Introduction
The Aquatic Warbler Acrocephalus paludicola, once an abundant species with an extensive range throughout European fen mires, has undergone substantial population decline and is presently the only globally threatened migratory passerine on the European continent (BirdLife International 2024b; Flade et al. Reference Flade, Malashevich, Krogulec, Poluda, Preiksa, Végvári, Tanneberger and Kubacka2018; Schulze-Hagen Reference Schulze-Hagen, Glutz, Blotzheim and Bauer1991). Drainage and overgrowth of fen mires by bush communities or common reed Phragmites australis due to habitat succession and land-use changes has led to a significant shrinkage of the species’ breeding range to less than 400 km2 (Flade et al. Reference Flade, Malashevich, Krogulec, Poluda, Preiksa, Végvári, Tanneberger and Kubacka2018). The typical habitats of the species are open, mesotrophic sedge fens or wet meadows formed following fen drainage, devoid of trees and bushes or with sparsely scattered bushes, with low vegetation and water level not exceeding several centimetres above the ground (Leisler et al. Reference Leisler, Ley and Winkler1989). The breeding system with exclusively female care of the young implies high food abundance in the breeding habitat (Schulze-Hagen et al. Reference Schulze-Hagen, Flinks and Dyrcz1989).
Most studies aiming to assess local or country-wide population sizes, habitat quality, and habitat requirements of this once widespread species have relied on estimates of singing male densities (e.g. Kloskowski et al. Reference Kloskowski, Tanneberger, Marczakiewicz, Wiśniewska and Choynowska2015; Tanneberger et al. Reference Tanneberger, Flade, Preiksa and Schröder2010). Owing to the secretive behaviour of females, much less is known about the nesting biology and reproductive success of the species. Nesting success and the causes of nest failure especially deserve careful attention as they may provide information about key population threats (Dyrcz and Zdunek Reference Dyrcz and Zdunek1993a; Vergeichik and Kozulin Reference Vergeichik and Kozulin2006). Also, the research on Aquatic Warbler nesting biology in areas where the species is nowadays present has naturally focused on core breeding grounds in the Biebrza Valley (north-east Poland) and in Belarus (Dyrcz and Zdunek Reference Dyrcz and Zdunek1993a; Kubacka et al. Reference Kubacka, Oppel, Dyrcz, Lachmann, Barros, Costa and Kail2014; Vergeichik and Kozulin Reference Vergeichik and Kozulin2006); but see also Wawrzyniak and Sohns (Reference Wawrzyniak and Sohns1977) on an abandoned breeding area at Rietzer See, Brandenburg, and Tanneberger et al. (Reference Tanneberger, Bellebaum, Helmecke and Minets2013) on the scattered and vanishing Pomeranian population, both at the western range limit, east Germany/north-west Poland. Information on the breeding ecology and reproductive success in the other present strongholds of the Aquatic Warbler, including the southern range limit of the species, is needed. This is especially important because the habitats of these populations may be exposed to large-scale transformations, for example, the disturbance of the hydrological regime of floodplain mires via deepening of riverbeds in Ukraine or the planned construction of the transnational waterway E40 (Poluda Reference Poluda2017; Sutherland et al. Reference Sutherland, Atkinson, Broad, Brown, Clout and Dias2021).
We studied the breeding biology of the Aquatic Warbler in calcareous fens near Chełm, which form a Natura 2000 protected area, in south-east Poland. The calcareous fens, dominated by the Cladietum marisci sedge community, together with the sedge marshes of the Polesie National Park, located northwards, support the second largest population of Aquatic Warbler in Poland and globally form one of the species’ main breeding areas (Flade et al. Reference Flade, Malashevich, Krogulec, Poluda, Preiksa, Végvári, Tanneberger and Kubacka2018). We aimed to evaluate the reproductive parameters of the Aquatic Warbler, i.e. nesting phenology, nest and singing male densities, clutch size, and the success of hatching and fledging, and to compare breeding success parameters between the first- and second-brood period. We also attempted to determine the causes of nest losses and measured nest habitat characteristics to relate them to nest survival. For a declining species, in which only females care for offspring, assessment of reproductive success during the nest stage and elucidating the causes of egg/nestling losses can help to identify population threats that occur in breeding areas and provide knowledge necessary for guiding conservation strategies.
