As our understanding of breast cancer risk factors increases, so does interest in the influence of lifestyle factors. Nutrients, foods and dietary patterns have been explored in efforts to determine what dietary recommendations can be made to reduce breast cancer risk( 1 ). Several meta-analyses suggested a possible association between meat intake and breast cancer risk despite inconsistent results( Reference Missmer, Smith-Warner and Spiegelman 2 – Reference Alexander, Morimoto and Mink 4 ). One way dietary components may influence breast cancer risk is through hormonal pathways. For example, dietary fibre( Reference Monroe, Murphy and Henderson 5 ) and dairy foods( Reference Brinkman, Baglietto and Krishnan 6 ) were shown to influence endogenous sex hormone levels. More evidence that a low-meat diet may affect steroid hormones comes from dietary pattern studies. In the Nurses’ Health Study( Reference Fung, Hu and Barbieri 7 ), a better Alternative Healthy Eating Index score was associated with lower plasma levels of oestradiol (E2), and oestrogen levels were higher among women with a food pattern high in meat( Reference Fung, Schulze and Hu 8 ). In a study of postmenopausal women( Reference Aubertin-Leheudre, Hamalainen and Adlercreutz 9 ), vegetarians had lower plasma levels of oestrogens than omnivores. Another comparison of vegetarians and omnivores found lower circulating free E2 and testosterone in vegetarians even after controlling for body weight( Reference Karelis, Fex and Filion 10 ). In a randomized dietary trial of women on a Mediterranean diet characterized by high vegetable intake, a significant decrease in total oestrogens was observed( Reference Carruba, Granata and Pala 11 ).
To date, most studies of diet and oestrogen metabolism were conducted in postmenopausal women due to fluctuations during the menstrual cycle that challenge steroid hormone assessment in premenopausal women. However, one study reported few differences in hormonal and dietary profiles by menopausal status( Reference Karelis, Fex and Filion 10 ). The role of endogenous oestrogens in breast cancer aetiology among premenopausal women is less understood than in postmenopausal women; testosterone and progesterone appear to play an important role( Reference Eliassen, Missmer and Tworoger 12 – Reference Kaaks, Berrino and Key 14 ). For premenopausal women within the Nurses’ Health Study, higher urinary oestrone (E1) and E2 levels were associated with a significant 50 % lower risk, suggesting that a higher urinary excretion of parent oestrogens may be protective( Reference Eliassen, Spiegelman and Xu 15 ). Differences in the major metabolic pathways for oestrogens, i.e. the more carcinogenic 4-OH and 16α-OH metabolites and the less harmful 2-OH metabolites, may also contribute to breast cancer risk( Reference Obi, Vrieling and Heinz 16 ).
To better understand the relationship between diet, especially low v. high meat intake, and oestrogen among premenopausal women, we combined data from two previous studies that collected multiple serum and urine samples( Reference Maskarinec, Franke and Williams 17 , Reference Maskarinec, Ollberding and Conroy 18 ) and compared serum E1 and E2 and nine urinary oestrogen metabolites from 272 premenopausal women stratified by meat intake. Neither of the two interventions detected an effect of soya on serum oestrogen levels( Reference Maskarinec, Franke and Williams 17 , Reference Maskarinec, Ollberding and Conroy 18 ); a small change in urinary oestrogens was seen in BEAN2( Reference Morimoto, Conroy and Pagano 19 ) but not when both studies were analysed together( Reference Maskarinec, Morimoto and Heak 20 ).
