Initiated over a decade ago (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010), the research domain criteria (RDoC) framework was introduced in response to a growing disconnect between clinical neuroscience and the classification used to understand psychiatric disorder. In particular, the approach taken by clinical neuroscience focused on dynamic and elastic processes that functioned over time to generate and sustain patterns of cognition, emotion, and behavior. The data generated by this approach had limited translation to front-line treatment as they were generally incompatible with a traditional nosological approach that uses diagnostic categories determined by a combinatorial selection of associated symptoms. RDoC aims to provide researchers with a data-driven, theoretically agnostic framework to integrate behavior and biology across levels of analysis. This approach reframed research priorities with the goal of yielding more nuanced classification of psychological health and dysfunction, emphasizing mechanistic validity rather than diagnostic reliability, a core feature of the prevailing approach. RDoC is currently considered a guide for basic research, although long-term applications aim to inform individualized treatment (Cuthbert & Insel, Reference Cuthbert and Insel2010). Despite its promise, RDoC has core gaps in approach that may limit its utility for prevention and intervention. Specifically, the RDoC matrix undervalues ontogenetic development, which circumscribes our understanding of the etiologies, trajectories, and maintaining mechanisms of psychopathology risk.
In this paper, we provide evidence to support the claim that integrating temperament research – defined as a young child's proclivities in experiencing and demonstrating affect, attention, activity, and regulation (Shiner et al., Reference Shiner, Buss, Mcclowry, Putnam, Saudino and Zentner2012) – into the RDoC framework will advance our understanding of the mechanistic origins that underlie psychopathology from early childhood and shape trajectories well into adulthood. Whether defined by fine-grained emotions (e.g., anger) or broad phenotypes (e.g., dysregulated fear), a young child's temperament can serve as an indicator of potential psychological health or dysfunction that is evident as early as the first year of life. Decades of research in this area has aimed to describe the genetic, neural, cognitive, emotional, and behavioral manifestations of temperament traits, while considering their interaction over time in response to varied early experiences. Indeed, temperament traits reliably predict childhood psychopathology risk, often as a function of internal (cognitive, emotional) or external (environmental) influences (see Stifter & Dollar, Reference Stifter, Dollar and Cicchetti2016 for review). Adopting a trait-based approach to early individual differences in psychopathology risk will provide a practical guide for researchers to integrate a life span neurodevelopmental perspective into the largely static RDoC matrix. A better understanding of the etiology and early emergence of psychopathology will, in turn, inform practice with both children and adults as clinicians incorporate first-line treatments sensitive to specific clinical trajectories.
The ideas we present here build upon recent calls for integrating developmental psychopathology and RDoC perspectives via a trait-based, “neural system first” approach to pediatric research (Beauchaine & Hinshaw, Reference Beauchaine and Hinshaw2020; Garber & Bradshaw, Reference Garber and Bradshaw2020). Temperament traits reflect transdiagnostic, neurobiologically based vulnerabilities that transact with environmental influences to shape behavioral development (e.g., Beauchaine, Zisner, & Sauder, Reference Beauchaine, Zisner and Sauder2017; Clauss, Avery, & Blackford, Reference Clauss, Avery and Blackford2015). This transaction between the individual and the environment is a central tenet of life span approaches to psychopathology risk (Crowell, Puzia, & Yaptangco, Reference Crowell, Puzia and Yaptangco2015). In illustrating this approach, we focus on birth through preschool age due to the rapid, widespread, and environmentally sensitive neural and behavioral development that defines this period of life. Indeed, the dynamic biology–environment interplay that takes place during this period yields individual differences in neurobehavior that support emotion, cognition, and behavior for years to follow. Across early life, these differences are thought to be elaborated upon and biologically embedded via epigenetic, morphological, and physiological mechanisms (Gottlieb, Reference Gottlieb2007). The protracted rate of neurodevelopment (beginning in utero) leaves a young child's developing brain sensitive to proximal and distal external factors, such as positive and negative aspects of the caregiving context (e.g., Bick & Nelson, Reference Bick and Nelson2017; Cui et al., Reference Cui, Mistur, Wei, Lansford, Putnick and Bornstein2018). Leveraging detailed information on specific risk traits during these early years of vulnerable brain development may aid RDoC-focused researchers and clinicians in identifying transdiagnostic and modifiable targets for intervention that reduce a young child's psychopathology risk prior to symptom onset (Wakschlag et al., Reference Wakschlag, Perlman, Blair, Leibenluft, Briggs-Gowan and Pine2018).