Methods
Study areas
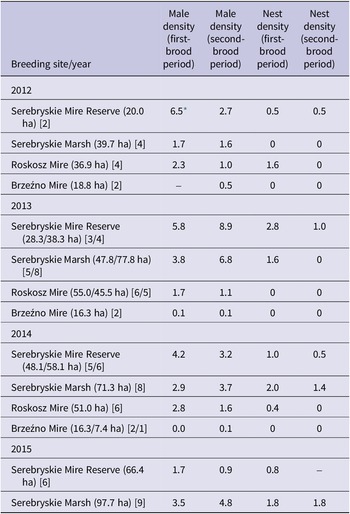
The study was carried out from mid-May till early August in 2012–2015 in calcareous fens near Chełm in south-east Poland, which are alkaline wetlands formed on calcium carbonate-rich deposits. The fens developed as a result of the accumulation of organic and mineral matter in karst depressions shaped by postglacial waters in calcareous substrates (Wołejko et al. Reference Wołejko, Pawlaczyk and Stańko2019), and comprise four sites, the Serebryskie Mire Reserve (area 377 ha), calcareous meadows Serebryskie Marshes (area 606 ha), Roskosz Mire (606 ha), and Brzeźno Mire (255 ha) (Figure 1). These four sites, situated close to each other, are part of a historical fen complex, presently encompassing mesotrophic fens and transition zones to meadow communities; they are all covered by the Special Protection Area “Chełm Calcareous Marshes” PLB060002 (4,309 ha) and a large part of the fens is covered by the Special Area of Conservation “Chełm Marshes” PLH060023 (2,124 ha). The alkaline substrate implies development of unique calciphilous vegetation. The areas occupied by the Aquatic Warbler are typically dominated by the sedge association Cladietum marisci, with vast patches of the great fen-sedge Cladium mariscus (Cyperaceae) (Figure 2a) interspersed with tall sedge Magnocaricion associations, mainly Caricetum elatae, C. distichae, and C. buxbaumii, and with meadow grasses on the edges of the fens. A distinct feature of the habitat is the prevalence of the great fen-sedge, a highly productive, large-sized (above-ground shoots of about 1 m in height) perennial, which, at least in eastern Poland, is strongly dependent on a calcareous substrate; the three Chełm reserves are one of the most important strongholds of this relict sedge in Europe (Buczek Reference Buczek2005). However, the individual sites are under diverse habitat threats and require different management. In some years, water levels in the Brzeźno and Roskosz Mires were too high for the Aquatic Warbler (Buczek and Buczek Reference Buczek and Buczek2017; Grzywaczewski et al. Reference Grzywaczewski, Bochniak, Wiącek, Łapiński and Morelli2017), while the Serebryskie Marsh, the only (in large part) privately owned and extensively utilised breeding site, is a relatively “flat” (without sedge tussocks) and dry area with the lowest share of Cladium, threatened by encroachment of meadow vegetation from the edges. The Chełm calcareous fens support in total an estimated 200–270 singing Aquatic Warbler males (Buczek and Buczek Reference Buczek and Buczek2017; Grzywaczewski Reference Grzywaczewski2015). The fens also provide breeding habitats for a variety of marshland birds (Buczek and Buczek Reference Buczek and Buczek2017) and have been identified as an Important Bird and Biodiversity Area (IBA) site (BirdLife International 2024a; Grimmett and Jones Reference Grimmett and Jones1989).

Figure 1. Location of the Aquatic Warbler Acrocephalus paludicola breeding sites covered in this study: 1. Serebryskie Mire Reserve; 2. Serebryskie Marshes; 3. Roskosz Mire; 4. Brzeźno Mire.

Figure 2. (a) Aquatic Warbler Acrocephalus paludicola male singing in a Cladium mariscus patch; 4 July 2015, Serebryskie Marsh (Photograph: M. Gągała) (b) Nearly fledged young Aquatic Warbler Acrocephalus paludicola; 25 June 2015, Serebryskie Marsh (Photograph: J. Wołoszkiewicz)
Fieldwork was conducted on plots of about 10 ha. Each year, 2–5 plots were situated within each fen site. The plots were representative of the species’ breeding habitat and together covered a significant part of the habitat suitable for the Aquatic Warbler within the individual sites. Individual plots typically included habitats varying in terms of the stage of vegetation succession (i.e. areas open and covered with shrubs) and human management/disturbance (at different successional stages after mowing or fire). In the Brzeźno Mire, the smallest breeding site, the surveyed plots were approximately the same in consecutive years, while in the other breeding sites the total size of plots varied and the plots partly overlapped between years. In 2012–2014, study plots were located in all four breeding sites; owing to the gradual abandonment of the Brzeźno Mire by the Aquatic Warbler and strong population decline in the Roskosz Mire (see Discussion), in 2015, plots were situated only in the two remaining sites.
Timing of breeding
Based on observations of nesting females, especially the marked females in 2014–2015 (see below), we divided the breeding season into the “first-brood period” (clutches initiated before 18 June in 2013, before 7 June in 2014, and before 20 June in 2015), and the “second-brood period” (clutches initiated after 1 July in 2013, after 13 June in 2014, and after 29 June in 2015). In each year, there was a gap of 1–2 weeks during which no new nests were found. In 2012, only a few nests were detected, in May and July. Broods started during the second-brood period were principally re-nesting attempts after clutch/brood loss or after successful first breeding (replacement clutches after early breeding failure could be laid during the first-brood period as well), although in most cases, we could not reliably determine whether a late-initiated nest was a first or a second breeding attempt of a given female in the season.
Aquatic Warbler male counts
The density of singing Aquatic Warbler males is indicative of both nest density and fledgling production in an area (Kubacka et al. Reference Kubacka, Oppel, Dyrcz, Lachmann, Barros, Costa and Kail2014). Observers mapped singing males (Figure 2a) within the selected plots as described by Kloskowski and Krogulec (Reference Kloskowski and Krogulec1999); the counts were carried out from 19h30 to 21h30, that is, peak times of singing activity around the sunset (Dyrcz and Zdunek Reference Dyrcz and Zdunek1993a). As numbers of singing Aquatic Warbler males show seasonal peaks which can be related to the main periods of egg laying by females (reviewed by Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018), four counts were typically conducted, two during the first- (“early”; between late May and early June) and two during the second-brood period (“late”; between late June and early July). However, for logistical reasons a few plots were surveyed only during the early or late period. Early and late counts were typically separated by 3–4 weeks. The singing male numbers obtained from counts on individual plots were combined to produce average male densities for the entire breeding sites during the early and late breeding season.
Nest surveys
Nests were located by systematic searching of plots and parting ground vegetation following observation of females whose behaviour indicated nest proximity (i.e. alarming, carrying food). Typically, females give alarm calls on detection of humans in the vicinity of the nest (Schulze-Hagen Reference Schulze-Hagen, Glutz, Blotzheim and Bauer1991). Potentially breeding females were observed using binoculars and spotting scopes, usually by 1–2 field workers, to facilitate nest location. During each field visit, locations of individual females were marked using coloured ribbons and recorded on orthophoto maps. Detected nests (Figure 2b) were marked with small ribbons in a way allowing quick re-detection by researchers but not facilitating nest detection by potential predators; the location of the nests was also recorded using a GPS. As it was extremely difficult to detect nests prior to or at the onset of egg laying, we calculated the clutch initiation date by backdating from known dates (typically hatching dates or fledging dates). We assumed that one egg was laid per day, incubation lasted 15 days (Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018), and the hatching to fledging period lasted 14 days (Wawrzyniak and Sohns Reference Wawrzyniak and Sohns1977).
