not found elsewhere. The temperature tolerance range of mosses growing on Z. abelicea was rather wide but most species were indicative of moderately warm conditions (values 4 & 5), with only two species indicative of rather cool conditions (values 2 & 3; Homalothecium sericeum and Orthotrichum rupestre) and two species tolerating hot to extremely hot temperatures (value 8; Habrodon perpusillus and Leptodon smithii). Gerakari was the only site in which species tolerating hot temperatures (value 8) were found, while also hosting at the same time cool and intermediate species. For continentality, all species show values between oceanic to subcontinental (values 3–5). However, species with value 3 were found only in Gerakari. Regarding humidity, species are indicative of arid conditions (values 2 & 3) to humid (values 4 & 5) but not wet conditions. The species with the highest value (5; Habrodon perpusillus) was found only in Gerakari, but this latter site also hosted the full range of values. As for pH of substratum, species are indicative of moderately acidic substrata (value 5) to weakly acidic to weakly basic (value 7) substrata.

Fig. 8. Ecological indicator values for mosses recorded on Zelkova abelicea trees at different study sites on Crete following Düll (Reference Düll, Ellenberg, Weber, Düll, Wirth, Werner and Paulissen1991). Detailed information can be found in that publication and Supplementary Material Table S4 (available online). Only the observed values are described here. A, light; 5 = half-shade, 6 = between 5 and 7, 7 = half-light, 8 = light. B, temperature; 1 = cold, 2 = between 1 and 3, 3 = cool, 4 = between 3 and 5, 5 = moderately warm, 6 = between 5 and 7, 7 = warm. C, continentality; 2 = oceanic, 3 = between 2 and 4, 4 = suboceanic, 5 = intermediate. D, humidity; 1 = strongly arid, 2 = between 1 and 3, 3 = arid, 4 = between 3 and 5, 5 = humid. E, pH of substratum; 5 = moderately acidic, 6 = between 5 and 7, 7 = weakly acidic to weakly basic. The midlines of the boxplots show the median, the boxes show the 1st and 3rd quartiles and the whiskers extend up to 1.5 times the interquartile range while values exceeding this threshold are plotted as open circles.
Discussion
Diversity and distribution of epiphytic lichens and bryophytes
The diversity of epiphytic lichens and bryophytes growing on Zelkova abelicea was investigated in this study for the first time, and over the whole distribution range of the phorophyte tree species.
Our study revealed that the diversity of epiphytic lichens and bryophytes growing on Z. abelicea was rather high, with a total of 70 species recorded (60 lichen and 10 bryophyte species). Individual records included up to 20 lichen and four bryophyte species per tree. All previous studies focusing on or including epiphytic lichens or bryophytes of other Cretan phorophytes reported lower species numbers (e.g. Gradstein Reference Gradstein1971; Spribille et al. Reference Spribille, Schultz, Breuss and Bergmeier2006; Christensen Reference Christensen2007, Reference Christensen2014; Vondrák et al. Reference Vondrák, Guttová and Mayrhofer2008). However, higher or equivalent species counts are known from several phorophytes in other areas of the Mediterranean (e.g. Zedda & Sipman Reference Zedda and Sipman2001; Aragón et al. Reference Aragón, Sarrión and Martínez2004). This suggests that insufficient attention has been given to Cretan epiphytes and that probably more species are still to be recorded after further in-depth sampling. Indeed, the discovery of 10 lichen species previously unrecorded for Crete, of which three are also new for the whole Greek territory, shows how little is known about the epiphytic biodiversity of phorophytes on Crete, as already emphasized by Christensen (Reference Christensen2007, Reference Christensen2014). However, the present study did not reveal any epiphyte taxa exclusively restricted to Z. abelicea and most of the epiphytes recorded in this study are also encountered on other phorophyte species in Greece or the Mediterranean (Ros-Espin et al. Reference Ros-Espin, Mazimpaka, Abou-Salama, Aleffi, Blockeel, Brugués, Cros, Dia, Dirkse and Draper2013; Arcadia Reference Arcadia2022).