The RDoC Framework
Despite its relatively short tenure, the composition, merits, and shortcomings of the RDoC framework have been discussed extensively (Beauchaine & Hinshaw, Reference Beauchaine and Hinshaw2020; Casey, Oliveri, & Insel, Reference Casey, Oliveri and Insel2014; Cuthbert & Insel, Reference Cuthbert and Insel2010; Franklin, Jamieson, Glenn, & Nock, Reference Franklin, Jamieson, Glenn and Nock2015; Garber & Bradshaw, Reference Garber and Bradshaw2020; Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010; Lilienfeld, Reference Lilienfeld2014). A comprehensive review of the RDoC literature is beyond the scope of this paper. We briefly describe the RDoC framework and highlight major critiques as they pertain to research with pediatric samples.
The core component of the RDoC approach is the matrix, which provides researchers with a structured guide for investigations into basic dimensions of human functioning. The RDoC matrix consists of six major domains – negative valence, positive valence, cognitive, social processes, arousal/regulatory, and sensorimotor systems – that are further divided into several more specific constructs (e.g., acute threat, reward valuation, affiliation, and attachment), each of which is operationalized across multiple levels of analysis – molecules, cells, neural circuits, physiology, behavior, and self-report. The matrix is directly informed by research and therefore designed to be continuously evolving as new findings emerge (Cuthbert & Insel, Reference Cuthbert and Insel2010).
RDoC's emphasis on dimensional constructs permits researchers to explore patterns of functioning ranging from normal to atypical both within and between constructs. RDoC also promotes the identification of biobehavioral vulnerabilities that cut across diagnostic categories, acknowledging the heterogeneity inherent to psychological dysfunction. For example, heightened response to threat, disrupted reward processing, and attentional biases have each been implicated as mechanisms that contribute to both anxiety and mood disorders (e.g., Buss & Qu, Reference Buss, Qu, Pérez-Edgar and Fox2018; Casey et al., Reference Casey, Oliveri and Insel2014; Dillon et al., Reference Dillon, Rosso, Pechtel, Killgore, Rauch and Pizzagalli2014; Jarcho & Guyer, Reference Jarcho, Guyer, Pérez-Edgar and Fox2018). These areas of impairment cut across dimensions of positive valence, negative valence, and cognitive systems and are controlled by cortical–subcortical neural circuitry, which, in turn, is thought to modulate peripheral physiology and behavior. The RDoC domains (rows) and levels of analysis (columns) foster the identification of mechanisms underlying psychological health and dysfunction. However, since the triggers and consequences of maladaptive traits vary widely across individuals, these mechanisms must also be contextualized within the environment and across development in order to capture fully the impact on individual functioning.
The RDoC matrix has yielded promising insight into adult psychopathology; nevertheless, several limitations are worth noting. First, certain units of analysis are inextricably linked (e.g., circuits and physiology), raising questions as to whether these systems should be defined independently in the framework. Second, impairments across domains of functioning (e.g., negative valence systems, cognitive systems) exhibit strong convergent validity but often lack discriminant validity. Deficits in these systems frequently co-occur and are shared across psychopathologies, which serves the transdiagnostic goals of the framework. However, some (e.g., Shankman & Gorka, Reference Shankman and Gorka2015) argue that more focus is needed on how impairments across systems diverge as predictors of symptom clusters. For example, threat–safety discrimination, a component of fear conditioning relevant to the negative valence system, has been associated with familial risk for anxiety but not depression, while reduced sensitivity to reward, part of the positive valence system, was linked to depression but not anxiety (Nelson et al., Reference Nelson, McGowan, Sarapas, Robison-Andrew, Altman, Campbell and Shankman2013). More research clarifying the specificity of deficits within and across domains as predictors of psychological dysfunction is warranted.
