Obesity and glucose intolerance are major risk factors for various diseases, such as cancer, depression, diabetes and CVD( Reference De Pergola and Silvestris 1 – Reference Golay and Ybarra 3 ). Excessive energy (food) intake is a critical cause of obesity. In response to every meal ingestion, various gut hormones are immediately released from enteroendocrine cells to regulate postprandial responses, including gut motility, pancreatic endocrine and exocrine secretion, and satiety induction( Reference Campbell and Drucker 4 , Reference Troke, Tan and Bloom 5 ). Since some gut hormones have anorexigenic and insulinotropic action, enteroendocrine hormone mimetics is thought to be a new therapy for obesity and/or diabetes( Reference Troke, Tan and Bloom 5 , Reference Irwin and Flatt 6 ).
Postprandial glycaemia is tightly regulated not only by insulin action but also by the gastric emptying rate( Reference Meier, Gallwitz and Salmen 7 ). Glucagon-like peptide-1 (GLP-1) has critical roles in maintaining postprandial glycaemia through its insulinotropic effect and gastric inhibitory effect( Reference Holst 8 ). Secretion of GLP-1 is stimulated by luminal nutrients, including glucose, fatty acids, proteins, protein hydrolysates and amino acids( Reference Rocca, LaGreca and Kalitsky 9 , Reference Higuchi, Hira and Yamada 10 ), indicating that postprandial GLP-1 release represents sensitivity to luminal nutrients in the gut. Because of these physiological functions of GLP-1, incretin-based therapy using GLP-1 receptor agonists or dipeptidyl peptidase-IV inhibitors is increasingly used for the treatment of diabetes( Reference Rathmann, Kostev and Gruenberger 11 , Reference Wysham, Grimm and Chen 12 ).
Although the insulinotropic effect of GLP-1 under a normal condition and improvement of glucose tolerance under a diabetic condition by GLP-1-based therapies are well recognised, changes (reduced, enhanced or unchanged) in nutrient-induced GLP-1 secretion in type 2 diabetic patients are still controversial( Reference Nauck, Vardarli and Deacon 13 – Reference Alssema, Rijkelijkhuizen and Holst 15 ). In a high-fat (HF) diet-induced obesity animal model, GLP-1 secretory response to glucose was decreased( Reference Gniuli, Calcagno and Dalla Libera 16 , Reference Anini and Brubaker 17 ), but the response to fatty acids remained unchanged( Reference Paulsen, Larsen and Hansen 18 ). However, it has not yet been characterised whether the GLP-1 secretory response to ‘meal’ is decreased or increased during the progression of diet-induced obesity. In the present study, rats were fed with a high-fat and high-sucrose (HF/HS) diet to induce obesity. To examine the physiological response to meal ingestion during the progression of obesity, a ‘normal diet’ was orally given to rats every week for the measurement of postprandial plasma glucose, insulin and GLP-1 levels using the meal tolerance test (MTT) rather than loading a glucose solution (oral glucose tolerance test).
Materials and methods
Animals
Male Sprague–Dawley rats (5 weeks old) were purchased from Japan SLC. The experiments were performed in a temperature-controlled room maintained at 23 ± 2°C with a 12 h light–12 h dark cycle (light period 08.00–20.00 hours). The rats were fed an American Institute of Nutrition (AIN)-93G (control) diet for 1 week as an acclimatisation period, and then divided into three groups based on body weight. The control and HF/HS groups were, respectively, fed ad libitum with the AIN-93G diet or a fat/sucrose-rich diet (for the composition of each diet, see Table 1). Generally, intake of a HF/HS diet is lower than the intake of the control diet due to the high energy density of the HF/HS diet, which would result in a relatively lower intake of protein, minerals and vitamins in the HF/HS group compared with the control group, and deficiency in these nutrients could, in turn, affect the expression of nutrient transporters and receptors( Reference Liuzzi, Blanchard and Cousins 19 – Reference Chen, Pan and Wong 21 ). To compensate the effect of lower protein/mineral/vitamin intake in the HF/HS group, a food-restricted group was included in the present study. The rats in the food-restricted group were fed the control diet with the same amount (in g) as that consumed by the HF/HS group on the previous day to examine the effects of reduced intake of nutrients, such as protein, minerals and vitamins. All rats had free access to water throughout the experiment. The study was approved by the Hokkaido University Animal Committee, and the animals were maintained in accordance with the guidelines for care and use of laboratory animals at Hokkaido University.
Table 1 Composition of the experimental diets

HF/HS, high-fat and high-sucrose; AIN, American Institute of Nutrition.
* TK-16 (Matsutani Chemical Industry Company Limited).
