Vitamin D insufficiency is a challenge worldwide, and an estimated 20–30 % of the world population are vitamin D-insufficient(Reference Karampela, Sakelliou and Vallianou1). Several risk factors have been associated with low vitamin D status, and one of them is obesity(Reference Karampela, Sakelliou and Vallianou2). Multiple hypotheses have been proposed to explain the link between low vitamin D status and obesity. These include the sequestration of vitamin D in adipose tissue due to its lipophilic molecular structure, volumetric dilution, limited exposure to sunlight and impaired hepatic 25-hydroxylation of vitamin D3 (Reference Karampela, Sakelliou and Vallianou2). Vitamin D is a calciotropic hormone and is essential for bone health and calcium (Ca) homoeostasis(Reference Bouillon, Marcocci and Carmeliet3). Many studies have suggested that low vitamin D status may be influencing the phenotype in many lifestyle-related diseases such as metabolic syndrome, diabetes and CVD(Reference Wimalawansa4–Reference Cosentino, Campodonico and Milazzo6). This indicates that vitamin D insufficiency in individuals with obesity who are at increased risk of developing lifestyle-related diseases should be prevented by screening and supplementation. However, the daily recommended intake of vitamin D has, in many studies, been shown to be inadequate for individuals with obesity to reach sufficient vitamin D levels(Reference Pereda and Nishishinya7), and in overweight infertile men with Klinefelter syndrome, supplementation with calcidiol rather than vitamin D3 seems to be required(Reference Ferlin, Selice and Di Mambro8). It has been suggested that men with obesity and impaired gonadal function may be particularly prone to persistently low vitamin D status despite vitamin D3 supplementation(Reference Foresta, Calogero and Lombardo9). This study aims to determine the sufficiency of high-dose vitamin D3 supplementation in achieving adequate serum calcidiol (25OHD) status among infertile men with overweight and obesity.
Materials and methods
Study design and intervention
This investigation is based on secondary analyses in a predefined subgroup from the Copenhagen Bone-Gonadal Study (CBG study). The CBG study is a single-center, double-blinded, randomised clinical trial conducted at the Department of Growth and Reproduction, Rigshospitalet, Denmark (NCT01304927), designed to investigate the effect of vitamin D3 supplementation on semen quality in infertile men with vitamin D insufficiency(Reference Blomberg Jensen, Gerner Lawaetz and Petersen10). The study was approved by the Danish Medicines Agency (approval no. 2010-024588-42), the Danish National Committee on Health Research Ethics (approval no. H-4-2010-138), and the Danish Data Protection Agency (approval no. 2010124801) and monitored by the Good Clinical Practice (GCP) unit, Copenhagen University Hospital. Informed consent was obtained from all participants. Study design and power calculations are described in the primary manuscript(Reference Blomberg Jensen, Gerner Lawaetz and Petersen10). In brief, 1427 infertile men were referred for an andrological examination. Inclusion criteria were male factor infertility defined by more than 12 months of inability to conceive and impaired semen quality as determined by WHO criteria(Reference Kumari, Singh and Kumari11). Moreover, all men had a serum 25OHD ≤ 50 nmol/l at screening and no serious co-morbidities. BMI was not part of the inclusion or exclusion criteria. A total of 307 men were included and randomised to vitamin D3 or placebo treatment for 150 days. Men allocated to active treatment initially received an oral bolus of 300 000 mg of vitamin D3, followed by daily supplementation with 1400 mg of vitamin D3 and 500 mg of Ca (Tablets, Ferrosan/Pfizer). Men allocated to placebo received an oral bolus of oil and non-Ca-containing placebo tablets for 150 days. All participants were instructed not to consume vitamin D supplements > 400 mg daily during the trial. In this cohort, we have previously reported that BMI correlates with serum 25OHD and serum parathyroid hormone (PTH), and serum 25OHD was significantly higher and serum PTH was significantly lower in men with normal weight compared with men with overweight and obesity(Reference Holt, Petersen and Dinsdale12).
Biochemical analysis
Blood sampling was performed between 8·00 and 10·00 a.m. Serum was analysed immediately for PTH and ionised Ca. The remaining analyses were conducted on frozen serum samples from day 1 and after trial completion. Serum 25OHD was measured by isotope dilution LC–MS/MS, with an inter-assay CV < 10 %. PTH levels were measured using the Cobas 8000 (Roche) with a CV < 4 %, and ionised Ca was measured using the Konelab 30i (Thermo Fisher Scientific) with a CV < 2 %. Whole-body dual-energy x-ray absorptiometry (DXA) scan was performed on day 1 and on day 150 with light clothing to determine total body composition, including total fat mass in percentage (Lunar Prodigy, GE Healthcare). Reproductive hormones were also measured and have previously been published(Reference Blomberg Jensen, Gerner Lawaetz and Petersen10,Reference Holt, Yahyavi and Kooij13,14) .