To help in identifying the factors limiting breeding success (e.g. predation, nest flooding) and in collecting data on breeding parameters, such as fledgling number, V8 DTY (Shenzhen) or GOPRO (Hero) mini-cameras were set in 2014 and 2015 at 18 and 36 nests, respectively. Unless detected later, each focal brood was recorded at least once in the first and second post-hatching week. A small camera lens was placed by the nest so as not to interfere with feeding and not to facilitate nest location by predators; the cameras did not seem to disturb the females. The recordings were made at all times of the day between 07h00 and 21h00. Camera batteries allowed recording for up to about 5 hours. The average time of recording was 2.3 hours (range 0.5–4.7 hours); recordings shorter than 30 minutes were discarded. All active nests were visited at 3–4-day intervals, and in 2015 the recorded nests were visited more frequently due to the replacing of batteries and memory cards in the cameras. Later, we calculated the frequency of brood feeding (female nest visits with food) from the obtained recordings. To be able to compare our data with earlier studies on nestling provisioning, we used only the observations from the period 6–9 days after brood hatching (e.g. Schulze-Hagen et al. Reference Schulze-Hagen, Flinks and Dyrcz1989).
Since 2013, nestlings were marked with standard metal rings at the age of 7–8 days (exceptionally at a later age when a nest was found at a later brood stage). During the 2014 and 2015 seasons females were caught in mist-nets set close to nests and also marked with metal and alphanumeric plastic colour rings (total N = 47 females mist-netted within the study plots), which allowed the identification of marked individuals from a distance.
While detectability of Aquatic Warbler females is low on a single visit and the proportion of undetected females remains unknown, we assume that, by searching the study plots for alarming females repeatedly throughout the whole season, we found most nests. First, we did not miss nests of detected females, because we always found them. Second, while we must have failed to detect some females, this number appears low. For example, in 2015, when Aquatic Warbler males were mist-netted far from the already detected nests, in all the study plots and throughout the entire nesting season, only three previously unknown females were captured (relative to 33 females ringed at their nests).
Nest habitat characteristics
We used three categories of nest placement: nests placed directly on the ground (typically a few centimetres above the soil, in dry, flat areas); nests built on tussocks; and nests suspended (typically above water) and interwoven in dead or living Carex/Cladium vegetation (cf. a more complex classification in Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018). Nests of the two latter types were placed higher above the ground compared with the first type.
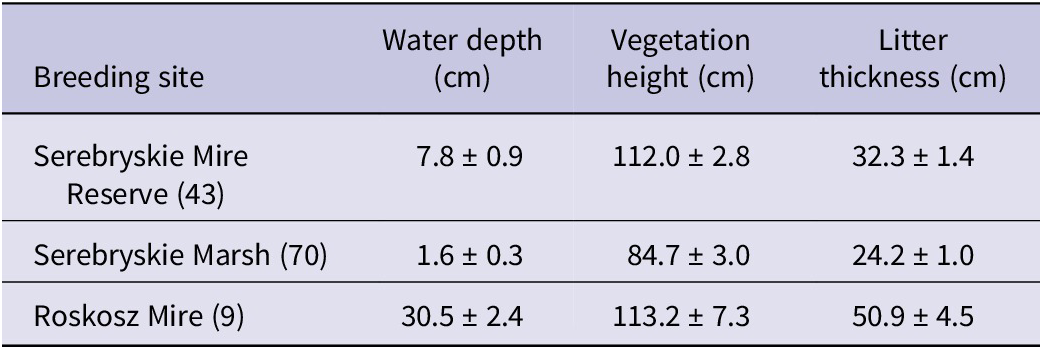
Within 50 cm of a detected nest, we measured three habitat parameters that are known to be key features of habitat selection by Aquatic Warbler in other breeding areas (Kloskowski et al. Reference Kloskowski, Tanneberger, Marczakiewicz, Wiśniewska and Choynowska2015; Tanneberger et al. Reference Tanneberger, Flade, Preiksa and Schröder2010): height of the vegetation (measured at the highest plant twigs); water depth; thickness of litter layer (both measured from ground level). The measurements were carried out at four points around the nest and averaged. Additionally, we recorded the height of the nest above the ground (measured to the nest bottom). Nest height and water depth were measured with a ruler to the nearest centimetre. The height of living vegetation and litter were recorded to the nearest 10 cm.
Statistical analysis
We compared the breeding parameters between the first- and the second-brood period using generalised linear mixed models (GLMMs) to control for the non-independence of observations within years and study sites. Generally, we did not focus on differences in breeding parameters between years since the study plots varied in habitat quality (both between and within study sites) and the between-year variation was likely biased by the selection of plots in individual years. Hence, study year and study site were considered as random factors. We used GLMMs with normal error distribution for clutch size and with Poisson error distribution for the production of fledglings from successfully hatched nests. Since the number of fledglings produced per nest can be highly dependent on clutch size or the number of hatched eggs, fledging success (the number of fledged nestlings) was modelled as a binomial response variable in a GLMM using the number of hatched nestlings as the binomial denominator. Thus, the number of “failures” within this binomial response, i.e. number of fledglings that could have been produced but were not, was due exclusively to the post-hatching mortality. The year 2012 was dropped from the models due to a relatively small sample of detected nests and unbalanced data (only one nest was found during the second-brood period; cf. Figure 3). Also, we omitted the hatching success from the comparisons due to the low numbers of nests found in which an egg did not hatch.