† JustFibre (Morimura Bros., Inc.).
Experimental protocol for the meal tolerance test
A MTT was conducted every week to examine postprandial glycaemic and GLP-1 responses after single meal (control diet) ingestion throughout the experiment. The rats were fasted for 6 h (09.00–15.00 hours)( Reference Andrikopoulos, Blair and Deluca 22 – Reference Ayala, Samuel and Morton 24 ), and then orally administrated the AIN-93G (3 g/kg body weight) diet suspended in deionised water (0·167 g/ml, 18 ml/kg body weight) by a feeding tube (6 Fr; Atom Medical Company). The suspension contained acetaminophen (100 mg/kg body weight) to evaluate the gastric emptying rate( Reference Heading, Nimmo and Prescott 25 , Reference Maida, Lovshin and Baggio 26 ). Blood samples (120 μl) from the tail vein were collected before (0 min) and at 15, 30, 60, 90 and 120 min after the oral meal administration. Blood samples were immediately mixed with aprotinin (final concentration 500 KIU/ml; Wako Pure Chemical Industries Limited) and heparin (final concentration 25 IU/ml; Nacalai Tesque, Inc.) on ice. Plasma was separated from the blood samples by centrifugation at 2300 g for 10 min at 4°C, and then frozen at − 80°C until measurements were taken. Plasma glucose and acetaminophen levels were measured using a glucose CII-test kit (Wako) and acetaminophen detection kit (Kanto Chemical Company, Inc.), respectively. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula:
where 1 mg insulin = 26 IU.
Blood and tissue collection at the final day
After overnight fasting, the rats were anaesthetised using sodium pentobarbital (Somnopentyl; Kyoritsu Seiyaku Company) on day 56. The waist circumference length (mid-line girth) of individual rats was measured as an obesity parameter that reflects the amount of adipose tissue( Reference Soliman, Wagih and Algaidi 27 , Reference Schroeder, Zagoory-Sharon and Shbiro 28 ). Portal blood was collected into a syringe containing heparin (final concentration 25 IU/ml), aprotinin (final concentration 540 KIU/ml) and dipeptidyl peptidase 4 (DPP-IV) inhibitor (final concentration 50 μm; Millipore). Mucosal samples were collected from the middle (approximately 10 cm) duodenum, jejunum, ileum and colon, respectively, after washing out the luminal content with cold saline. Caecal mucosa was collected from the whole caecal tissue after washing out the caecal content with cold saline. These samples were immediately frozen with liquid N2, and stored at − 80°C until RNA extraction.
Plasma hormone measurement
Plasma GLP-1 concentrations (25 μl) were measured with the Total GLP-1 enzyme immunoassay (EIA) Kit (intra- and inter-assay variations < 5 and < 12 %, respectively; Millipore), according to the manufacturer's instructions. Plasma insulin concentrations (10 μl) were measured with an insulin-ELISA kit (intra- and inter-assay variations < 5 and < 5 %, respectively; AKRIN-010T; Shibayagi), according to the manufacturer's protocols. Plasma collected on day 50 was diluted two times to adjust for a standard curve. For the measurement of plasma cholecystokinin (CCK) and gastrin levels, plasma was extracted as described in a previous study( Reference Rehfeld 29 ). In brief, one volume of plasma sample was mixed with two volumes of 99·5 % ethanol. The mixture was incubated on ice for 30 min, and then centrifuged at 9300 g for 10 min at 4°C. The supernatant was transferred to a new tube and evaporated in a vacuum centrifuge. The dried extracts were stored at − 80°C until analysis. After reconstituting into an equivalent volume by assay buffer, plasma concentrations of CCK (50 μl) and gastrin (100 μl) were measured according to the manufacturer's protocols.
Because the primary antiserum in the CCK EIA Kit (intra- and inter-assay variations < 5 and < 14 %, respectively; Phoenix Pharmaceuticals, Inc.) cross-reacts (100 %) not only with sulphated and non-sulphated CCK-8( Reference Maida, Lovshin and Baggio 26 – Reference Jackson, Leahy and McGowan 33 ), but also with gastrin-1, we measured plasma gastrin concentration using the human gastrin-1 EIA kit (intra- and inter-assay variations < 9 and < 7 %, respectively; Assay Designs, Inc.). The primary antiserum in the Human Gastrin-1 EIA Kit has high reactivity with rat gastrin-1 (70·7 %), human gastrin-1 (100 %) and human mini gastrin (74·6 %), but it slightly reacts with CCK-8 (2·67 %).