The three newly reported species for Greece (Anisomeridium polypori, Candelariella efflorescens and Polycauliona polycarpa) are all species that are found in other nearby countries of the Mediterranean (see e.g. Yazici & Aptroot Reference Yazici and Aptroot2008; Bilovitz et al. Reference Bilovitz, Stešević and Mayrhofer2010; Yavuz & Çobanoğlu Reference Yavuz and Çobanoğlu2018; John et al. Reference John, Güvenç and Türk2020; Nimis & Martellos Reference Nimis and Martellos2021), and therefore their presence in Greece is not surprising. Two other potentially new species for Crete, Leptogium cf. cochleatum and Ochrolechia szatalaensis, were recorded. The identity of the former is uncertain, whereas O. szatalaensis was considered by Kukwa (Reference Kukwa2011) to be a synonym of O. macrospora Vers., a species that was previously recorded from Crete by Christensen & Svane (Reference Christensen and Svane2007). However, their specimen was reported as having large spores (68–100 μm) whereas the specimen we examined had spores that were smaller, less than 60 μm, which is typical for O. szatalaensis. The chemical reactions of our specimen were consistent with the diagnosis of O. szatalaensis reported by Kukwa (Reference Kukwa2011). Therefore, we believe that the O. macrospora specimen of Christensen & Svane (Reference Christensen and Svane2007) may belong to another taxon, and that our specimen is the first record of O. szatalaensis for Crete, but further investigations are needed to clarify this.
Lichen and bryophyte richness is influenced by a multitude of factors which are often difficult to disentangle, such as precipitation, temperature, light, air humidity, water availability, substratum characteristics, land-use and landscape history, stand structure and size, phorophyte species and surrounding vegetation (Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2009; Pinho et al. Reference Pinho, Bergamini, Carvalho, Branquinho, Stofer, Scheidegger and Máguas2012; Aranda et al. Reference Aranda, Gabriel, Borges, Santos, de Azevedo, Patiño, Hortal and Lobo2014; Medina et al. Reference Medina, Albertos, Lara, Mazimpaka, Garilleti, Draper and Hortal2014; Cardós et al. Reference Cardós, Martínez, Calvo and Aragón2016; Henriques et al. Reference Henriques, Borges, Ah-Peng and Gabriel2016). Extensive pasturelands have been found to have high lichen biodiversity because of the simultaneous presence of sensitive species which would disappear in more eutrophicated sites, and of nitrophytic species associated with locally higher atmospheric ammonia due to the activity of grazing animals (Śliwa Reference Śliwa and Godzik2006; Pinho et al. Reference Pinho, Bergamini, Carvalho, Branquinho, Stofer, Scheidegger and Máguas2012). Tree age is known to be an important factor in sustaining lichen and bryophyte biodiversity since epiphytes have had more time to establish on older trees and/or because of age-dependent changes in bark qualities (Johansson et al. Reference Johansson, Rydin and Thor2007; Ranius et al. Reference Ranius, Johansson, Berg and Niklasson2008; Fritz et al. Reference Fritz, Brunet and Caldiz2009; Lie et al. Reference Lie, Arup, Grytnes and Ohlson2009; Nascimbene et al. Reference Nascimbene, Marini, Motta and Nimis2009; Király et al. Reference Király, Nascimbene, Tinya and Ódor2013). A study of the demographic structure of Z. abelicea populations showed that not only arborescent trees but also dwarfed individuals can be several centuries old (Fazan et al. Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012), and thus dwarfed individuals may act as important, but often overlooked, phorophytes.
There were also spatial patterns of richness. Zelkova abelicea of the Levka Ori mountain range show the highest diversity of epiphytes since 53 out of 60 species of lichen and 7 out of 10 species of bryophyte were found there. However, this may be expected since the highest number of trees (17 trees, i.e. 47% of the total), including both arborescent and dwarfed individuals, was sampled there. This mountain range (with its suitable climatic conditions; Goedecke & Bergmeier Reference Goedecke and Bergmeier2018) hosts the most developed and abundant number of Z. abelicea stands (Kozlowski et al. Reference Kozlowski, Frey, Fazan, Egli, Bétrisey, Gratzfeld, Garfì and Pirintsos2014) and Cardós et al. (Reference Cardós, Martínez, Calvo and Aragón2016) has found that well-developed and large tree stands tend to have higher lichen and bryophyte diversity than small, fragmented or isolated tree patches. Furthermore, in the Levka Ori, a higher lichen diversity than elsewhere was found on individual trees, with up to 20 species per tree (Fig. 5). There are no previous comparisons of epiphytic flora between mountain ranges of Crete. However, Christensen (Reference Christensen2014) found a higher diversity of lichens on Platanus orientalis L. in western Crete compared to trees situated in central Crete, which he attributed to the higher precipitation occurring in western Crete compared to more eastern sites, and in some cases also differences in land-use practices. The Levka Ori is the highest rainfall area of the island (Varouchakis et al. Reference Varouchakis, Corzo, Karatzas and Kotsopoulou2018; Agou et al. Reference Agou, Varouchakis and Hristopulos2019) and an increased lichen diversity due to a positive correlation with precipitation has also been noticed by other researchers (e.g. Giordani Reference Giordani2006; Svoboda et al. Reference Svoboda, Peksa and Veselá2010).