Another challenge comes from the practicalities of conceptualizing and conducting RDoC-informed research without reverting to the prevailing model of clinical science. Indeed, despite being the target of investigation, biological and behavioral dysfunctions are still frequently analyzed as correlates of symptom profiles from traditional diagnostic categories, often controlling for comorbidities, eschewing the very complexity that the National Institute of Mental Health (NIMH) aims to capture. Despite explicit statements that RDoC is not intended as a diagnostic tool, attempts to link biobehavioral vulnerabilities with specific disorders in a one-to-one manner are common. Third and finally, the strong emphasis on biological underpinnings in the RDoC framework has been interpreted by some as biological reductionism (see for example, Berenbaum, Reference Berenbaum2013), framing expectations that specific units of analysis, particularly neural circuitry, should be prioritized when examining mechanisms underpinning psychological dysfunction.
In presentation and conceptualization, the RDoC matrix is also to be linked to developmental and environmental considerations. This link, however, has garnered far less attention from researchers. As the RDoC documentation states, “many areas of the child psychopathology literature (such as reward sensitivity, emotional dysregulation, behavioral inhibition) serve as a more compatible model for a dimensionally based approach compared to the highly specified categories of adult psychopathology” (NIMH, 2018). To this end, there has been a recent push to incorporate developmental perspectives more explicitly in the RDoC approach, particularly with regard to developmental pathophysiology (Franklin et al., Reference Franklin, Jamieson, Glenn and Nock2015; Mittal & Wakschlag, Reference Mittal and Wakschlag2017). Still, the RDoC matrix presents dimensions of human behavior as largely static, lacking clear guidelines on how developmental considerations should be integrated into the matrix.
RDoC's atheoretical approach was intended to free researchers from constraints of prevailing clinical diagnostic categories. For example, rather than focusing on correlates of depressive disorder, researcher A may choose to investigate the neurobiological underpinnings of ruminative thoughts, while researcher B explores trajectories of pathophysiology related to disrupted sleep, both of which are symptoms of depression. Advancements from each of these hypothetical researchers would provide insight into processes central to many forms of psychological dysfunction beyond simply depression, and may ultimately identify malleable points of intervention.
By situating itself as an atheoretical approach, however, RDoC may have limited its capacity to advance knowledge on the ontogeny of psychological dysfunction. Clearly articulating one's theoretical perspective serves two purposes: to consolidate extant evidence and to propose plausible explanations that can be subsequently tested. RDoC need not exclusively adhere to a single theoretical perspective. Flexible integration of principles from multiple theoretical perspectives may provide a robust representation of a construct of interest, facilitating multidisciplinary research that furthers knowledge on a topic. Integrating complementary theoretical perspectives is particularly important when considering developmental processes, given the complexity inherent to understanding social, emotional, cognitive, and biological system maturation across the life span. Thus, to better understand development and psychopathology risk, RDoC may benefit from a move away from its atheoretical approach and instead consider how multiple, neurodevelopmentally informed theories can be incorporated into the framework.
These limitations may contribute to the protracted integration of development into the RDoC matrix. To harness the advantages RDoC provides for examining origins and mechanisms of psychological functioning, researchers might consider trait vulnerabilities that are predictive of, but not restricted to, adult psychopathology presentation. A trait-based, “neural systems first” approach to childhood psychopathology risk (Beauchaine & Constantino, Reference Beauchaine and Constantino2017; Beauchaine & Hinshaw, Reference Beauchaine and Hinshaw2020) leverages detailed, multiple-levels-of-analysis information on a specific trait (e.g., anger) and considers it in dynamic interaction with other well-described traits (e.g., cognitive control) over time. This approach emphasizes the utility of neurobiologically informed, transdiagnostic traits as starting points to understand childhood psychopathology risk. In this way, temperament researchers are uniquely positioned to infuse development into the RDoC matrix – emphasizing individual differences in dimensional neurobehavioral proclivities examined with an appreciation for maturational and environmental influences that shape functioning over time and across levels of analysis.