Real-time quantitative PCR
Total RNA was extracted by using the RNeasy Mini Kit (Qiagen), according to the manufacturer's protocol. RNA concentrations were determined by optical densitometry at 260 nm. RNA quality was assessed by the ratio of 260/280 nm (>1·8). Complementary DNA was synthesised using the ReverTra Ace Quantitative PCR with a genome DNA remover (Toyobo Company Limited), according to the manufacturer's protocol. Gene expression levels were determined by TaqMan gene expression assays (Life Technologies Company) with rat gene-specific, predesigned TaqMan primers and probe sets (proglucagon (Gcg): Rn00562293_m1, Cck: Rn00563215_m1). PCR amplification and fluorescence data collection were performed with the Mx3000P Real-Time PCR System (Agilent Technologies, Inc.). The mRNA expression level was calculated with a standard curve determined from several concentrations of complementary DNA. The concentration of samples was corrected with glyceraldehyde 3-phosphate dehydrogenase (Gapdh) (Rn99999916_s1) mRNA as a reference gene. Data are presented as the relative expression level to that of the control group.
Statistical analysis
All results are expressed as means with their standard errors. In the MTT, data were analysed by a three-way ANOVA with treatment, time and day as main factors (SPSS Japan). When there were significant main effects or interaction, a two-way ANOVA (treatment and time) was performed to identify the main effects on each day. Data on AUC, HOMA-IR, mRNA expression and portal hormone levels were analysed by a one-way ANOVA (treatment) or two-way ANOVA (treatment and day). Significant differences among the groups or time points were determined by Student's t test, Tukey–Kramer's or Dunnett's post hoc test (P< 0·05). The AUC of plasma glucose, insulin and GLP-1 levels during the MTT was calculated by the trapezoidal rule.
Results
Effect of the high-fat and high-sucrose diet on body weight, food intake, waist circumference, fat accumulation and liver weight
Body weight was increased in the HF/HS group, with significant differences being observed from day 30 compared with the control group (Fig. 1). At the end of the experiment (day 56) (Table 2), the body weight of the HF/HS group was significantly higher than that of the control and food-restricted groups. The total food intake of the HF/HS group was significantly lower than that of the control group, while total energy intake was significantly higher in the HF/HS group than in the other two groups. To confirm the effect of micronutrient deficiency caused by the decreased intake of the HF/HS diet, a food-restricted group was included in the experiment. The energy intake of the food-restricted group was significantly lower than that of the control and HF/HS groups, but the weight of total food intake in the restricted group was similar to that in the HF/HS group. This indicates that the total intake of protein, vitamins and minerals did not differ between the food-restricted and HF/HS groups. Similar to the results reported for a HF diet( Reference Sumiyoshi, Sakanaka and Kimura 30 , Reference Buettner, Parhofer and Woenckhaus 31 ), the chronic intake of the HF/HS diet in the present study significantly increased body weight, waist circumference, visceral fat, and liver weight.

Fig. 1 Daily changes in body weight. Rats were fed the control diet ad libitum (![]() ), a restricted amount of the control diet (
), a restricted amount of the control diet (![]() ) and the high-fat and high-sucrose diet ad libitum (
) and the high-fat and high-sucrose diet ad libitum (![]() ), except on the day of the meal tolerance test. Body weight was measured every morning. Values are means (n 8–9 rats per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test).
), except on the day of the meal tolerance test. Body weight was measured every morning. Values are means (n 8–9 rats per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test).
Table 2 Body weight, total food intake, waist, visceral adipose tissue weight and liver weight on day 56 after chronic intake of the high-fat and high-sucrose (HF/HS) diet (Mean values with their standard errors; n 8–9 rats per group)
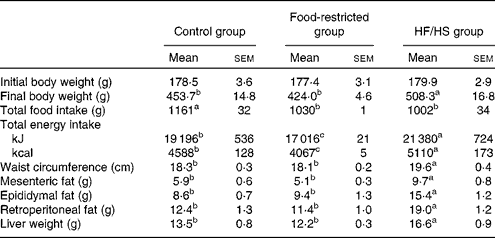
a,b,cMean values with unlike superscript letters were significantly different (P< 0·05; Tukey–Kramer's post hoc test).
Basal and postprandial glycaemic responses during the meal tolerance test
In the present study, we used the MTT rather than the oral/intraperitoneal glucose tolerance test to evaluate postprandial glucose tolerance and GLP-1 secretion( Reference Ahlkvist, Vikman and Pacini 32 ). It should be noted that the control diet was orally administered in all the groups during the MTT after 6 h deprivation of the respective experimental diets. The MTT was conducted every week to monitor 8-week changes in postprandial responses during the establishment of obesity or glucose intolerance (Table 3) .