Methods
Study population
The original Breast, Estrogen, and Nutrition (BEAN1) study, conducted in 2000–2003, randomized 220 women to intervention and control groups; 190 women contributed at least four samples to the present analysis( Reference Maskarinec, Franke and Williams 17 ). The BEAN2 trial was conducted in 2007–2010 as a cross-over study with 6 months each on a high- and a low-soya diet separated by a 1-month washout period( Reference Maskarinec, Morimoto and Conroy 21 ). Of the ninety-six randomized women, eighty-two participants completed both diet periods. Eligibility criteria for both studies included a normal mammogram, no breast implants, no oral contraceptives, not pregnant, no previous cancer diagnosis, intact uterus and ovaries, regular menstrual periods and low soya intake. For BEAN2, the participants also had to produce at least 10 μl nipple aspirate fluid, one of the study outcomes( Reference Maskarinec, Morimoto and Conroy 21 ). The same dietary intervention protocol was used in both studies; the high-soya diet consisted of two servings of soya foods providing approximately 50 mg of isoflavones per day. During the low-soya diet, participants continued their regular diet and were counselled to minimize soya intake. The protocols of both studies were approved by the University of Hawaii Committee on Human Studies and by the Institutional Review Boards of the participating clinics. All women signed an informed consent form before entry into the trial and gave written permission to use frozen samples for future analyses. A Data Safety Monitoring Committee reviewed the progress of the studies, reasons for dropouts and any reported symptoms annually.
Data collection
All participants completed a baseline FFQ validated for a multiethnic population( Reference Stram, Hankin and Wilkens 22 ); in a calibration study, the correlations between FFQ and 24 h recall data were 0·57–0·74 for nutrient densities. The questionnaire also included information on demographic characteristics, anthropometric measures and reproductive health. To assess adherence to the study protocol, all participants completed seven unannounced 24 h dietary recalls. In BEAN1, all recalls during the 2–year period were conducted by telephone( Reference Maskarinec, Franke and Williams 17 ), whereas in BEAN2, trained staff collected the first recall in person during the screening visit and three recalls by telephone during each diet period( Reference Maskarinec, Morimoto and Conroy 21 ). The 24 h recalls were scheduled randomly in intervals of a few weeks or months, and used standardized protocols, standard probes and a three-pass method to obtain a detailed account of all foods and beverages consumed during the previous day. The dietitian inquired about preparation methods and additions and probed about easily forgotten foods. Both weekdays and weekend days were captured. Recalls were conducted at multiple points during the study years to reflect seasonal variation in food selection. The FFQ and the 24 h recalls were analysed utilizing the Food Composition Table maintained by the Nutrition Support Shared Resource at our Center( Reference Murphy 23 ); the databases represent an extensive list of local foods consumed by the various ethnic populations of Hawaii and the Pacific.
Oestrogens in blood and urine
Collection of serum and urine samples was attempted to occur during the mid-luteal phase (3–11 d before the next menstruation). However, due to scheduling problems, 14 % and 24 % of visits in BEAN1 and BEAN2, respectively, occurred outside the luteal phase. Based on the available information, it was not possible to estimate the exact cycle day. In BEAN1, timing was determined using ovulation kits and confirmed retrospectively by serum progesterone levels( Reference Maskarinec, Franke and Williams 17 ), while in BEAN2 the cycle day was estimated based on the last menstruation date and confirmed by the onset date of the next menstruation obtained via telephone contact with participants( Reference Maskarinec, Morimoto and Conroy 21 ). All specimens were stored at −80°C after aliquoting. Using validated RIA, five repeated serum samples for BEAN1 and three samples for BEAN2 were analysed for E1 and E2 in 0·5 ml serum( Reference Goebelsmann, Bernstein and Gale 24 ). Based on blinded samples, the inter-assay CV were 17·7 % for E1 and 11·2 % for E2 in BEAN1 and 15·0 % for both E1 and E2 in BEAN2( Reference Maskarinec, Franke and Williams 17 , Reference Maskarinec, Morimoto and Conroy 21 ).