Temperament in Early Life
Temperament traits across early childhood provide the foundation for many facets of adult functioning – from cognition, motivation, and attention to personality and psychopathology (Pérez-Edgar & Fox, Reference Pérez-Edgar and Fox2018; Rothbart & Bates, Reference Rothbart, Bates, Eisenberg, Damon and Lerner2006; Stifter & Dollar, Reference Stifter, Dollar and Cicchetti2016). Contemporary definitions of temperament highlight biologically based individual differences in behavior and emotionality that are reflected in moderately stable traits. The emphasis is on “moderately,” as temperament traits are malleable and reflect maturational and environmental influences across early life. Thus, trait expression reflects a complex interplay between genetic disposition, neurobehavioral activity, and the early environment, with an emphasis on the caregiving context as a determinant of functional development (Shiner et al., Reference Shiner, Buss, Mcclowry, Putnam, Saudino and Zentner2012).
Modern temperament research has been informed by two core principles (Shiner et al., Reference Shiner, Buss, Mcclowry, Putnam, Saudino and Zentner2012; Stifter & Dollar, Reference Stifter, Dollar and Cicchetti2016), described specifically below.
(a) Modern temperament research focuses on the individual through development. A central aim of temperament research is to describe traits and phenotypes that predict psychological health and dysfunction. In doing so, this work strives to characterize a child's strengths and weaknesses in light of environmental demands within and across developmental windows. This information, in turn, helps to predict developmental trajectories for specific processes and outcomes. Indeed, it is this predictive and explanatory power that makes temperament research particularly beneficial within the context of RDoC. Inherent in temperament research is also an acknowledgement that manifest emotions and behaviors may change over time in conjunction with trait or skill development in other domains. Supporting the current paper's focus on early development, a rich literature base demonstrates that temperament in the first 5 years of life is characterized by both stability and change (e.g., Braungart-Rieker, Hill-Soderlund, & Karrass, Reference Braungart-Rieker, Hill-Soderlund and Karrass2010; Bridgett, Laake, Gartstein, & Dorn, Reference Bridgett, Laake, Gartstein and Dorn2013; Gartstein & Hancock, Reference Gartstein and Hancock2019), which provides the necessary malleability for scientific and clinical utility.
(b) Modern temperament research cuts across multiple levels of analysis. Temperament research is by its very nature an investigation into individual difference across interdependent levels of analysis. Historically, the temperament literature implemented an RDoC approach, even before there was an RDoC. That is, while the literature is rooted in extensive descriptions of behavior, temperament traits have been linked to interrelated structural and functional differences across domains in the developing brain. Indeed, a core tenet of temperament research is that traits are biologically based and, to varying degrees, heritable (Saudino & Micalizzi, Reference Saudino and Micalizzi2015; Shiner et al., Reference Shiner, Buss, Mcclowry, Putnam, Saudino and Zentner2012). Converging evidence from epidemiology and developmental psychobiology supports both genetic and experience-dependent (possibly beginning in utero) pathways to the development of temperament traits via adjustments to the structure and function of early neurobehavioral systems. Many temperament approaches were rooted in models that linked biological functioning and behavioral profiles – as can be seen in the amygdala model of behavioral inhibition (Kagan, Reznick, & Snidman, Reference Kagan, Reznick and Snidman1987; discussed below). This approach is embedded in the temperament literature as seen in systematic studies of sympathetic, parasympathetic, and neuroendocrine activity at rest and in response to challenge (see e.g., Buss, Morales, Cho, & Philbrook, Reference Buss, Morales, Cho, Philbrook and Calkins2015; Miskovic & Schmidt, Reference Miskovic and Schmidt2012; Nigg, Reference Nigg2006; Stifter & Dollar, Reference Stifter, Dollar and Cicchetti2016 for reviews). Biological and behavioral activity does not, however, occur in a vacuum. To this end, contemporary temperament research also aims to understand the way in which environmental factors, such as parenting or poverty, modulate the trajectory of trait expression (and possible psychopathology risk) for a young child.
Inherent to each of the principles is the notion that temperament researchers seek to understand the processes through which traits manifest and change over time, whether it is due to maturational or environmental influences, or an interaction (or transaction) between the two. These core principles are echoed across the varying theoretical perspectives on temperament, which can broadly be categorized into deductive (theory informs interpretation) and inductive (data informs theory) approaches (see Fu & Pérez-Edgar, Reference Fu, Pérez-Edgar and Wright2015 for review).