Statistical analysis
Descriptive statistics are presented as mean and standard deviations in Tables 1 and 2 and mean with 95 % CI in Table 3. Analyses in Tables 2 and 3 were performed according to predefined subgroups defined as BMI < 25 kg/m2 (normal weight), BMI 25–30 kg/m2 (overweight) and BMI > 30 kg/m2 (obesity). Differences between predefined subgroup analyses according to BMI were performed by the Kruskal–Wallis test for continuous variables, and the χ 2 test was used for differences in season of enrolment at baseline (Table 2) and by a comparison of means (independent t test) between men treated with vitamin D3 and placebo after the intervention (Table 3). The difference in average changes in serum 25OHD and serum PTH from baseline to after intervention, between vitamin D-treated and placebo-treated men in each subgroup according to BMI, was assessed by an independent t test (Fig. 1). Vitamin D insufficiency was defined as serum 25OHD ≤ 50 nmol/l and vitamin D sufficiency as serum 25OHD > 50 nmol/l(Reference Thacher and Clarke15–Reference Lips, Binkley and Pfeifer17). The study was designed to obtain an expected increase in serum 25OHD concentration of 50 nmol/l(Reference Blomberg Jensen, Gerner Lawaetz and Petersen10). In hindsight, we would have used a higher daily dosage instead of the initial megadose (300 000 mg). The rationale for using this was the risk of non-compliance in the active group which would lead to no major difference in vitamin D status between the active and the placebo arm. The dose regimen ensured a marked difference in vitamin D status, but it is doubtful if the initial megadose is beneficial, and in this way, we may underestimate the positive effect of correcting vitamin D insufficiency. No observations were excluded. A P-value < 0·05 was considered significant. All statistical analyses were performed by IBM SPSS Statistics version 28.
Table 1 Baseline characteristics on day 1
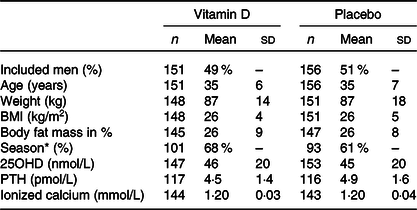
25OHD, calcidiol. PTH, parathyroid hormone.
* Season at day 1: Winter or spring.
Data presented as mean (SD) unless otherwise indicated.
Table 2. Baseline characteristics stratified according to BMI
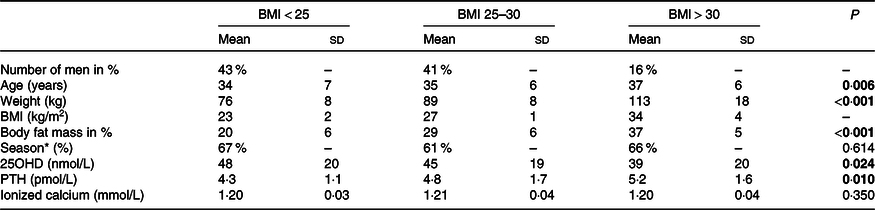
25OHD, calcidiol. PTH, parathyroid hormone.
* Season at day 1: Winter or spring.
Data presented as mean (SD) unless otherwise indicated.
P-value: Differences between groups are evaluated with Kruskal–Wallis test, unless differences in Season where χ 2 test was used.
All significant findings are highlighted as bold.
Table 3. Outcome after intervention according to baseline BMI

25OHD, calcidiol. PTH, parathyroid hormone.
Subgroup analyses of outcome according to BMI at baseline.
Data are presented as mean ± 95 % CI.
P-value: differences are evaluated by an independent t test.
All significant findings are highlighted as bold.