In both studies, repeated overnight urine samples were collected in containers with added ascorbic and boric acid to control bacterial growth( Reference Maskarinec, Morimoto and Heak 20 ). For BEAN1, the baseline and the final samples (24 months) were analysed for 173 women after 7–10 years of storage. For the seventy-nine BEAN2 participants, three samples (baseline, end of low-soya diet and end of high-soya diet at 6 or 13 months) were analysed after 0–3 years of storage. The samples were divided into three sets and analysed during 2010. Consistency across rounds was checked by including external urines. The predominant steroidal oestrogens in premenopausal women( Reference Eliassen, Ziegler and Rosner 25 ), namely E1, E2, 2-OHE1, 2-OHE2, 2-MeOE1, 4-OHE1, oestriol (E3), 16-keto-E2 and 16α-OHE1, were measured by LC–MS (model Exactive; Thermo Fisher Scientific, Waltham, MA, USA) using five labelled internal standards as described previously( Reference Franke, Custer and Morimoto 26 ). Six less common metabolites that constituted 6·5 % of all metabolites in an analysis among premenopausal women were not assessed( Reference Eliassen, Ziegler and Rosner 25 ). Ascorbic acid was added during hydrolysis and derivatization to prevent artificial oxidation of sensitive analytes. This urine pool from premenopausal women repeated on nine different days revealed CV of 4–21 % depending on the analyte concentrations. Urinary creatinine concentrations were measured using a Roche-Cobas MiraPlus clinical chemistry autoanalyser (Roche Diagnostics, Switzerland). Urinary isoflavonoids as a biomarker for soya intake were assessed by HPLC in BEAN1( Reference Maskarinec, Franke and Williams 17 ) and by LC–MS in BEAN2( Reference Maskarinec, Morimoto and Conroy 21 ). All urinary measurements were expressed per mg creatinine to adjust for urine volume.
Statistical analysis
The SAS statistical software package version 9·2 was used for the statistical analysis. Based on the literature( Reference Haddad and Tanzman 27 ) and on mean values for the seven 24 h recalls, we defined vegetarians as women who ate less than 30 g/d in the combined categories of red meat, poultry and fish, and pescatarians as those consuming red meat and poultry less than 20 g/d but fish greater than 10 g/d. All other women were considered non-vegetarians. Because of the small numbers, the eighteen vegetarians and nineteen pescatarians were combined into one group of semi-vegetarians for statistical analysis. We calculated the sum of the nine urinary metabolites measured in both studies (total urinary oestrogens), the relative percentages for the three metabolic pathways (2-, 4- and 16α-OH) based on molar concentrations, and the ratio of 2-OHE1 to 16α-OHE1. We applied mixed models to incorporate the covariance structure of the repeated measures within individuals and to allow for the varied lengths and sample collection times of the two studies and the inclusion of women with partially missing data to compute least-square means adjusted for age, BMI, ethnicity, total energy intake, parity, study status (BEAN1 or BEAN2), dietary assignment (low v. high soya), time of sample collection (study month) and menstrual cycle phase (within or outside the luteal phase). With one exception (2-OH pathway), serum and urinary measures were log-transformed due to non-normal distributions prior to assessing the significance of the difference between groups. Because soya food consumption did not significantly modify oestrogen levels in serum( Reference Maskarinec, Franke and Williams 17 , Reference Maskarinec, Ollberding and Conroy 18 ) or urine( Reference Maskarinec, Morimoto and Heak 20 ), we included all time points into the current analysis. However, we performed sensitivity analyses to examine the same models after excluding samples collected during the high-soya period and samples not taken during the luteal period.
Results
The ethnic distribution of the 272 participants, aged 41·9 (sd 4·5) years at randomization, was 41 % White (n 112), 36 % Asian (n 98), primarily Japanese, and 23 % Other (n 62), primarily Native Hawaiian (Table 1). Differences in dietary intake between BEAN1 and BEAN2 participants were seen at baseline and persisted throughout the study period. Using dietary recall data, nearly 14 % of participants (n 37) were categorized as semi-vegetarians, consisting of 60 % Whites (n 22), 32 % Asians (n 12) and 8 % other ethnic groups (n 3). The two groups were similar in age (P = 0·78), but semi-vegetarians had a lower BMI (23·9 (sd 5·1) v. 26·3 (sd 5·3) kg/m2; P = 0·01) and non-significantly lower urinary creatinine (868 (sd 372) v. 1140 (sd 152) mg/l; P = 0·50) than non-vegetarians.