With regard to the bryophyte flora, Gerakari on Mt Kedros appears to be the most suitable place in our study and contained by far the highest diversity of bryophytes, with eight out of 10 species recorded there, as well as the highest number of bryophytes per tree. On Mt Kedros, the sampling site is located in an open forest on a steep and shaded north-facing slope at the foot of a cliff. This site has a relatively high precipitation and high potential run-off or percolation (Goedecke & Bergmeier Reference Goedecke and Bergmeier2018) and had the lowest heat load value (following McCune & Keon Reference McCune and Keon2002) of all analyzed Z. abelicea sites, conditions that seem to be favourable to the development of a rich moss community.
The lowest epiphytic diversity (only 15 species of lichen and no bryophytes) was found at Rouvas on Mt Psiloritis, although this is also the site in which the lowest number of trees (n = 2) was sampled. Some areas of Mt Psiloritis have been previously found to have a low number of endemic vascular species due to a supposedly stronger human impact on the vegetation there than elsewhere (Legakis & Kypriotakis Reference Legakis and Kypriotakis1994). This strong anthropogenic impact coupled with locally adverse microclimatic conditions could account for the low epiphytic diversity recorded for this area.
Only mosses and no liverworts were found during our study. This almost certainly reflects the more pronounced drought intolerance of most epiphytic liverworts (Bischler Reference Bischler2004). Three of the four most frequently recorded bryophyte species have a widespread distribution in temperate Europe (Düll Reference Düll1984, Reference Düll1985). The largest areas on the trunks of Z. abelicea were occupied by Leucodon sciuroides which was also the most frequently recorded species. Several of the less frequently recorded bryophytes are oceanic or sub-oceanic species (Düll Reference Düll1984, Reference Düll1985) and occur throughout the Mediterranean basin (Ros-Espin et al. Reference Ros-Espin, Mazimpaka, Abou-Salama, Aleffi, Blockeel, Brugués, Cros, Dia, Dirkse and Draper2013). The majority of bryophytes growing on the trunk of Z. abelicea trees are light-loving species (Düll Reference Düll, Ellenberg, Weber, Düll, Wirth, Werner and Paulissen1991) and are not found in densely forested areas.
Bryophytes did not occur at all in three sites (Rouvas, Viannou, Thripti), all situated in central or eastern Crete, whereas all sites in western Crete recorded bryophytes. With the exception of Katharo, all sampled trees east of Mt Kedros hosted no bryophytes. As suggested for the lichen flora, Gradstein (Reference Gradstein1971) evokes the west-east decreasing gradient in precipitation as a major factor influencing the distribution of bryophytes in Crete, although in his study some bryophytes were found uniquely in the central or eastern Cretan mountains. This explanation seems to be verified by our study. It is possible that the three sites without epiphytic bryophytes present environmental conditions that are not suitable for their growth, although further field investigations and more in-depth sampling should be undertaken to confirm this finding. Katharo stands out amongst the other eastern sites because the sampled trees were located on the border of a cultivated plateau, and thus epiphytic bryophytes there could possibly benefit from agricultural activities or moisture due to irrigation.
Factors influencing the species composition of epiphytic communities on Zelkova abelicea
The permutation tests for the db-RDA showed that longitude, topography and browsing pressure were significant in differentiating epiphytic communities whereas altitude was not. The db-RDA showed that the three sites of Impros, Niato and Thripti were clearly distinguished in terms of composition of epiphytic community. Moreover, Rouvas with the addition of one tree from Viannou and one from Katharo were also differentiated.