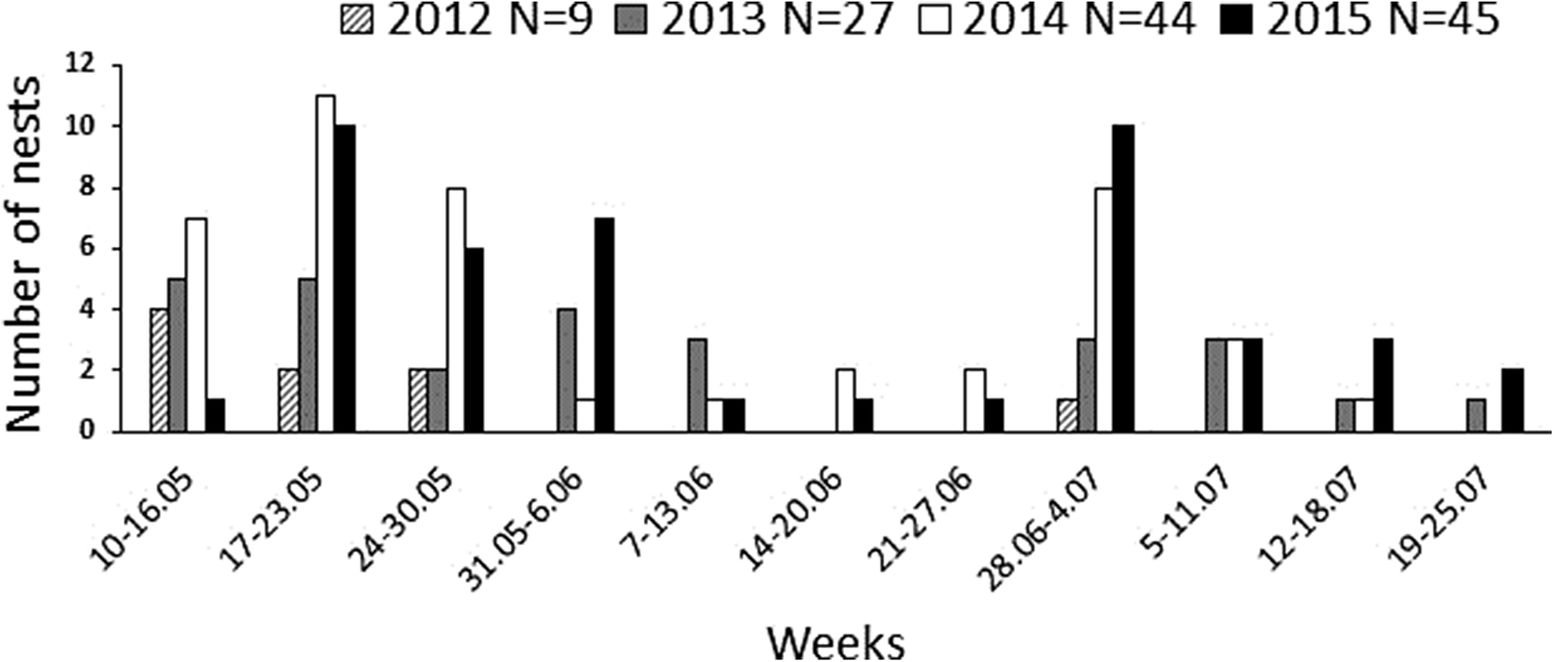
Figure 3. Aquatic Warbler Acrocephalus paludicola clutch initiation dates plotted at weekly intervals.
Nest habitat parameters were investigated using Principal Components Analysis (PCA), as the data were intercorrelated and their use as separate explanatory variables in statistical models would lead to multicollinearity issues. We first checked for intercorrelations between nest habitat features using Spearman’s correlations (some of the parameters were not normally distributed; all P <0.0001). Next, we explored the relationship between individual nest variables using a PCA based on a correlation matrix. We retained only the first PCA axis, which corresponded to 75.4% of the total variation in the nest habitat characteristics and reflected the increasing height of the nest over the ground, a gradient from thin to thick litter layer, from low to high vegetation, and from dry ground to high levels of water stagnating in the area surrounding the nest. The obtained gradient of nest characteristics was strongly correlated with all variables that contributed to the PCA (Spearman’s correlations, all P <0.0001). In the next step, a binomial GLMM was used to explore the association between the probability of nest survival to fledging (failure or at least one chick fledged successfully) and the nest habitat features. The scores of the first PCA axis were used as the explanatory variable in a model with the study site as a random factor. As the GLMM failed to converge with year as a random effect, we removed this term from the model. The GLMMs were performed using GenStat 15 (VSN International Ltd).
Apart from the GLMM estimates taking into account the local breeding sites, year, and time of season, we calculated the mean ± standard error (SE) of the clutch size and the hatching and fledging success for the total area and study period. Also, for the total data set, we calculated daily nest mortality rates and the probability of nest survival (throughout incubation and nestling periods combined) using the traditional Mayfield method (Mayfield Reference Mayfield1975), to allow comparisons with earlier studies on the breeding biology of the Aquatic Warbler in core nesting areas in the central parts of the species’ range.
Sample sizes differed between the analyses because data were not collected equally for all breeding parameters (e.g. many nests were detected only at the chick stage, in a few nests we failed to determine the number of fledglings, and in one nest we did not ascertain whether any chicks fledged). Nests detected outside the study plots were considered only in the analyses not based on nest density.