Table 3 P values for the effects of diet, time and day in the meal tolerance test (MTT)*

Tr, treatment; Ti, time; D, day; GLP-1, glucagon-like peptide-1.
* Data obtained from the MTT were analysed by three-way ANOVA.
Basal glucose levels were significantly higher in the HF/HS group than in the other two groups after day 20 (Fig. 2(a)). Postprandial glucose levels were higher in the HF/HS group than in the other two groups throughout the experimental period due to increased basal glucose levels (Fig. 2(a)). Significant treatment effects were observed on days 6 and 13 for postprandial glycaemic response (Δglucose; Fig. 2(b)). On day 6, significantly higher glycaemic responses compared with the basal level (0 min) were observed at 15 and 60 min in the HF/HS group, but only at 15 min in the control group. Similarly, the control group showed a significant increment from the basal level only at 15 min, but the HF/HS group showed an increment at 15, 30 and 60 min on day 13. Although a significant effect was not detected by the two-way ANOVA with treatments and days, the one-way ANOVA and post hoc test demonstrated the significant effect of HF/HS diet treatment on the AUC of Δglucose on day 13 compared with the control group (Fig. 2(c)).

Fig. 2 Postprandial glycaemic responses during the meal tolerance test (MTT). The control diet (American Institute of Nutrition (AIN)-93G) suspended in water was administered orally in rats (3 g/kg body weight) after 6 h fasting on days 6, 13, 20, 27, 34, 41 and 50. Rats were fed the control diet ad libitum (![]() ), a restricted amount of the control diet (
), a restricted amount of the control diet (![]() ) and the high-fat and high-sucrose (HF/HS) diet ad libitum (
) and the high-fat and high-sucrose (HF/HS) diet ad libitum (![]() ), except on the day of the MTT. Blood samples from the tail vein were collected before (0 min) and after (15, 30, 60, 90 and 120 min) the meal load, and plasma glucose levels were measured. (a) Absolute glucose levels and (b) changes from basal levels (Δglucose). (c) AUC of Δglucose (
), except on the day of the MTT. Blood samples from the tail vein were collected before (0 min) and after (15, 30, 60, 90 and 120 min) the meal load, and plasma glucose levels were measured. (a) Absolute glucose levels and (b) changes from basal levels (Δglucose). (c) AUC of Δglucose (![]() , control;
, control; ![]() , food-restricted;
, food-restricted; ![]() , HF/HS). Values are means (n 6–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti), day (D) and the interaction of treatment and time (Tr × Ti) or day (Tr × D) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that at baseline (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test). To convert glucose in mg/dl to mmol/l, multiply by 0·0555.
, HF/HS). Values are means (n 6–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti), day (D) and the interaction of treatment and time (Tr × Ti) or day (Tr × D) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that at baseline (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test). To convert glucose in mg/dl to mmol/l, multiply by 0·0555.
Basal insulin, homeostasis model assessment of insulin resistance and postprandial insulin secretion during the meal tolerance test
Basal insulin levels in the HF/HS group gradually increased from day 13 to day 50 (Fig. 3(a)), and were significantly higher than those in the other two groups on days 34 and 50. HOMA-IR was also significantly higher in the HF/HS group than in the other two groups (Fig. 3(c)) after day 34. Postprandial insulin levels in the HF/HS group were significantly higher than those in the control group at 15, 30 and 60 min in each MTT (Fig. 3(b)). Furthermore, a significant difference in the AUC of Δinsulin levels between the HF/HS and control groups was observed on days 34 and 50, and its levels were increased by the chronic intake of the HF/HS diet (Fig. 3(d)).

Fig. 3 Postprandial insulin secretion during the meal tolerance test (MTT) and fasting homeostasis model assessment of insulin resistance (HOMA-IR). The control diet (American Institute of Nutrition (AIN)-93G) suspended in water was administered orally in rats (3 g/kg body weight) after 6 h fasting on days 13, 34 and 50. Rats were fed the control diet ad libitum (![]() ), a restricted amount of the control diet (
), a restricted amount of the control diet (![]() ) and the high-fat and high-sucrose (HF/HS) diet ad libitum (
) and the high-fat and high-sucrose (HF/HS) diet ad libitum (![]() ), except on the day of the MTT. Blood samples from the tail vein were collected before (0 min) and after (15, 30, 60, 90 and 120 min) the meal load, and plasma insulin levels were measured. (a) Absolute insulin levels and (b) changes from basal levels (Δinsulin). (c) HOMA-IR was calculated as described in the Materials and methods section. (d) AUC of Δinsulin (
), except on the day of the MTT. Blood samples from the tail vein were collected before (0 min) and after (15, 30, 60, 90 and 120 min) the meal load, and plasma insulin levels were measured. (a) Absolute insulin levels and (b) changes from basal levels (Δinsulin). (c) HOMA-IR was calculated as described in the Materials and methods section. (d) AUC of Δinsulin (![]() , control;
, control; ![]() , food-restricted;
, food-restricted; ![]() , HF/HS). Values are means (n 7–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti), day (D) and the interaction of treatment and time (Tr × Ti) or day (Tr × D) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that at baseline (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test).