Altitude was not significant in influencing species composition of the epiphytic community, despite the fact that it is known, alongside precipitation, to be a major factor influencing species composition in the Mediterranean (see e.g. Loppi et al. Reference Loppi, Pirintsos and De1997; Mucina et al. Reference Mucina, Valachovic, Dimopoulos, Tuibsch and Pisut2000; Matos et al. Reference Matos, Pinho, Aragón, Martínez, Nunes, Soares and Branquinho2014; Medina et al. Reference Medina, Albertos, Lara, Mazimpaka, Garilleti, Draper and Hortal2014; Vieira et al. Reference Vieira, Aguiar, Portela, Monteiro, Raven, Holmes, Cambra, Flor-Arnau, Chauvin and Loriot2016; Sevgi et al. Reference Sevgi, Yılmaz, Çobanoğlu Özyiğitoğlu, Tecimen and Sevgi2019). This is probably because all investigated sites were situated within a narrow altitudinal range (i.e. only 170 m between the highest and lowest sites). Furthermore, longitude, or geographical position on Crete, is highly reflective of precipitation patterns, with a general west to east decreasing trend (Varouchakis et al. Reference Varouchakis, Corzo, Karatzas and Kotsopoulou2018; Agou et al. Reference Agou, Varouchakis and Hristopulos2019), although small-scale climatic conditions such as orographic effects, cloud and dew accumulation and snow cover (see Goedecke & Bergmeier Reference Goedecke and Bergmeier2018) also probably play an important role. Niato and Impros have completely different precipitation patterns despite being geographically close (Fig. 1). Niato is located on the windward side of the Levka Ori, in a doline surrounded by mountains where pockets of fog may persist and in an area which receives abundant levels of rainfall. Impros is on the dry leeward southern side of the same mountain range, on a slope overlooking the Libyan Sea. The importance of nocturnal dew or humidity rising from the sea is unknown for all sites and might be an important and overlooked factor that explains the compositional differences between these nearby sites. Indeed, the lichen community of Impros, although showing a wide range of EIVs, includes several lichen taxa that are highly sensitive to air humidity and solar irradiation (Fig. 7 and see below).
Local effects such as topography (i.e. slope or doline) may also play an important role in community composition. Niato and Thripti are the only two sampling sites situated on a flat mountain doline and not on a slope. However, coincidentally, these two sites were the only two places visited during the study where no arborescent trees were found, and thus only dwarfed shrubby Z. abelicea individuals were sampled. As a result, we cannot disentangle the influence of topography from that of tree morphology here. However, dolines have different pedological conditions (e.g. deeper soils, different soil pH and nutrient content) than sloped areas (Egli Reference Egli1993) and dwarfed individuals have a different architecture than arborescent trees; the former may host epiphytic lichens and bryophytes found necessarily closer to the ground and living under different microclimatic and biotic (e.g. browsing) influences. Dwarfed trees or otherwise low-growing shrubs seem to have been often overlooked in previous studies. Here, neither Niato nor Thripti showed lower epiphytic species numbers compared to other sites where arborescent trees were sampled. This is particularly true for Niato where 31 lichen species and four bryophyte species were found, showing a relatively high overall diversity. These results underline the importance of dwarfed, overbrowsed individuals as refugia for epiphytic floras. This is consistent with the results of Spribille et al. (Reference Spribille, Schultz, Breuss and Bergmeier2006) who found species-rich epiphytic lichen communities on trees in overbrowsed and dwarfed communities, and of Grube et al. (Reference Grube, Lindblom and Mayrhofer2001) who state that thorny cushion plants provide interesting microhabitats for epiphytic lichens. Moreover, Pirintsos et al. (Reference Pirintsos, Loppi, Dalaka and De Dominicis1998) showed that lichen community composition in overbrowsed dwarfed shrublands was influenced mainly by shrub height and shrub density (i.e. gaps between shrubs) which influence microclimatic conditions for lichen growth, but also depended on the phorophyte species present. Furthermore, epiphytic lichen communities have been found to change depending on the height at which they grew on the trunk (e.g. Pirintsos et al. Reference Pirintsos, Diamantopoulos and Stamou1993; Asplund et al. Reference Asplund, Sandling, Kardol and Wardle2014). Epiphytic communities living on dwarfed Z. abelicea individuals may benefit from microclimatic conditions linked with this specific tree morphology and which are probably different to those of arborescent trees. Despite their small size, dwarfed Z. abelicea individuals were also found to be in some cases older than arborescent trees (Fazan et al. Reference Fazan, Stoffel, Frey, Pirintsos and Kozlowski2012), and old trees are known to have more developed and more species-rich epiphyte communities (Nascimbene et al. Reference Nascimbene, Marini and Nimis2010). This could be explained by the fact that they offer more diverse microhabitats (Nordén et al. Reference Nordén, Jordal and Evju2018), provide more time for the colonization and establishment of species-rich communities or possess different substratum qualities (Lie et al. Reference Lie, Arup, Grytnes and Ohlson2009). Nevertheless, our study contains a bias, since epiphytes were collected only from the trunk on arborescent trees and not from the canopy, while being collected from both the trunk and canopy of dwarfed individuals. Trunk and canopy epiphytic communities have been shown to be quite different in many cases (e.g. McCune et al. Reference McCune, Rosentreter, Ponzetti and Shaw2000; Ellis Reference Ellis2012; Maceda-Veiga & Gómez-Bolea Reference Maceda-Veiga and Gómez-Bolea2017), and therefore further sampling should be undertaken to assess if this could also be the case for Z. abelicea and whether this could lead to different results than those presented here.