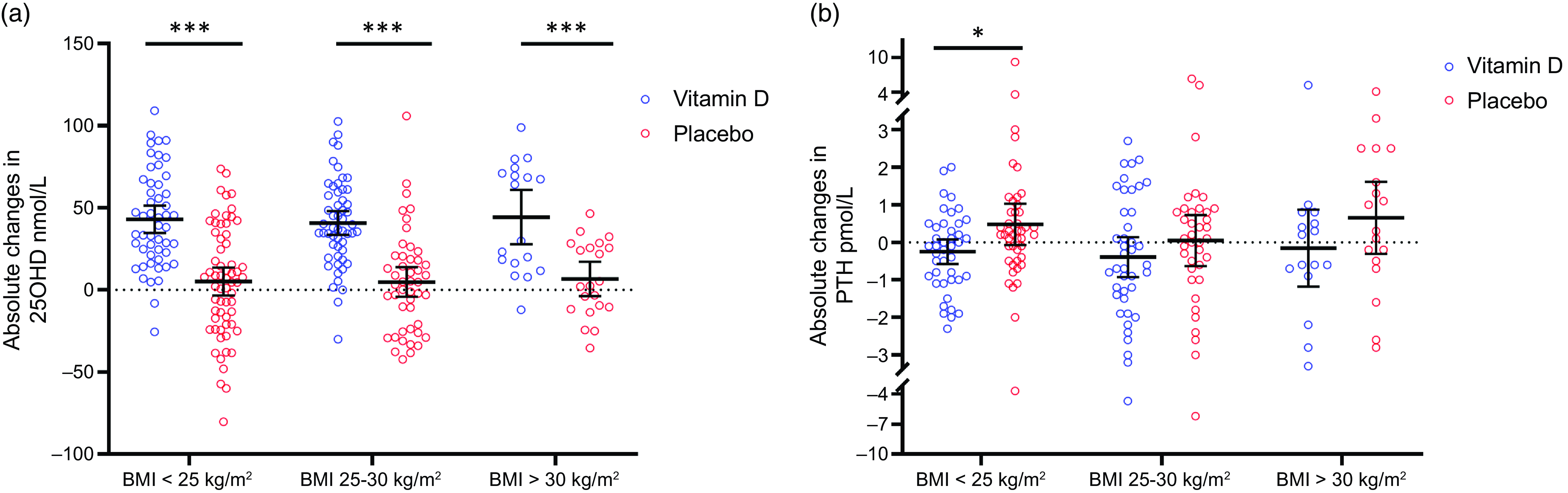
Fig. 1. Absolute changes in serum 25OHD and serum PTH from baseline to after the intervention according to baseline BMI (BMI normal: < 25 kg/m2, BMI overweight: 25–30 and BMI obese > 30 kg/m2). Circles represent individual changes (blue circles represent vitamin D-treated men and red circles represent placebo-treated men). Black lines represent mean ± 95 % CI. *P < 0·05 and ***P < 0·001.
Results
Baseline BMI and vitamin D status
At baseline, included men were on average overweight (BMI = 26 kg/m2) and vitamin D-insufficient with a serum 25OHD of 45 nmol/l, and the majority enrolled during winter and spring (Table 1). In a predefined subgroup according to baseline BMI (normal weight < 25 kg/m2, overweight 25–30 kg/m2 and obesity > 30 kg/m2), serum 25OHD was higher in men with normal weight compared with men with overweight and obesity (48 nmol/l v. 45 nmol/l and 39 nmol/l, respectively; P = 0·024). In contrast, serum PTH was significantly lower in men with normal weight compared with men with overweight and obesity (4·3 pmol/l v. 4·8 pmol/l and 5·2 pmol/l, respectively; P = 0·010). No differences were found in serum ionised Ca between the groups (1·20 mmol/l v. 1·21 mmol/l and 1·20 nmol/l, respectively; P = 0·350). There were no differences in season of enrolment between men with normal weight, overweight and obesity. Men with normal weight were significantly younger than men with overweight and obesity (34 years v. 35 years and 37 years, respectively; P = 0·006). As expected, men with normal weight had a lower weight (76 kg v. 89 kg and 113 kg, respectively; P < 0·001) and body fat mass (%) compared with men with overweight and obesity (20 % v. 29 % and 37 %, respectively; P < 0·001) (Table 2). There were no differences in any of the presented variables at baseline, between men treated with vitamin D and placebo-treated men within each subgroup according to BMI (data not shown).