Results
General breeding parameters
Aquatic Warbler females most often laid 4–6 eggs. Two clutches, detected at a late egg-laying stage, eventually contained three eggs. The mean clutch size for the entire study area was 4.8 ± 0.1 (n = 74). In 25 of 113 (22.1%) nests with hatching success (hatched brood size greater than zero) 1–2 unhatched eggs were found. In 64 nests with known clutch size, 6.4% (± 1.5) of eggs failed to hatch. The total hatching success (whether a nest successfully hatched at least one egg) was relatively high with 92.5% (calculated for 53 nests detected at the incubation stage). The total nest fledging success (whether any chicks fledged) was 76.6% in 124 nests with known outcome, and 82.6% in 115 nests with at least 1 egg hatched (9 clutches lost during the incubation and 20 entirely failed broods during the nestling stage). The mean fledgling number was 2.7 ± 0.2 fledglings per nest (n =115 nests where the number of fledglings was determined) and 3.0 ± 0.2 in successfully hatched nests (n =106). Mean fledgling production per successfully fledged nest (in which at least one chick fledged) was 3.7 ± 0.1 (n = 86). The overall daily mortality rate calculated by the Mayfield method was 0.02 losses per nest-day, hence the probability of survival throughout the entire nesting period was 56.9%. Brood feeding frequency at the age of 6–9 days post-hatch obtained from 80.3 hours of video recordings (10 nests in 2014 and 13 nests in 2015) was 15.5 ± 1.0/hour, i.e. on average about every 3.9 minutes. The hourly feeding rate per nestling was 4.0 ± 0.3.
Early and late broods
Altogether, during the four study years we detected 80 nests in the first-brood period and 45 nests in the second-brood period (Figure 3) with mean clutch sizes 5.0 ± 0.1 (n = 50) and 4.2 ± 0.1 (n = 24), respectively. The earliest eggs were laid between 10 and 12 May in all study years. The latest clutches were initiated between 17 and 25 July except in 2012, in which only one late brood was detected, with the first egg estimated to be laid on 1 July. Of the colour-ringed females, seven were observed to initiate second broods after successful fledging of a first clutch (two females ringed during the first breeding attempt in 2014 and five of 18 colour-marked females with successful first nests in 2015). In these females, on average 14.9 ± 2.1 days (range 7–23 days) elapsed between the fledging of the first brood and the laying of the first egg of the second clutch, and the period between initiating the first and the second brood ranged from 38 to 52 days. The second-brood nests of the marked females were not begun until 27 June. The double-brooded females and also three females observed nesting in both 2014 and 2015 seasons exhibited fidelity to the breeding site; the second-brood nests were found within the same or adjacent plots. Clutch size was lower during the second-brood period and the late broods produced marginally non-significantly fewer fledglings than the early broods (Table 1 and Figure 4). Also, the post-hatching fledging success, specified as the number of fledglings produced given the number of hatched young, was lower in the second-brood period (Table 1).
Table 1. Results of generalised linear mixed models (GLMMs) relating Aquatic Warbler Acrocephalus paludicola clutch size, number of fledglings in successfully hatched nests, and number of fledglings produced given the number of eggs hatched to the time of breeding season (first- vs second-brood periods) in 2013–2015. Mean effect size with standard error of differences (SED) is reported. First-brood period was used as the reference level. Year and site identity were fitted as random terms. Sample sizes (number of nests) are shown in parentheses


Figure 4. Clutch size (open bars) and fledging success (filled bars) of the Aquatic Warbler Acrocephalus paludicola during the first- and second-brood period in 2013–2015. Data shown are generalised linear mixed model (GLMM) means and 95% confidence intervals. The fledging success estimates were back-calculated. Sample size (number of nests) is given above each column.
Male densities
Singing male densities, averaged from the first- and second-brood periods, varied between study sites when compared using counts from years 2012–2014, in which all four sites where surveyed (Friedman ANOVA χ 2 = 8.79, df = 3, P = 0.032) (Table 2). The counts (pooled per breeding site) did not reveal any differences in male densities between the first- and second-brood period, medians 2.8 (lower-upper quartile 1.7–4.1) vs 1.6 (1.0–3.7), respectively (Wilcoxon Z = 0.2, N = 12, P = 0.859; data from all study years). Within the individual sites, male numbers appeared to be higher during the first-brood period in one year, while a reverse tendency was observed in another year (Table 2). The highest male densities per plot, with 16.0 and 16.1 males/10 ha, were observed at two neighbouring plots during the second-brood period in the Serebryskie Mire Reserve. The highest densities per plot in the Serebryskie Marsh were also recorded during the second-brood period, i.e. 12.8 males/10 ha in 2014. The plots with the highest densities during the first-brood period contained 11.1 males/10 ha in the Serebryskie Mire Reserve and 9.7/10 ha in the Serebryskie Marsh in 2014. In the Roskosz Mire in the highest-density plot 5.3 and 4.3 males per 10 ha were recorded during the first- and the second-brood period, respectively. In the Brzeźno Mire the highest-density plot contained 2.8 males/10 ha during both brood periods in 2014.
Table 2. Densities of singing Aquatic Warbler Acrocephalus paludicola males and nests (per 10 ha) during the first- and second-brood period in individual breeding sites on Chełm calcareous fens in 2012–2015. The total areas of surveyed plots are shown in parentheses; double values indicate that the numbers of plots (given in square parentheses) differed between the two periods of the breeding season. Dashes indicate no survey effort

* Singing males were counted on only one 10-ha plot during the first-brood survey.