Table 1 Characteristics of BEAN1 and BEAN2 participants at baselineFootnote *
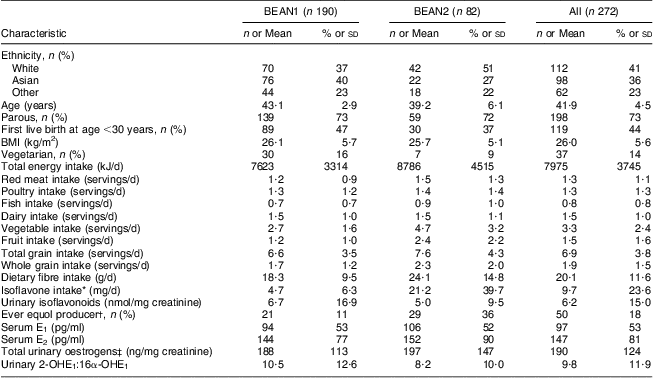
BEAN, Breast, Estrogen, and Nutrition; E1, oestrone; E2, oestradiol; E3, oestriol.
* Dietary intakes are estimated from a 1-year FFQ; isoflavone intake is estimated from a 24 h recall.
† Equol producer status is based on detecting urinary daidzein excretion ≥2 nmol/mg and urinary equol:daidzein ≥0·018 in at least one of the urine samples collected throughout the study.
‡ Sum of E1, E2, 2-OHE1, 2-OHE2, 2-MeOE1, 4-OHE1, E3, 16-keto-E2 and 16α-OHE1.
Mean intake levels according to the dietary recalls during the study period were in agreement with most FFQ-based values (Table 2). Both the FFQ analysis and the recalls showed that semi-vegetarians consumed less meat, poultry and fish than non-vegetarians; these differences were significant except for fish based on recalls. According to both assessments, semi-vegetarians consumed more vegetables, whole grains and dietary fibre than non-vegetarians although not all differences were statistically significant. At the same time, semi-vegetarians reported lower total energy intakes according to both methods and lower total grain intake based on the FFQ values. Adjustment for BMI attenuated the differences in total energy intake but did not eliminate them. Intakes of dairy and fruit did not differ much by dietary group.
Table 2 Dietary intakes among 272 BEAN1 and BEAN2 study participants
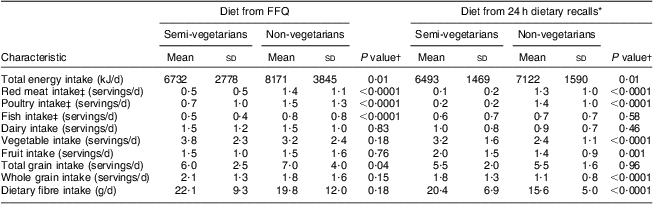
BEAN, Breast, Estrogen, and Nutrition.
*Mean of seven unannounced 24h dietary recalls over 2 years (BEAN1) and 13 months (BEAN2).
†Obtained from Student t tests.
‡1 serving ≈ 30 g.
When E1 and E2 levels were compared by meat-eating status, serum but not urinary levels were significantly lower in semi-vegetarians than non-vegetarians (Table 3). Whereas the respective differences for serum E1 and E2 were 85 v. 100 pg/ml and 140 v. 154 pg/ml (P = 0·04 for both), the urinary E1 and E2 values were similar in both groups. The small difference in total urinary oestrogens (183 v. 200 pmol/mg creatinine) was not statistically significant (P = 0·27), nor were any differences observed for the other metabolites or the ratio 2-OHE1:16α-OHE1 (10·9 v. 11·6; P = 0·36). Repeating the analyses for urinary E1, E2 and E3 using absolute values instead of percentages did also not reveal any differences by dietary pattern (data not shown). Dividing non-vegetarians into low and high meat consumers did not indicate any trend with increasing meat intake (data not shown).