BMI and vitamin D status after intervention
After the intervention, 130 men treated with vitamin D3 had higher serum 25OHD (89 nmol/l v. 51 nmol/l; P < 0·001), lower PTH (4·1 pmol/l v. 5·2 pmol/l; P < 0·001) and higher serum ionised Ca (1·21 mmol/l v. 1·20 mmol/l; P = 0·010) compared with 138 placebo-treated men, as reported previously(Reference Blomberg Jensen, Gerner Lawaetz and Petersen10,Reference Holt, Petersen and Dinsdale12) . Moreover, there were no differences in weight, BMI or body fat mass (%) after the intervention between the two groups(Reference Holt, Petersen and Dinsdale12). To explore the effect of high-dose vitamin D3 supplementation on vitamin D status in men with obesity, we looked at subgroups of men according to baseline BMI (Table 3). Men with normal weight, overweight and obesity treated with vitamin D3 had higher serum 25OHD compared with corresponding placebo-treated men (BMI < 25 kg/m2: 92 nmol/l v. 53 nmol/l, BMI = 25–30 kg/m2: 87 nmol/l v. 49 nmol/l and BMI > 30 kg/m2: 85 nmol/l v. 48 nmol/l; P < 0·001 for all, respectively). Serum PTH was lower in all three subgroups of men treated with vitamin D3 compared with corresponding placebo-treated men (BMI < 25 kg/m2: 4·0 pmol/l v. 4·9 pmol/l; P = 0·007, BMI = 25–30 kg/m2: 4·1 pmol/l v. 5·1 pmol/l; P = 0·010 and BMI > 30 kg/m2: 4·5 pmol/l v. 6·1 pmol/l; P = 0·035). Only men with normal weight treated with vitamin D3 had a higher serum ionised Ca compared with corresponding placebo-treated men (BMI < 25 kg/m2: 1·21 mmol/l v. 1·20 mmol/l; P = 0·040), BMI = 25–30 kg/m2: 1·21 mmol/l v. 1·21 mmol/l; P = 0·431 and BMI > 30 kg/m2: 1·21 mmol/l v. 1·19 mmol/l; P = 0·146). There were no differences in weight, BMI or body fat mass (%) between vitamin D3 and placebo-treated men in the three groups (Table 3). Men with normal weight, overweight and obesity treated with vitamin D3 had an average increase in serum 25OHD of more than 40 nmol/l, in contrast to the placebo-treated men where serum 25OHD was on average increased by about 5 nmol/l in each group (Fig. 1(a)). The changes in serum PTH from baseline to day 150 were only different in men with normal weight treated with vitamin D3 compared with placebo-treated men (ΔPTH: −0·3 v. 0·5; P = 0·026), whereas there was no difference in men with overweight and obesity treated with vitamin D3 compared with placebo-treated men (ΔPTH: −0·4 v. 0·0; P = 0·301 and −0·3 v. 0·7, P = 0·167) (Fig. 1(b)). In placebo-treated men, serum PTH was significantly lower in men with normal weight compared with men with obesity at day 150, whereas no differences were found in serum 25OHD between the three subgroups (data not shown).
Discussion
This study shows that high-dose vitamin D3 supplementation is adequate to correct vitamin D insufficiency in men with overweight and obesity. Noteworthy, high-dose supplementation was able to suppress serum PTH concentration with similar potency and magnitude in men with overweight and obesity compared with men with normal weight. The concomitant changes in both serum 25OHD and PTH of similar magnitude suggest that the effect of high-dose supplementation on Ca homoeostasis is independent of BMI. One explanation for our finding could be the initial oral megadose of 300 000 mg of vitamin D3 followed by a daily dosage of 1400 mg for 150 days that secures rapid restoration of vitamin D status and maintenance throughout the study duration. The estimated half-life of vitamin D3 is 1 day, while it is 15 days for 25OHD in serum(Reference Hollis and Wagner18,Reference Jones, Assar and Harnpanich19) . The short half-life of vitamin D3 is due to its lower affinity for the vitamin D binding protein (DBP) and possible storage in the abdominal subcutaneous adipose tissue(Reference Didriksen, Burild and Jakobsen20). Camozzi et al. found that individuals with obesity had a longer period of adequate serum 25OHD status compared with individuals with normal weight after a single high-dose bolus of vitamin D3 supplementation of 300 000 mg(Reference Camozzi, Frigo and Zaninotto21), which indicates different kinetics in normal v. high BMI following high-dose supplementation. Obesity is evidently a risk factor for having low vitamin D status, which may be due to sequestration in adipose tissue, volumetric dilution, and limited exposure to sunlight, etc.