Ecological indicator values
Since no database including Greece has been compiled so far, the EIVs used in this study were those compiled for Italy (lichens) and Central Europe (mosses). Some authors have successfully used EIVs for vascular plants outside of their original range (e.g. Körner et al. Reference Körner, Dupouey, Dambrine and Benoit1997). In addition, Christensen (Reference Christensen2014) argues that, at least for lichens, the Italian database can also be used in a Greek context. However, other authors (e.g. Godefroid & Dana Reference Godefroid and Dana2006) have shown differences in EIVs for vascular plants between Mediterranean countries, and this is important to keep in mind when interpreting the data presented in this paper.
Significant differences in lichen assemblages in terms of bark pH, air humidity and eutrophication were recorded among sites. These differences are most probably related to variations in intensity of pastoral activities. Indeed, an increased presence of browsing animals will trigger a rise in eutrophication levels through higher deposition of nitrogen which in turn will raise bark pH. Bark pH is known to be an important factor in determining epiphytic lichen composition (van Herk Reference van Herk2001) and will increase through the emission of ammonia (NH3) as a result of practices such as animal husbandry (Paoli et al. Reference Paoli, Pirintsos, Kotzabasis, Pisani, Navakoudis and Loppi2010), but also due to dust deposition and dry conditions (Loppi & De Dominicis Reference Loppi and De1996; Loppi et al. Reference Loppi, Pirintsos and De1997). However, bark pH also depends on phorophyte species, tree age, position of epiphytes on the tree and soil type (Kermit & Gauslaa Reference Kermit and Gauslaa2001). Paoli et al. (Reference Paoli, Pirintsos, Kotzabasis, Pisani, Navakoudis and Loppi2010) state that due to the intense and widespread livestock grazing that occurs throughout Crete, the whole island is affected to some extent by habitat eutrophication through the deposition of nitrogen, but it is clear that local disparities exist among sites. Furthermore, the presence of species tolerant to lower air humidity in some areas may be explained not only by more xeric growth conditions but also by the prevalence of nitrogen-tolerant species, since nitrogen-sensitive species are also often sensitive to air humidity (Hauck & Wirth Reference Hauck and Wirth2010).
Despite the effects of grazing and localized eutrophication, Pinho et al. (Reference Pinho, Bergamini, Carvalho, Branquinho, Stofer, Scheidegger and Máguas2012) found that extensive pasturelands could maintain high lichen species diversity due to the concomitant presence of nitrophilous and non-nitrophilous (sensitive) lichen species. However, the maintenance of the latter, albeit with a decrease in abundance, occurs only up to a certain degree of land use intensity, after which these species disappear. This phenomenon can be observed, for example, in Omalos where lichen assemblages signal the highest values of bark pH and aridity, rather high values of eutrophication and high values of poleotolerance. This all tends to point toward a strong influence of pastoral activities on the local lichen assemblages, with the predominance of eutrophication- and poleotolerant species. The values of aridity that are higher than elsewhere and the high proportion of nitrogen-tolerant species are probably linked in Omalos. However, the presence of species such as Ochrolechia szatalaensis, a strict indicator of no eutrophication, and of several species with low poleotolerance or low to medium aridity tolerance demonstrates that sensitive species can still be maintained locally, and possibly thrive in Cretan Z. abelicea stands.
No significant differences were found between sites when considering the EIVs of mosses, but most sites contained only a small number of species, from which it is difficult to extract conclusions. Nevertheless, compared to the other study sites, Gerakari showed a moss community that included, besides generalists, more shade-tolerant species, oceanic species and species tolerating less arid conditions. Although these features could be a sampling artefact since Gerakari also hosts the highest number of moss species (8 out of 10 species), it is probably reflective of local site conditions. Indeed, Gerakari also hosts lichen species indicative of higher air humidity in comparison with the rest of the study sites. These findings are probably due to the fact that the site is situated on a shaded north-facing slope at the foot of a cliff. Goedecke & Bergmeier (Reference Goedecke and Bergmeier2018) previously stated that the site had the lowest heat load value of all analyzed Z. abelicea sites.