Table 3 Serum and urinary oestrogen levels by meat-eating status among 272 BEAN1 and BEAN2 study participants
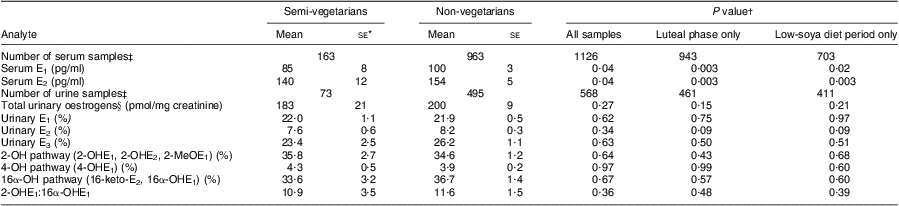
BEAN, Breast, Estrogen, and Nutrition; E1, oestrone; E2, oestradiol; E3, oestriol.
*Results are least-square means and standard errors obtained from mixed models and adjusted for ethnicity, BMI, age, total energy intake, parity, study month, luteal phase (yes or no), study (BEAN1 or BEAN2) and dietary assignment (low or high soya).
†P values were computed on log-transformed variables except for the 2-OH pathway.
‡Number of samples analysed differed between serum and urine because more serum samples were measured.
§Sum of E1, E2, 2-OHE1, 2-OHE2, 2-MeOE1, 4-OHE1, E3, 16-keto-E2 and 16α-OHE1.
When the analysis was restricted to the 943 serum or 461 urine samples collected during the luteal phase of the menstrual cycle, the associations by dietary pattern were strengthened. The differences between non-vegetarians and semi-vegetarians were greater for serum E1 (78 v. 95 pg/ml; P = 0·003), serum E2 (126 v. 146 pg/ml; P = 0·003), total urinary oestrogens (171 v. 193 pmol/mg; P = 0·15) and urinary E2 (7·0 v. 8·1 %; P = 0·09). Similarly, the differences between semi-vegetarians and non-vegetarians persisted after excluding all samples collected during the high-soya diet period and limiting the analysis to the remaining 703 serum or 411 urine specimens; the respective values were 82 v. 100 pg/ml (P = 0·02) for serum E1, 131 v. 156 pg/ml (P = 0·003) for serum E2 and 187 v. 201 pmol/mg (P = 0·21) for total urinary oestrogens. The results for individual urinary oestrogens, metabolic pathways and the ratio 2-OHE1:16α-OHE1 remained non-significant in these sensitivity analyses.
Discussion
In the current comparison among premenopausal women, those with minimal meat intake (vegetarians and pescatarians) had lower levels of circulating E1 and E2 than non-vegetarians, but no significant differences in the amount or the relative proportion of urinary oestrogen metabolites were seen. The fact that limiting the analysis to luteal samples strengthened the associations for serum oestrogen levels confirms the importance of controlling for timing within the menstrual cycle when studying sex hormones among premenopausal women. The dietary analysis indicated that the semi-vegetarian group consumed less food and total energy than non-vegetarians with higher intakes of whole grains, fibre and vegetables. These findings agree with other reports showing that vegetarians consume less saturated fat by replacing animal-based foods with lower-fat and energy-dense plant-based foods and tend to weigh 3–20 % less( Reference Lanou and Svenson 28 ).
To date, the research on meat intake and breast cancer risk has been inconsistent( Reference Missmer, Smith-Warner and Spiegelman 2 – Reference Alexander, Morimoto and Mink 4 ). Several meta-analyses reported relatively weak associations primarily from case–control studies; however, hormone receptor status and timing of meat consumption in early v. later life were suggested as areas that need additional research( Reference Alexander, Morimoto and Mink 4 , Reference Michels and Willett 29 ). Dietary patterns and their assessment of overall intake v. a single food or nutrient appear to be a promising avenue for gaining new insight into the relationship between high meat intake and breast cancer risk( Reference Fung, Hu and Barbieri 7 – Reference Carruba, Granata and Pala 11 ). These studies indicate that patterns high in meat and low in plant-based foods may be associated with higher circulating sex steroid levels in women, but other studies reported little difference in oestrogen levels in relation to meat consumption( Reference Brinkman, Baglietto and Krishnan 6 , Reference Thomas, Davey and Key 30 ). Given the difficulties of assessing dietary intake and hormone status accurately, observational studies may not be able to provide a conclusive answer to whether diet affects sex steroid levels. It may take dietary trials, such as the Mediterranean diet intervention described above( Reference Carruba, Granata and Pala 11 ), to further explore this question.