Perhaps no deductive approach to temperament aligns with these guiding principles, or the structure of the RDoC matrix, as directly as Rothbart's psychobiological model (Rothbart & Derryberry, Reference Rothbart, Derryberry, Lamb and Brown1981). A dimensional approach, Rothbart's model argues that reactivity and self-regulation are neurobiological processes that are intimately linked and can be operationalized via three broad temperament dimensions, namely, negative affect, surgency, and effortful control. These broad dimensions can be divided into finer grained traits (e.g., anger/frustration, activity level, inhibitory control) that develop over time. In particular, this perspective emphasizes both micro-longitudinal –threshold, latency, and rate of recovery of a behavioral response – and macro-longitudinal – maturational and environmental influences on trait expression (e.g., Braungart-Rieker et al., Reference Braungart-Rieker, Hill-Soderlund and Karrass2010; Gartstein & Hancock, Reference Gartstein and Hancock2019) – change processes in its conceptualization of temperament.
The integration of temporal dynamics is reflected in the model of temperament by Goldsmith and Campos as well (Goldsmith & Campos, Reference Goldsmith, Campos, Emde and Harmon1982), which focused on developing emotional capacities as a defining feature of temperament. This perspective posits that emotion and the regulation of emotion are intertwined concepts that are difficult to disentangle without consideration of the temporal dynamics of emotions (Campos, Frankel, & Camras, Reference Campos, Frankel and Camras2004; Cole, Martin, & Dennis, Reference Cole, Martin and Dennis2004; see Pérez-Edgar, Reference Pérez-Edgar, LoBue, Pérez-Edgar and Buss2019 for counter argument). These authors have also argued for the heritable nature of emotional expression in early childhood, a view that is integral to Buss and Plomin's criterion model of temperament as well (Buss & Plomin, Reference Buss and Plomin1975; Reference Buss and Plomin1984). Each of these models converges with the suggestion that trait expression is, to varying degree, inherited and appears as a stable profile in infancy, even though they may differ in what constitutes temperament traits in the first years of life.
These dimensional models of temperament are contrasted by typological models. Typological models consider a behavioral phenotype as an emergent property that is more than the sum of its constituent behaviors, and this leads to categories, or “types,” of children. This approach is exemplified by the behavioral-style model of Thomas and Chess (Thomas & Chess, Reference Thomas and Chess1977) and Kagan's biotypological model (Kagan, Reference Kagan1994), both of which may be considered inductive approaches. In their foundational temperament study, Chess and Thomas (Chess & Thomas, Reference Chess, Thomas, Strelau and Angleitner1984; Thomas & Chess, Reference Thomas and Chess1977) categorized (most) children into one of three categories – easy, slow to warm, and difficult – based on nine dimensions of behavior identified during extensive interviews with parents. With these types in mind, Thomas and Chess argued that the most adaptive childhood outcomes would occur when the temperamental qualities of a child were congruent with environmental characteristics suited for that temperament type, a concept they referred to as “goodness of fit.” Maladaptive childhood outcomes were thought to be a consequence of a mismatch between a young child's proclivities and their environment, rather than either of these factors in isolation (Thomas & Chess, Reference Thomas and Chess1977).
Kagan and colleagues (Kagan, Reference Kagan2018; Pérez-Edgar & Fox, Reference Pérez-Edgar and Fox2018) took a different approach to identifying another behavioral phenotype, behavioral inhibition. Based on initial observations of toddlers, and emerging animal research, Kagan and colleagues proposed that individual differences in this behavioral phenotype were attributable to a hyper-reactive amygdala to novelty (Kagan et al., Reference Kagan, Reznick and Snidman1987). Over the past four decades, researchers have continued to garner data to characterize behavioral inhibition, a temperament trait defined by reticence in response to unexpected or unfamiliar stimuli. This trait is identifiable by the age of 2 (Garcia-Coll, Kagan, & Reznick, Reference Garcia-Coll, Kagan and Reznick1984), with a developmental precursor, high negative reactivity, evident in the first year of life among approximately 20% of infants (Fox, Henderson, Rubin, Calkins, & Schmidt, Reference Fox, Henderson, Rubin, Calkins and Schmidt2001; Fox, Snidman, Haas, Degnan, & Kagan, Reference Fox, Snidman, Haas, Degnan and Kagan2015). A continued emphasis on a multiple-levels-of-analysis approach has led to detailed information on the neural, physiological, cognitive, emotional, and social correlates and underpinnings of this temperament trait (see Pérez-Edgar & Fox, Reference Pérez-Edgar and Fox2018 for a detailed discussion). This work is particularly useful from the RDoC perspective as behavioral inhibition is one of the most reliable predictors of childhood internalizing of symptoms, specifically increasing the risk for social anxiety six-fold (Clauss & Blackford, Reference Clauss and Blackford2012).