The sites of Niato, Thripti and to a lesser extent Impros have some particularities in terms of lichen EIVs and confirm the db-RDA results (Fig. 6). Niato and Thripti show lower values of bark pH and eutrophication. In addition, Niato has a lichen assemblage that is more sensitive to air humidity and human disturbance than Thripti, although the latter also contains lichens that are very sensitive to human disturbance. At Impros, lichen EIVs point towards a wider range of tolerance to solar irradiation compared to all other sites. Both lichens preferring more shaded conditions and lichens tolerant to high solar irradiation are found there. These findings could reflect the dwarfed nature of some of the sampled trees (Niato and Thripti) but could also be an indicator of lower than expected pastoral activities, possibly resulting from the remoteness of the three sites.
Rouvas, on the contrary, seems to have lost its most sensitive lichens. The site is distinguished by the low number of lichen species (only 15 spp.) found there, an absence of bryophytes as well as its levels of poleotolerance that are higher than elsewhere, comparatively high eutrophication level and presence of lichens tolerating high air aridity levels. These results might be explained by a locally sparser forest cover, a more arid environment or, as suggested by Legakis & Kypriotakis (Reference Legakis and Kypriotakis1994), Lyrintzis (Reference Lyrintzis1996) and Hostert et al. (Reference Hostert, Röder, Hill, Udelhoven and Tsiourlis2003), could be indicative of a stronger disturbance than elsewhere due to locally intensive agropastoral practices or other human activities. In our study, the site of Rouvas seems to be the least favourable site for epiphytic lichens on Z. abelicea, although sampling more trees in Rouvas would be needed to confirm this finding.
Conclusions
The diversity and distribution of epiphytic lichens and bryophytes using Zelkova abelicea as a phorophyte were studied for the first time over the whole distribution range of this tree species. The rather high diversity of epiphytes recorded and the number of previously unrecorded species for Greece and Crete alike show how much is still unknown about epiphytes on Crete in general, but also about the epiphytic communities hosted by Z. abelicea. Differences in community composition and species diversity between sites was reflective of many differences in local conditions, across scales relating to climate, topography, land use, pastoral activities and tree morphology (dwarfed or arborescent). Our study also highlighted the importance of possibly very old, dwarfed trees as key hosts of specific epiphytic communities. Dwarfed trees were found to have different but equally rich communities as arborescent Z. abelicea trees. We were able to show that although some areas seem to experience a relatively strong influence of human activities, they nevertheless maintained a high diversity of species due to the co-occurring presence of both eutrophication-tolerant and -sensitive species. Our study paves the way for further and more in-depth research to explain the patterns observed.
Acknowledgements
We would like to thank H.-R. Siegel for permission to use his photograph in Fig. 2. We also wish to thank the anonymous reviewers who greatly helped to improve the quality of the previous versions of this paper. Study permits were granted by the Greek Ministry of Environment under permit nos 174101/5060 and 155924/1184.
Author Contributions
Conceptualization: D. Gwiazdowicz, G. Kozlowski and L. Fazan; plant material collection: D. Gwiazdowicz, D. Ghosn and H. Remoundou; data analyses: Y. Fragnière, G. Kozlowski, W. Fałtynowicz and L. Fazan; bryophyte identification: A. Rusińska and P Urbański; lichen identification: W. Fałtynowicz; writing, review and editing: L. Fazan, G. Kozlowski, D. Gwiazdowicz, G. Garfì, S. Pasta, W. Fałtynowicz, A. Rusińska. All authors have read and agreed to the published version of the manuscript.
Author ORCIDs
Laurence Fazan, 0000-0002-2981-1806; Yann Fragnière, 0000-0003-4167-379X; Dariusz J. Gwiazdowicz, 0000-0002-0064-2316; Wiesław Fałtynowicz, 0000-0003-3636-6218; Dany Ghosn, 0000-0003-1898-9681; Paweł Urbański, 0000-0002-5199-8021; Guiseppe Garfì, 0000-0003-0466-4288; Salvatore Pasta, 0000-0003-3265-9072; Gregor Kozlowski, 0000-0003-4856-2005.
Availability of Data and Material
The raw dataset is available online as Supplementary Material Table S5.
Funding
This research was partially funded by Fondation Franklinia.
Supplementary Material
To view Supplementary Material for this article, please visit https://doi.org/10.1017/S0024282922000159.