(Reference Karampela, Sakelliou and Vallianou2). However, other studies have suggested that obesity may directly influence vitamin D metabolism, through down-regulation of CYP2R1 activity in the liver and adipose tissue(Reference Wamberg, Christiansen and Paulsen22–Reference Elkhwanky, Kummu and Piltonen24), which would imply that individuals with obesity will struggle to achieve and maintain a sufficient vitamin D status. A previous study suggested that men with impaired testicular function have lower serum levels of 25OHD due to impaired local testicular CYP2R1 activity(Reference Foresta, Calogero and Lombardo9). Moreover, men with impaired testicular function and obesity would require supplementation with calcidiol instead of vitamin D3 because of their reduced ability to convert vitamin D3 into 25OHD by testicular 25-hydroxylation(Reference Ferlin, Selice and Di Mambro8). In our study, high-dose vitamin D3 supplementation was sufficient to reach adequate serum 25OHD concentrations, despite participating men had impaired semen quality and thereby various degrees of impaired testicular function. Furthermore, all infertile men included in the trial were vitamin D-insufficient at screening with an average serum 25OHD concentration of 35 nmol/l(Reference Blomberg Jensen, Gerner Lawaetz and Petersen10). The placebo-treated men experienced an improvement in their vitamin D status with an increase in serum 25OHD from 35 nmol/l at screening to 51 nmol/l at day 150 despite no active treatment. This suggests that the increased sun exposure to infertile men including during winter or spring is sufficient to generate an adequate increase in vitamin D status despite having impaired gonadal function. This observation questions, along with the marked increase in serum 25OHD in the vitamin D group, that the testes exert an essential influence on systemic serum 25OHD levels. We propose that the lower serum 25OHD levels in hypogonadal men are facilitated by the lower serum testosterone causing male obesity and metabolic syndrome(Reference Pivonello, Menafra and Riccio25), factors known to be associated with impaired vitamin D status(Reference Karampela, Sakelliou and Vallianou2) and which further may induce a down-regulation of hepatic CYP2R1 activity(Reference Roizen, Long and Casella23,Reference Elkhwanky, Kummu and Piltonen24) resulting in lower serum 25OHD concentrations.
It should be noted that 500 mg of Ca was added as a daily supplement to the used vitamin D dosage. Ca supplementation lowers serum PTH levels(Reference Sadideen and Swaminathan26), and since PTH regulates the vitamin D metabolising enzymes (CYP27B1 and CYP24A1)(Reference Meyer, Lee and Carlson27), the combined supplementation may affect serum 25OHD concentration. The lower serum PTH in men with obesity treated with vitamin D3 may be of clinical interest, since it has been shown that high serum PTH levels are associated with mortality in patients with type 2 diabetes(Reference Brandtner, Muendlein and Leiherer28). Our results are in line with a recent systematic review investigating the optimal dosages of vitamin D supplementation for individuals with obesity, concluding that the recommended vitamin D doses might need to be tripled to 1200 mg(Reference Pereda and Nishishinya7). There are some important limitations to this study. All participants were instructed not to consume vitamin D supplements > 400 mg daily during the trial, although it is plausible that some participants in the placebo group did not follow this instruction. Seasonal variation in serum 25OHD is another limitation that also will be influenced by ethnicity and sun exposure, which also affects vitamin D status in the placebo group. We have no data on sun exposure or ethnicity, but MBJ included all patients and > 80 % were Caucasian, which is in accordance with a report from the Danish authorities showing that 88·9 % of the Danish population was of Danish origin. Finally, co-treatment with 500 mg of Ca may affect serum PTH and serum 25OHD and thereby potentially be a confounder of the reported results. One of the major strengths of the study was the use of LC-MS/MS to determine serum 25OHD, and all men had a serum 25OHD ≤ 50 nmol/l at screening, and finally the dosage used induced the expected increase in serum 25OHD without causing toxicity.
In conclusion, we show that high-dose vitamin D3 supplementation is sufficient to reach adequate serum 25OHD levels in a predefined subgroup of infertile men with obesity. This suggests that vitamin D3 supplementation is able to correct vitamin D status, but it remains unclear whether the recommended low-dose vitamin D3 can restore vitamin D adequately in individuals with obesity.
Acknowledgements
This work was funded by Rigshospitalet, the Novo Nordisk Foundation, the Aase & Ejnar Danielsens Foundation, FSS and Candy Foundation.
M. B. J., A. J. and N. J. designed the randomised clinical trial; M.B.J. conducted the trial; R. H. and M. B. J. analysed the data; R. H. and S. K. Y. conducted the statistical analyses; R. H. and M. B. J. wrote the paper; R. H. and M. B. J. take primary responsibility for the final content. All authors (R. H., M. J. J, S. K. Y, S. Q., A. J., N. J. and M. B. J.) have participated in the revision of the manuscript. All authors read and approved the final manuscript.
The authors have no conflicts of interest to declare.