Building on this line of research, Buss and colleagues (Buss, Reference Buss2011; Buss et al., Reference Buss, Davis, Kiel, Brooker, Beekman and Early2013; Buss, Davis, Ram, & Coccia, Reference Buss, Davis, Ram and Coccia2018) provided support for a dysregulated fear profile – a pattern of behavior characterized by high levels of fear in both low- and high-threat contexts. This construct expands upon Kagan's behavioral inhibition profile by identifying high-risk toddlers based on when (e.g., low-threat situation) and what type of fearful behavior is displayed, in addition to the intensity of a fear response (Buss & Kiel, Reference Buss, Kiel, Vasa and Roy2013). In this way, dysregulated fear considers situational specificity as a key component to identifying young children at risk for psychopathology. In doing so, researchers have shown that dysregulated fear is a strong predictor of childhood internalizing of symptoms above and beyond behavioral inhibition (Buss, Reference Buss2011; Buss et al., Reference Buss, Davis, Kiel, Brooker, Beekman and Early2013; Reference Buss, Davis, Ram and Coccia2018). It is worth noting that the constructs of behavioral inhibition and dysregulated fear have each been identified via dimensional analyses and latent profile analysis (Buss, Reference Buss2011; Loken, Reference Loken2004), lending support for their robust representation as predictors of psychopathology risk in early life.
More recently, researchers have sought to incorporate advanced statistical methods to identify a finite number of distinct, homogeneous groups of children defined by a shared constellation of behaviors (e.g., Beekman et al., Reference Beekman, Neiderhiser, Buss, Loken, Moore, Leve and Reiss2015; Karalunas et al., Reference Karalunas, Fair, Musser, Aykes, Iyer and Nigg2014; Ostlund et al., Reference Ostlund, Pérez-Edgar, Shisler, Terrell, Godleski, Schuetze and Eidenin press; Scott et al., Reference Scott, Lemery-Chalfant, Clifford, Tein, Stoll and Goldsmith2016). In many ways, this data-driven approach leverages the strengths of both continuous and categorical conceptualization of temperament. Namely, continuous ratings of several temperament traits (typically parent-report data, but also observed data) are used to identify a finite number of subgroups of children (typically 3–5) who have constellations of traits in common, via person-centered analytic approaches, such as latent profile analysis. A non-exhaustive list of sample characteristics for studies that have used this approach with children under the age of 5 is presented in the Supplement.