, HF/HS). Values are means (n 7–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti), day (D) and the interaction of treatment and time (Tr × Ti) or day (Tr × D) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that at baseline (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test).
Basal and postprandial glucagon-like peptide-1 levels during the meal tolerance test
Postprandial GLP-1 secretion in the HF/HS and control groups was significantly higher than its basal levels but not in the food-restricted group on days 13 and 34 (Fig. 4(a) and (b)). GLP-1 levels at 15 min were significantly higher in the HF/HS group than in the control and food-restricted groups on day 50 (Fig. 4(a) and (b)). Furthermore, the AUC of GLP-1 levels in the HF/HS group on day 50 was significantly increased from day 13, which was significantly higher than that in the control group (Fig. 4(c)). The food-restricted group had the lowest basal and postprandial GLP-1 levels among all the groups in each MTT (Fig. 4(a) and (b)).

Fig. 4 Postprandial glucagon-like peptide-1 (GLP-1) secretion during the meal tolerance test (MTT). The control diet (American Institute of Nutrition (AIN)-93G) suspended in water was administered orally in rats (3 g/kg body weight) after 6 h fasting on days 13, 34 and 50. Rats were fed the control diet ad libitum (![]() ), a restricted amount of the control diet (
), a restricted amount of the control diet (![]() ) and the high-fat and high-sucrose (HF/HS) diet ad libitum (
) and the high-fat and high-sucrose (HF/HS) diet ad libitum (![]() ), except on the day of the MTT. Blood samples from the tail vein were collected before (0 min) and after (15, 30, 60, 90 and 120 min) the meal load, and plasma total GLP-1 levels were measured. (a) Absolute GLP-1 levels and (b) changes from basal levels (ΔGLP-1). (c) AUC of Δtotal GLP-1 (
), except on the day of the MTT. Blood samples from the tail vein were collected before (0 min) and after (15, 30, 60, 90 and 120 min) the meal load, and plasma total GLP-1 levels were measured. (a) Absolute GLP-1 levels and (b) changes from basal levels (ΔGLP-1). (c) AUC of Δtotal GLP-1 (![]() , control;
, control; ![]() , food-restricted;
, food-restricted; ![]() , HF/HS). Values are means (n 7–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti), day (D) and the interaction of treatment and time (Tr × Ti) or day (Tr × D) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that at baseline (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test).
, HF/HS). Values are means (n 7–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti), day (D) and the interaction of treatment and time (Tr × Ti) or day (Tr × D) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that at baseline (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test).
Postprandial gastric emptying rate during the meal tolerance test
The rate of gastric emptying affects postprandial glycaemia, and dysregulation of gastric emptying has been reported in obese patients( Reference Jackson, Leahy and McGowan 33 ) and diet-induced obese rodents( Reference Li, Ma and Wang 34 ). The acetaminophen (paracetamol) absorption test is used to assess the gastric emptying rate because acetaminophen is absorbed in the small intestine( Reference Heading, Nimmo and Prescott 25 , Reference Maida, Lovshin and Baggio 26 ). On days 6 and 13, acetaminophen concentrations at 15 and 60 min after preload of the control diet suspension were significantly lower in the HF/HS group than in the food-restricted group (Fig. 5(a) and (b)). On day 34, acetaminophen concentrations in the HF/HS group at 15 and 30 min were significantly lower than in the control group (Fig. 5(e)). However, on days 41 and 50, significant differences among the treatments were not observed (Fig. 5(f) and (g)).

Fig. 5 For Figs 5(d), 5(e) and 5(f) – insert x axis label: Time (min) in plasma acetaminophen concentrations during the meal tolerance test (MTT). Acetaminophen (100 mg/kg body weight) was orally administered with the control diet (American Institute of Nutrition (AIN)-93G; 3 g/kg body weight) in the MTT to assess the gastric emptying rate after 6 h fasting on days (a) 6, (b) 13, (c) 20, (d) 27, (e) 34, (f) 41 and (g) 50. Rats were fed the control diet ad libitum (![]() ), a restricted amount of the control diet (
), a restricted amount of the control diet (![]() ) and the high-fat and high-sucrose diet ad libitum (
) and the high-fat and high-sucrose diet ad libitum (![]() ), except on the day of the MTT. Changes in plasma acetaminophen levels are shown. Values are means (n 6–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti) and the interaction of treatment and time (Tr × Ti) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the food-restricted group (P< 0·05; Tukey–Kramer's post hoc test).