The current analysis was limited by the relatively low proportion of women who maintained a vegetarian or pescatarian diet. As in all studies among premenopausal women, fluctuations of hormone levels during the menstrual cycle challenge the interpretation of hormonal measurements. However, standardization of specimen collection during the luteal phase and the ability to exclude values not considered luteal allowed us to control for cyclical variations in hormone levels. It is also well known that oestrogen levels in serum do not necessarily reflect concentrations in the breast. Confounding by physical activity( Reference Campbell, Foster-Schubert and Alfano 31 ) and other lifestyle factors may have affected our results, but physical activity was not assessed in our studies. The major strength of the current analysis is the repeated measurement design, both for 24 h dietary recalls and for hormonal measures. This approach allowed us to capture long-term dietary behaviour and hormonal exposure better than a single measurement at one point in time. As indicated by the lack of an effect of the soya diet on hormonal outcomes( Reference Maskarinec, Franke and Williams 17 , Reference Maskarinec, Ollberding and Conroy 18 , Reference Maskarinec, Morimoto and Heak 20 ) and the results of the sensitivity analysis, the intervention design did not affect our findings. Although the classification of dietary status was based on intake as estimated by 24 h dietary recalls and not self-defined eating patterns, past research indicates that even self-defined vegetarians eat some meat; one study reported that two out of three self-defined vegetarians consumed some meat and often more than 10 g/d( Reference Haddad and Tanzman 27 ). The agreement between food intake assessed by 24 h recalls and FFQ provides additional validity to our classification despite the well-documented shortcomings of both methods; we found consistent differences in reported dietary patterns of non-vegetarians v. vegetarians/pescatarians. Small differences are to be expected because the baseline FFQ and the 24 h recalls covered different time periods and because some change in food consumption occurred as a result of the dietary intervention.
Our findings suggest that semi-vegetarians have lower serum oestrogen levels than non-vegetarians and agree with current dietary recommendations for cancer prevention published by the American Institute for Cancer Research, ‘To choose mostly plant foods, limit red meat, and avoid processed meat’( 1 ). Given that an estimated 30–35 % of all cancers may be due to dietary factors( Reference Lanou and Svenson 28 ), such advice may have a strong potential for cancer-preventive effects. However, considering the inconsistent literature related to meat consumption as a risk factor for breast cancer, the relatively small number of participants in the semi-vegetarian group, the null findings for urinary oestrogen concentrations and the wide variability of oestrogen values, the present study has to be interpreted with caution. Future investigations need to look at a larger population of premenopausal women who maintain a vegetarian or pescatarian diet and donate specimens at well-defined times during the menstrual cycle and/or conduct randomized dietary modification trials.
Acknowledgements
Sources of funding: This work was supported by R01CA80843 and by a Postdoctoral Training grant R25CA098566 from the National Cancer Institute, which had no role in the design, analysis or writing of this article. Conflicts of interest: None of the authors has a conflict of interest to declare. Authors’ contributions: G.M. and A.A.F. designed the studies and obtained funding. B.E.H. and G.M. planned the analyses. B.E.H., Y.M. and F.B. performed the statistical programming. B.E.H. and F.B. drafted the paper and prepared the tables. B.E.H. and Y.M. conducted the literature review. A.A.F. and F.Z.S. provided laboratory results and interpreted the results. All authors provided feedback on the initial draft of the manuscript and approved the final version.