Studies using this approach broadly categorize young children into six temperament profiles. Akin to the behavioral inhibition typology identified by Kagan and colleagues, one commonly identified profile is defined by high fearfulness and low regulatory abilities (Beekman et al., Reference Beekman, Neiderhiser, Buss, Loken, Moore, Leve and Reiss2015; Caspi & Silva, Reference Caspi and Silva1995; Gartstein et al., Reference Gartstein, Prokasky, Bell, Calkins, Bridgett, Braungart-Rieker and Seamon2017; Janson & Mathiesen, Reference Janson and Mathiesen2008; Putnam & Stifter, Reference Putnam and Stifter2005; Sanson et al., Reference Sanson, Letcher, Smart, Prior, Toumbourou and Oberklaid2009; Usai, Garello, & Viterbori, Reference Usai, Garello and Viterbori2009; Van Den Akker, Deković, Prinzie, & Asscher, Reference Van Den Akker, Deković, Prinzie and Asscher2010), and may thus be considered an inhibited/fearful group of young children. Another commonly observed profile, which might be referred to as negative reactive, is similar to the inhibited/fearful profile with the exception that, rather than being exclusively defined by extreme fear, these children exhibit high levels of negative affect (e.g., anger, sadness) more broadly across contexts (Beekman et al., Reference Beekman, Neiderhiser, Buss, Loken, Moore, Leve and Reiss2015; Janson & Mathiesen, Reference Janson and Mathiesen2008; Komsi et al., Reference Komsi, Räikkönen, Pesonen, Heinonen, Keskivaara, Järvenpää and Strandberg2006; Lin, Ostlund, Conradt, & Lagasse, Reference Lin, Ostlund, Conradt and Lagasse2018; Ostlund et al., Reference Ostlund, Pérez-Edgar, Shisler, Terrell, Godleski, Schuetze and Eidenin press; Planalp & Goldsmith, Reference Planalp and Goldsmith2020; Prokasky et al., Reference Prokasky, Rudasill, Molfese, Putnam, Gartstein and Rothbart2017). Researchers have also consistently observed what might be considered a dysregulated/irritable profile, that characterizes young children who display high negative affect, particularly anger, paired with high activity levels (Beekman et al., Reference Beekman, Neiderhiser, Buss, Loken, Moore, Leve and Reiss2015; Gartstein et al., Reference Gartstein, Prokasky, Bell, Calkins, Bridgett, Braungart-Rieker and Seamon2017; Lin et al., Reference Lin, Ostlund, Conradt and Lagasse2018; Ostlund et al., Reference Ostlund, Pérez-Edgar, Shisler, Terrell, Godleski, Schuetze and Eidenin press; Prokasky et al., Reference Prokasky, Rudasill, Molfese, Putnam, Gartstein and Rothbart2017; Sanson et al., Reference Sanson, Letcher, Smart, Prior, Toumbourou and Oberklaid2009), reminiscent of trait irritability (Beauchaine et al., Reference Beauchaine, Zisner and Sauder2017; Wakschlag et al., Reference Wakschlag, Perlman, Blair, Leibenluft, Briggs-Gowan and Pine2018).
Conversely, an exuberant/fearless profile characterized by high levels of positive affect, activity, and approach oriented behavior has also been observed (Gartstein et al., Reference Gartstein, Prokasky, Bell, Calkins, Bridgett, Braungart-Rieker and Seamon2017; Prokasky et al., Reference Prokasky, Rudasill, Molfese, Putnam, Gartstein and Rothbart2017; Putnam & Stifter, Reference Putnam and Stifter2005; Sanson et al., Reference Sanson, Letcher, Smart, Prior, Toumbourou and Oberklaid2009; Usai et al., Reference Usai, Garello and Viterbori2009; Van Den Akker et al., Reference Van Den Akker, Deković, Prinzie and Asscher2010). Lastly, many studies using this approach observe either an average/low reactive profile, defined by a modest level of emotionality, attention, and activity (Beekman et al., Reference Beekman, Neiderhiser, Buss, Loken, Moore, Leve and Reiss2015; Caspi & Silva, Reference Caspi and Silva1995; Gartstein et al., Reference Gartstein, Prokasky, Bell, Calkins, Bridgett, Braungart-Rieker and Seamon2017; Janson & Mathiesen, Reference Janson and Mathiesen2008; Lin et al., Reference Lin, Ostlund, Conradt and Lagasse2018; Planalp & Goldsmith, Reference Planalp and Goldsmith2020; Prokasky et al., Reference Prokasky, Rudasill, Molfese, Putnam, Gartstein and Rothbart2017; Putnam & Stifter, Reference Putnam and Stifter2005; Usai et al., Reference Usai, Garello and Viterbori2009; Van Den Akker et al., Reference Van Den Akker, Deković, Prinzie and Asscher2010), or a well-regulated profile, defined by relatively low levels of negative affect as well as high levels of regulatory abilities, such as effortful control (Beekman et al., Reference Beekman, Neiderhiser, Buss, Loken, Moore, Leve and Reiss2015; Caspi & Silva, Reference Caspi and Silva1995; Gartstein et al., Reference Gartstein, Prokasky, Bell, Calkins, Bridgett, Braungart-Rieker and Seamon2017; Janson & Mathiesen, Reference Janson and Mathiesen2008; Komsi et al., Reference Komsi, Räikkönen, Pesonen, Heinonen, Keskivaara, Järvenpää and Strandberg2006; Lin et al., Reference Lin, Ostlund, Conradt and Lagasse2018; Planalp & Goldsmith, Reference Planalp and Goldsmith2020; Prokasky et al., Reference Prokasky, Rudasill, Molfese, Putnam, Gartstein and Rothbart2017).