), except on the day of the MTT. Changes in plasma acetaminophen levels are shown. Values are means (n 6–9 rats per group), with their standard errors represented by vertical bars. P values for the effects of treatment (Tr), time (Ti) and the interaction of treatment and time (Tr × Ti) were calculated by a two-way ANOVA are shown in each subfigure. * Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test). † Mean value was significantly different from that of the food-restricted group (P< 0·05; Tukey–Kramer's post hoc test).
Portal peptide hormone levels after 8 weeks of high-fat and high-sucrose diet treatment
On day 56, we collected portal vein samples from overnight fasted rats to evaluate the effect of the HF/HS diet on basal gut hormone levels. Portal GLP-1 concentration was significantly higher in the HF/HS group than in the control and food-restricted groups (Fig. 6(a)). Although significant difference between portal insulin concentrations in the HF/HS and control groups was determined with student's t test (P= 0·010), there are insignificant changes of insulin levels among all the groups (Fig. 6(b)). Because the CCK EIA kit is able to detect both CCK and gastrin, we measured both CCK and gastrin levels. Portal CCK and gastrin levels did not differ among the three groups (Fig. 6(c) and (d), respectively).

Fig. 6 Fasting peptide hormone levels in the portal vein of rats fed the respective test diets for 8 weeks. Portal blood was collected from the rats after overnight fasting on day 56. The levels of (a) total glucagon-like peptide-1 (GLP-1), (b) insulin, (c) cholecystokinin (CCK) and (d) gastrin were measured by respective EIA kits. Values are means (n 8–9 rats per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05; Tukey–Kramer's post hoc test). HF/HS diet, high-fat and high-sucrose diet.
Proglucagon and cholecystokinin mRNA expression in the gastrointestinal tract
To examine the effect of the HF/HS diet on gut hormone mRNA expression, intestinal mucosa was collected from various regions. Although the GLP-1 level in the portal vein was higher in the HF/HS group (Fig. 6(a)), Gcg mRNA expression did not differ by dietary treatment group for any of the regions (Fig. 7(a)–(d)). Cck mRNA expression was significantly increased in the jejunum depending on energy intake (Fig. 7(f)).

Fig. 7 Proglucagon (Gcg) and cholecystokinin (Cck) mRNA expression in the intestinal mucosa of rats fed the respective test diets for 8 weeks. Mucosa was collected from the (a, f) jejunum, (b, g) ileum, (c) caecum, (d) colon and (e) duodenum of rats after overnight fasting on day 56. The mRNA expression levels of (a–d) Gcg and (e–g) Cck were determined by real-time quantitative PCR. Data are presented as the relative expression level to that of the control group normalised to glyceraldehyde 3-phosphate dehydrogenase (Gapdh) mRNA expression. Values are means (n 8–9 rats per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the food-restricted group (P< 0·05; Tukey–Kramer's post hoc test). HF/HS diet, high-fat and high-sucrose diet.
Discussion
In the present study, we monitored postprandial GLP-1, insulin, glycaemia and gastric emptying in rats during the progression of diet-induced obesity in rats. Daily intake of a HF/HS diet increased postprandial glycaemic and insulin responses to a ‘normal diet’ (AIN-93G) under the MTT from the early period of the experiment (day 13). After day 20, the HF/HS diet increased fasting glucose and insulin levels compared with the control group, indicating that HF/HS feeding induced glucose intolerance accompanied by insulin resistance within 3 weeks in rats. Importantly, postprandial glucose response was not further impaired by the HF/HS diet, and postprandial GLP-1 and insulin responses to the meal in the HF/HS group gradually increased until the end of the experimental period. The present study revealed that the postprandial GLP-1 response to meal ingestion is increased during the progression of glucose intolerance and obesity, which may slow the establishment of diet-induced obesity.
Epidemiological studies have provided evidence that dietary fat intake is closely related to obesity( Reference Bray and Popkin 35 , Reference Hill and Peters 36 ). Therefore, HF diets have been widely used and recognised to induce diet-related obesity in animal experiments( Reference Buettner, Schölmerich and Bollheimer 37 , Reference Hariri and Thibault 38 ). Long-term feeding of a sucrose-rich diet has been shown to induce higher glucose levels compared with a HF diet as measured by the oral glucose tolerance test( Reference Sumiyoshi, Sakanaka and Kimura 30 ). The combination of a HF diet and a HS diet has also been used to induce obesity as a model of the Western diet( Reference Aoun, Michel and Fouret 39 , Reference Sato, Kawano and Notsu 40 ). Sucrose consists of glucose and fructose equally, and fructose is known as a highly lipogenic sugar. It has been reported that excessive consumption of commercial beverages containing glucose and fructose (high-fructose maize syrup: 50 % glucose and 50 % fructose) has been linked to the development of the metabolic syndrome( Reference Dekker, Su and Baker 41 ).