Nest densities
Total yearly densities of nests did not differ between study sites when compared using data from years 2012–2014 in which all four sites where surveyed (Friedman ANOVA χ 2 = 4.18, df = 3, P = 0.243; Table 2); an exception was the Brzeźno Mire, in which no nests were found (Table 2). When this site was omitted, nest densities per 10 ha (per year and breeding site) were higher during the first- than the second-brood period, medians 1.6 (lower-upper quartile 0.7–1.9) vs 0.5 (0–1.2), respectively (Wilcoxon Z = 2.2, N = 8, P = 0.028; data from all study years). The study plot with the highest densities of nests during the first-brood period hosted 16.5 nests/10 ha while the most densely settled plot during the second part of the season contained 6.7 nests/10 ha, both plots in the Serebryskie Marsh in 2014. In the Serebryskie Mire Reserve the most densely settled plot contained 8.1 nests/10 ha during the first-brood period and 5.1 nests/10 ha during the second part of the season in 2013. In contrast, in the Roskosz Mire the highest-density per plot was only 1.6 nests/10 ha observed during the first-brood period in 2012; during the second-brood period no nests were detected in this site during the 2012–2014 surveys. In another historical breeding site, the Brzeźno Mire, no nests were found in the entire suitable habitat area during the 2012–2014 surveys (Table 2). When the results were compared between the breeding sites, male densities were consistently higher than nest densities during both parts of the breeding season (Wilcoxon test, both P ≤0.003).
Nest habitat characteristics and nest losses
Nests placed on sedge or grass tussocks made up 50.4%, suspended nests interwoven in litter or living Carex/Cladium vegetation formed 45.4%, while nests built directly on the ground constituted only 4.1% of nests whose placement was described (N = 121). Altogether, only 45 nests (37.2%) were surrounded by Cladium, however, in the Roskosz Mire and Serebryskie Mire Reserve, which are dominated by Cladium, over half of the nests (27 of 50) were placed in Cladium patches.
The mean values of pooled nest habitat parameters are shown in Table 3. The GLMM indicated that nest survival to fledging was positively related to the PCA gradient of nest habitat (Wald χ 2 = 4.61, df = 1, P = 0.035). Although the PCA scores formed a gradient of increasing water depth in the surroundings of the nest, water levels exceeded 10 cm only at 21 (16.9%) nests, of which nine nests were found on the Roskosz Mire. Water levels by nests later destroyed by flooding varied between 4 cm and 21 cm. The strong positive correlations between the nest habitat parameters (Spearman r, all P <0.0001) showed that with increasing water level nests were placed higher in the vegetation and in areas with a thicker litter layer, which likely enhanced nest safety.
Table 3. Mean (± SE) characteristics of the Aquatic Warbler Acrocephalus paludicola nest habitat from the three main breeding sites within Chełm calcareous fens. Data from all study years (see Table 2 for the study time span at each site) were pooled per site. Sample sizes (numbers of nests with measured habitat variables) are given in brackets. Note that the figures roughly reflect the differences in breeding habitat features between individual sites
Of the 124 nests with known fate (whether or not chicks fledged) 29 (23.4%) failed to produce any fledglings. Nest flooding occurred in all the breeding sites where nests were detected and was responsible for the failure of four broods (13.8% of failed nests) during the first-brood period. Nestlings (or occasionally eggs shortly before hatching) were apparently predated in 16 nests (55.2% of losses), however, destroyed nest structure or chick remains were found in only six nests; of these, at one nest chick remains and metal rings were found in a regurgitated pellet of presumably the Western Marsh Harrier Circus aeruginosus. In most other nests eggs or chicks disappeared without clear destruction of nest structure, indicating small-sized predators/scavengers. In one nest a shrew Sorex sp. was captured by the camera; however, no predation occurred in this nest. In another nest a field slug Deroceras laeve was found on a freshly hatched chick, leaving bloody wounds on its head. Moreover, in 18 nests that fledged at least one chick, 1–4 chicks disappeared from the nest or were found dead outside the nest. Similarly, eggs/chicks disappeared gradually (over the intervals between at least three nest visits) from some later abandoned or flooded nests. It cannot be ruled out that females evicted dead nestlings from active nests, however, in six nests corpses of single chicks, obviously dead from reasons other than predation, as well as two broken eggs in one nest (the female did not abandon the clutch despite the presence of ants attracted to the successively damaged eggs) were not removed from the active nest. In eight (27.6% of losses) nests eggs or chicks were abandoned by females or the females were predated; none of these females was re-sighted in the study area again. We did not record brood starvation unless a nest had been deserted or female predated; in two broods partial brood starvation occurred. In one brood, shortly before expected fledging, all nestlings were obviously weakened, suffering from a disease or intoxication, and died although they were regularly offered food by the female.
Discussion
General results
Our study showed that the calcareous fens at the south-western range limit of the Aquatic Warbler provided a suitable habitat for this species. Clutch size was very similar to that found in Aquatic Warbler populations from more northern latitudes (reviewed by Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018). Some measures of fledging success show lower values in the Chełm calcareous fens than in the core breeding areas (Dyrcz and Zdunek Reference Dyrcz and Zdunek1993b; Kubacka et al. Reference Kubacka, Oppel, Dyrcz, Lachmann, Barros, Costa and Kail2014; Vergeichik and Kozulin Reference Vergeichik and Kozulin2006). Comparisons with other breeding grounds are difficult because they are fragmented into areas of varying habitat quality (i.e. with varying reproductive success) and due to a lack of variation estimates in some of the previous reports. The comparisons, at least with the Biebrza population, are additionally biased by the selection of the study plots intended to represent optimal habitats of the species at Biebrza, as also shown by comparatively low breeding densities in one “suboptimal” (drier and with very high tufts) Biebrza plot (Dyrcz and Zdunek Reference Dyrcz and Zdunek1993a), whereas in our study the plots were selected to be representative of the entire habitat potentially suitable for breeding. As the four breeding sites that made up the Chełm calcareous fens varied in breeding densities, they obviously included low-quality habitat patches (as indicated by the plots with no nests), especially in the Brzeźno and Roskosz Mires. However, breeding densities of both males and females (nests) in the Serebryskie Mire Reserve and Serebryskie Marsh were within the ranges reported from the main breeding grounds of the species (reviewed by Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018). Also, at the scale of c.10-ha plots, the best habitat patches near Chełm supported male and nest densities similar to the highest densities at the Biebrza River Valley and in Belarus (Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018). The proportion of nest losses (see below for the reasons for nest losses) was close to losses in the Biebrza Valley (where they were assumed to be low compared with other passerines breeding in the same area; Dyrcz and Zdunek Reference Dyrcz and Zdunek1993b) and lower than losses in Belarus, where, in some breeding sites and some years, high proportions of nests were destroyed, either due to flooding or predation (Vergeichik and Kozulin Reference Vergeichik and Kozulin2006). Finally, the brood feeding rates by females were similar to those observed in the Biebrza Marshes (Dyrcz Reference Dyrcz1993; Schulze-Hagen et al. Reference Schulze-Hagen, Flinks and Dyrcz1989), which points to high prey abundance in the calcareous fens.