As shown in Figs. 2 and 3, weekly monitoring of postprandial glycaemia and insulin responses revealed that glucose intolerance was induced in rats just after 2 weeks on the HF/HS diet. Significant differences in body weight between the control and HF/HS groups were observed from day 30 (Fig. 1), indicating that impairment of glucose homeostasis occurs in advance of body-weight increase. Generally, diet-induced obesity animal models are studied after feeding with high-energy diets for 8 weeks or longer. However, the present result suggests that postprandial glucose intolerance is immediately caused by a daily intake of a high-energy diet rich in fat and sucrose as is the case in the intravenous glucose tolerance test( Reference la Fleur, Luijendijk and van Rozen 42 ). The food-restricted group was fed the control diet with the same amount (in g) as that consumed by the HF/HS group (Table 2), so that both groups consumed the same amounts of protein, vitamins and minerals, and finally both groups had lower protein/vitamin/mineral intake compared with the control group. However, the food-restricted group did not show the similar phenotype to the HF/HS group on postprandial response, suggesting that excessive energy intake, rather than the reduced intake of protein, vitamins and minerals, has a large impact on the impairment of postprandial glycaemia. The food-restricted group showed almost similar postprandial glycaemia overall, but relatively smaller responses in insulin and GLP-1 secretion compared with the control group (Figs. 2–4), suggesting that restriction (90 %) of food consumption is beneficial for the improvement of glucose tolerance. However, it is possible that these results were observed due to the lower body weight and lower energy load in the food-restricted group than in the control group. Another limitation is that the food-restricted group had a longer fasting period because they finished the diet every day before they were given a fresh diet.
The effects of each macronutrient (carbohydrate, fat and protein) on gut hormone secretion have been reported, and the ratio of fat:protein is closely related to GLP-1 secretion in healthy subjects( Reference Carrel, Egli and Tran 43 , Reference Wikarek, Chudek and Owczarek 44 ). The intake of a mixed meal has a potent effect on GLP-1 secretion compared with solo administration of each macronutrient( Reference Buettner, Parhofer and Woenckhaus 31 ). It has been previously reported that the MTT represents a better indication of normal postprandial glucose and insulin responses compared with the oral glucose tolerance test in a population-based cohort( Reference Rijkelijkhuizen, Girman and Mari 45 ). In the present study, the MTT was conducted (rather than the widely used oral glucose tolerance test) to evaluate ‘postprandial’ glycaemic and gastrointestinal responses under a more physiological condition reflecting dietary exposure in normal life. As equivalent dietary components are used to compare the effect of the diet on obesity, as shown in the clinical study( Reference Bowen, Noakes and Clifton 46 , Reference Parnell and Reimer 47 ), all rats received a normal diet rather than the respective test diets in the MTT. For the HF/HS group, the control diet administered was different from the usual diet (HF/HS diet). However, all rats received the control diet during the acclimatisation period before feeding the respective test diets; therefore, having the control diet in the MTT was not for the first time even for the HF/HS group. In addition, all rats were subjected to oral administration of the water-suspended diet in the MTT. Although the composition of the diet was unchanged for the control group, the form and way of ingestion were changed from the usual ‘meal’ for all the groups. Therefore, we assume that the impact of changing diet composition from daily consuming the HF/HS diet on postprandial responses in the HF/HS group was smaller than the chronic effect of the HF/HS diet. Because daily postprandial responses would be an important factor that would affect metabolic status, it is interesting to know the daily glycaemic, insulin and GLP-1 responses in each group after having the respective test diets. However, if the MTT had been performed in such a way, interpretations of the observed result would be complicated with respect to nutrient sensing because both chronic and acute effects of the respective diet compositions could affect postprandial responses. It would be interesting to examine the postprandial response to the HF/HS diet or a single nutrient load in the control and HF/HS groups in the future.
Previous reports have demonstrated that the peak of GLP-1 secretion after oral glucose administration was decreased in diet-induced obesity( Reference Gniuli, Calcagno and Dalla Libera 16 , Reference Anini and Brubaker 17 ). In contrast, it has been reported that GLP-1 secretion in response to oral fatty acid administration was unchanged between diet-induced obesity rats and diet-resistant rats( Reference Paulsen, Larsen and Hansen 18 ). Interestingly, the present study showed that postprandial GLP-1 response (to a normal diet) was gradually increased, but not decreased, by chronic intake of the HF/HS diet compared with the control diet, and a significant difference was observed after 7 weeks (Fig. 4). The result suggests that chronic intake of the HF/HS diet altered the nutrient-sensing function of the gastrointestinal tract to be more sensitive to the mixed meal. Possibly, the different postprandial responses arose from different amounts of energy load( Reference Ayala, Samuel and Morton 24 ), because the meal was given depending on the body weight of individual rats (3 g/kg) in the MTT of the present study. Indeed, the body weight of the HF/HS group was about 50 g (12 %) higher than that of the control group, and energy load in the HF/HS group was 12 % higher than that in the control group. Although a similar difference in body weight (10 %) was already observed on day 34, postprandial GLP-1 response was 2-fold higher in the HF/HS group than in the control group (Fig. 4(b) and (c)). Furthermore, data (see online supplementary Fig. S1) comparing selected rats having higher body weight in the control group and those having lower body weight in the HF/HS group demonstrated that GLP-1 and insulin responses are apparently higher in the HF/HS group than in the control group, although there was no significant difference in body weight between the two groups.
The present results also demonstrated that fasting GLP-1 levels in the portal vein were increased in the HF/HS group (Fig. 6), but Gcg mRNA expression did not differ by dietary treatments in any of the intestinal regions (Fig. 7), which implies that GLP-1 secretion, but not mucosal GLP-1 production, was changed by the HF/HS diet. Despite the change (Δ) in postprandial plasma glucose concentrations was not increased from day 20 to day 50 (Fig. 2(b)), enhancement of postprandial and fasting GLP-1 levels with increased insulin secretion was observed (Figs. 3, 4 and 6). Although gut hormones, such as GLP-1, are immediately secreted in response to meal ingestion, adaptive changes to a chronic high-energy diet develop over time in peripheral insulin-targeting tissues such as adipose tissue, and liver and skeletal muscles. The physiological relevance of increased GLP-1 and nutrient sensitivity needs to be further studied in the future, which may contribute to the prevention of excessive plasma glucose elevation and slow the establishment of glucose intolerance and obesity with the enhancement of insulin secretion.
Changes in acetaminophen concentration were smaller in the HF/HS group compared with the control group during the MTT on day 34 (Fig. 5(e)), suggesting delayed gastric emptying in the HF/HS group. Such an effect might prevent excessive loading of nutrients in the small intestine in the HF/HS group. Several reports have demonstrated that the dosage of luminal nutrients, including fat and protein, is an important factor for GLP-1 secretion( Reference Hira, Mochida and Miyashita 48 , Reference Yoder, Yang and Kindel 49 ). On day 34, postprandial GLP-1 levels in the HF/HS group were similar to those in the control group, although gastric emptying was delayed in the HF/HS group (Figs. 4(b) and 5(e)). In contrast, increased postprandial GLP-1 secretion and unchanged postprandial gastric emptying were observed on day 50 (Figs. 4(b) and 5(g)). GLP-1 secretion depends on luminal nutrients that are emptied from the stomach, but gastric emptying is regulated by various factors, such as CCK, serotonin and GLP-1. Although significant treatment effects were detected on days 6, 13 and 34 by the two-way ANOVA, it is unclear how such changes in the gastric emptying rate appeared and contribute to postprandial hormone and glycaemic responses in the present study.
In summary, feeding rats with a HF/HS diet rapidly impaired postprandial glycaemic responses (i.e. within 2 weeks) in advance of increased weight gain. Postprandial GLP-1 secretion during the MTT was increased by HF/HS diet treatment after 7 weeks. Food restriction demonstrates that habitual excessive energy (fat and sucrose) intake is the main factor that contributes to changes in postprandial GLP-1 secretion. Although mRNA expression levels of gut hormones were unchanged, fasting GLP-1 and insulin levels in portal blood were increased by the HF/HS diet after 8 weeks. The present study revealed that chronic ingestion of a high-energy diet elevates postprandial GLP-1 and insulin responses to meal ingestion in rats. The boosted postprandial GLP-1 secretion by chronic high-energy diet treatment suggests enhanced sensitivity to luminal nutrients in the gut, which may slow the establishment of glucose intolerance and obesity.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515000550
Acknowledgements
The present study was supported by JSPS KAKENHI (grant no. 11J07124).
The authors' contributions are as follows: S. N., T. H. and H. H. designed the research; S. N. conducted the research and analysed the data; S. N. and T. H. wrote the paper; T. H. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.