Timing of breeding
Two peaks in egg-laying dates were observed during the breeding season. Both the first laying dates and the time span of the two stages of the breeding season largely match those reported from Brandenburg by Wawrzyniak and Sohns (Reference Wawrzyniak and Sohns1977) and from the Biebrza Marshes by Dyrcz et al. (Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018). Our observations of marked females confirmed that the two peaks corresponded to the first and second broods. The double-brooded females started the second brood within an average of two weeks of the first brood leaving the nest. Given that fledglings require about two weeks of further care until full independence (Schulze-Hagen Reference Schulze-Hagen, Glutz, Blotzheim and Bauer1991), and that the female builds a new nest for the second brood, breeding activities related to the second breeding attempt begin soon after (and sometimes even before) the cessation of care for the first-brood young. The interval between starting the first and the second brood was similar in range to that in the Brandenburg population studied by Wawrzyniak and Sohns (Reference Wawrzyniak and Sohns1977).
The average clutch size decreased while the fledgling production showed only a marginal tendency to decline between the first- and the second-brood period (cf. Dyrcz and Zdunek Reference Dyrcz and Zdunek1993a). We also found a greater fledgling success in the early broods when the effective clutch size (number of hatched eggs) was taken into account. Although late breeding attempts were less frequent and less successful than early broods, they can be important to sustain breeding populations and should be considered in conservation programmes, such as agri-environment schemes (cf. Arbeiter et al. Reference Arbeiter, Roth, Helmecke, Haferland, Tanneberger and Bellebaum2018).
Causes of failure at the nest stage
The main factors limiting nest survival were direct predation and nest desertion (the latter presumably also often related to predation risk or unrecognised predation on females), which together were responsible for over half of the nest failures. Ground-nesting birds are likely to suffer particularly high nest losses in open grassland-like habitats (Martin Reference Martin1993), but this is apparently not the case with the Aquatic Warbler (Dyrcz and Zdunek Reference Dyrcz and Zdunek1993b). However, in most predation cases, including partial clutch/brood losses, nest predators remained unidentified (cf. Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018); as the nest structure was often not damaged, small-bodied predators were likely. While Aquatic Warbler nests appear overall well hidden from large predatory birds or mammals, their location close to the ground makes them vulnerable to small ground predators. Shrews have been suggested as the main threat to nests by Vergeichik and Kozulin (Reference Vergeichik and Kozulin2006), however, a variety of other unspecialised predators, such as small rodents, snakes, or even slugs might contribute to egg/nestling losses (cf. Turzańska and Chachulska Reference Turzańska and Chachulska2017). Our data indicate that nests placed low above the ground and with low water levels suffered higher losses; water around the nest acts as a barrier reducing accessibility to many mammalian predators (Picman Reference Picman1988). Hence, during dry years the predation pressure of small-sized non-avian predators may increase, as they expand into habitats from which they are normally excluded by high stagnating water tables (Maxson and Oring Reference Maxson and Oring1978). Also, losses following researcher disturbance may have gone unnoticed (Götmark Reference Götmark and Power1992). The proportion of unhatched eggs (due to embryo death or fertilisation failure) was within the range reported from the Biebrza Valley and Belarus (Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018) and appears low compared with mean hatching failure percentage estimates for threatened bird species (Marshall et al. Reference Marshall, Balloux, Hemmings and Brekke2023). Still, the determination of the causes of hatching failure, comparing different populations, is warranted from the point of view of conservation genetics (Hemmings and Birkhead Reference Hemmings and Birkhead2016).
In our study nest losses due to flooding were relatively rare, however, flooding has been documented to be an important factor influencing nest survival in the species (reviewed by Dyrcz et al. Reference Dyrcz, Kozulin, Vergeichik, Kubacka, Tanneberger and Kubacka2018). Previous research indicated that habitat suitability for the Aquatic Warbler increases with increasing ground cover by water, but water levels should not exceed a few centimetres above the soil surface (Kloskowski et al. Reference Kloskowski, Tanneberger, Marczakiewicz, Wiśniewska and Choynowska2015; Kubacka et al. Reference Kubacka, Oppel, Dyrcz, Lachmann, Barros, Costa and Kail2014). The present data show that the risk of flooding applied to a relatively wide range of water depths at nest sites; in areas with high water levels nests were placed higher above the ground and in patches with greater litter thickness, indicating that pronounced water table fluctuations can be a greater problem for nests than generally elevated but fairly stable water levels. Nevertheless, nest placement may be highly important for clutch/brood survival. Nests suspended relatively high off the ground are likely better protected from small ground predators as well as from submersion in high water conditions than those placed a few centimetres above the ground; this applies in particular to habitats lacking a “tussock” structure, as nests placed in tussocks are naturally above the usual surface of the stagnating water.
Overall, Aquatic Warbler nest losses during the present study were relatively low. Given that no broods were witnessed to die of starvation (cf. Dyrcz and Zdunek Reference Dyrcz and Zdunek1993b), and only a low percentage of nests were flooded, we conclude that the Chełm fens provided stable breeding conditions; however, our research period did not cover any extreme weather events (cf. Kubacka et al. Reference Kubacka, Oppel, Dyrcz, Lachmann, Barros, Costa and Kail2014).
Habitats provided by calcareous fens and their protection challenges
The most distinctive feature of the Chełm fen vegetation, when compared with the other breeding areas of the Aquatic Warbler, is the domination of Cladium mariscus. This perennial species is highly resistant to breakage, presumably because of the abundance of mechanical tissues (Conway Reference Conway1936). Consequently, dead leaves and culms, unless submerged in water, create a long-lasting litter bed. When the production of live shoots declines, the standing dead biomass forms a thick layer preventing the growth of new Cladium and other plants (Buczek Reference Buczek2005). Both living and dead Cladium provide attractive nesting sites for the Aquatic Warbler, allowing females to build their nests above the ground. However, accumulation of the slow-rotting litter, often to a height of over 1 m, may make large patches of the habitat unsuitable for the species (Kloskowski et al. Reference Kloskowski, Tanneberger, Marczakiewicz, Wiśniewska and Choynowska2015). A dense layer of decaying ground litter is likely to hamper the locomotion of foraging birds. A flexible management approach, by periodically limiting the succession of Cladium fens, yet adapted to the current state of the sedge community, is required to maintain proper breeding conditions for the species.
Since the 1950s, Cladium fens in south-east Poland, most of them documented to support Aquatic Warbler populations, have decreased by about a half, mainly due to drainage (Buczek Reference Buczek2005). While our study plots did not cover the entire area of the fens, our results clearly indicated that the suitable habitat was shrinking. Some reserve-protected parts of the fens, previously known to host breeding Aquatic Warblers (Buczek and Buczek Reference Buczek and Buczek2017; Grzywaczewski Reference Grzywaczewski2015), especially the Brzeźno Mire, but also the Roskosz Mire, both with high water levels, were progressively abandoned. Despite their protection, the survival of the calcareous fens is threatened by changes in water conditions because of historical drainage infrastructure and limestone extraction for a cement plant (Wołejko et al. Reference Wołejko, Pawlaczyk and Stańko2019). On the other hand, high water levels mediated by obstruction of old drainage ditches, e.g. blocked by dams of beavers Castor fiber, made some parts of the fen habitats unsuitable for the Aquatic Warbler (Buczek and Buczek Reference Buczek and Buczek2017); in significant parts of these areas, reedbeds, known to be avoided by Aquatic Warblers in the core breeding grounds (Kloskowski and Krogulec Reference Kloskowski and Krogulec1999; Vergeichik and Kozulin Reference Vergeichik and Kozulin2006), have expanded. Since 2009, a gradually increasing part of the Chełm Natura 2000 areas has been covered by agri-environment schemes with postponed and partial/rotational mowing to halt vegetation succession. Their implementation, although effective in preventing overgrowth with bushes, has not always been successful in the recreation of favourable habitat conditions. In some areas, the scheme-prescribed interventions have been blamed for facilitating reed encroachment and damage to the cover of hummock mosses due to the impact of heavy machinery used on flooded mires in late summer (Buczek and Buczek Reference Buczek and Buczek2017). Parallel to agri-environment schemes as tools to protect avian diversity, the Cladietum marisci deserves to be addressed by conservation schemes dedicated specifically to this calciphilous community. However, not only the replacement of Cladietum sedges by reed, grass or bush vegetation in the succession process, but also the dominance of Cladietum at an advanced stage of succession, i.e. the prevalence of a thick layer of Cladium litter, brings unfavourable habitat changes for Aquatic Warbler populations. Adjustment of management strategies, including water level control, to the breeding requirements of the Aquatic Warbler should benefit the species as well as other marsh birds associated with the fens.
Acknowledgements
We thank Irene Arnaldos Giner, Krzysztof Beznar, Marine Boucaux, Magdalena Deruś, Belen Escudero, Flaviu Filip, Julien Foucher, Claire Frances, Michał Gągała, Mathilde Gely, Sagrario Gonzalez Cabezas, Nicolas Hillier, Grzegorz Kiljan, Karolina Kisiel, Nordine Kotbi, Jarosław Krogulec, Paul Lenrumé, Jose Alguazas Martinez, Orsolya Máthé, Sylwia Mielnicka, Sandra Morujo, Liza Olson, Ana Piñeiro, Conrado Requena Aznar, Edna Rita Correia, Leire Ruiz, Adrià Solé Cantero, Kamil Taras, Noëmie Thébaud, Fatima Torrico, Arkadiusz Wołoszkiewicz, Robert Wróblewski, and Sylwia Zgorzałek for assistance in the field, and Natalia Krajewska and Magdalena Suchora for their support with GIS data. The Regional Directorate of Environmental Protection in Lublin granted permissions (WPN.6401.70.2013.MO and WPN.6205.1.23.2014.MO) to carry out fieldwork in the Chełm reserves. Małgorzata Angel (Chełm Landscape Park) helped with accommodation during the fieldwork in the area. Our thanks are also extended to the ACROLA Team for inspiration and motivation and to Eugeniusz Mazurek for permission to access his lands. Funding was provided by the Polish Society for the Protection of Birds (LIFE09 NAT/PL/000260 project), the British Ornithologists’ Union (career development bursary to JKu in 2014, and ornithological research grant to JW in 2014), CEMEX foundation (grant to JW in 2014), and the Poznań University of Life Sciences (Grant Programme for Young Scientists, project no. 507.511.33 to JW in 2015).