The replicability of these temperament profiles despite differences in methodology (e.g., laboratory assessment, parent-report questionnaire), age, and risk status (e.g., prenatal substance exposure, Lin et al., Reference Lin, Ostlund, Conradt and Lagasse2018) underscores their robustness in early childhood.
Integrating Temperament, Development, and RDoC
We believe temperament research can advance both the refinement and application of RDoC as a conceptual structure and research tool in two meaningful ways: namely, through (a) the integration of principles of temperament theories (i.e., focus on individual over time and across levels of analysis) to inform etiologies, mechanisms, and trajectories of RDoC constructs in early childhood and (b) the identification of transdiagnostic risk traits and phenotypes that are evident prior to preschool age that reliably predict childhood psychopathology risk.
Illustrative examples
As stated by other developmental psychopathologists (Beauchaine & Hinshaw, Reference Beauchaine and Hinshaw2020; Garber & Bradshaw, Reference Garber and Bradshaw2020), the RDoC matrix would benefit from changes that embrace the complexity inherent in developmental research. One possibility would be to add a developmental qualifier that acknowledges temperamental precursors and trajectories that may contribute to individual differences to each RDoC construct. It may also be useful for identifying what systems are involved and/or might interact to shape the development of that temperamental precursor. For example, a qualifier could be added to describe temperament traits that are relevant to a specific RDoC construct, as well as when they emerge, how they change over time, and what role they play as interrelated neurodevelopmental antecedents to the construct. This approach would highlight gaps in our understanding of a developmental precursor to an RDoC construct, serving as a guide for future research endeavors.
These developmental qualifiers may also help distinguish behaviors that might be considered developmentally appropriate (e.g., the occasional temper tantrum among 2-year-olds) from worrisome patterns (e.g., prolonged tantrums at age 5), as well as points in development when behaviors move from normative to potentially problematic. Similarly to the RDoC matrix at large, these developmental qualifiers would evolve as additional longitudinal research is conducted and consensus about the causes, correlates, and contributions of specific traits is reached. Indeed, this approach would facilitate a seamless, semi-realtime integration of results from large-scale National Institutes of Health studies, such as the Environmental Influences on Child Health Outcomes, Adolescent Brain Cognitive Development, or Healthy Brain and Child Development projects, into the RDoC matrix. Crucially, no study to date has prospectively examined whether the inclusion of early emerging temperament traits into the RDoC framework will advance our understanding of childhood psychopathology risk. This reflects a gap in the literature and an essential next step for future research.
Let us consider two RDoC constructs from the negative valence system, frustrative nonreward and acute threat, as examples to illustrate how temperament research, beginning in the first year of life, can be incorporated as a developmental qualifier on the RDoC matrix. The frustrative nonreward and acute threat constructs are conceptually similar to irritability and behavioral inhibition, respectively. These two temperament traits are commonly included in ontogenetic models of developmental psychopathology (e.g., Beauchaine, Reference Beauchaine2015; Crowell et al., Reference Crowell, Puzia and Yaptangco2015). A comprehensive review of the neural and physiological mechanisms that contribute to childhood irritability and behavioral inhibition is beyond the scope of this paper. Thus, we note a few key findings from the literature to highlight the utility of core principles described above to infuse development into the RDoC matrix.
Irritability: A trait antecedent of frustrative nonreward
Frustrative nonreward is defined by negative reactivity in response to repeated failures at reward attainment (NIMH, 2018). Viewed through a developmental lens, this construct comprises emotional (anger, irritability) and behavioral (reactivity, aggression) expressions that may manifest differently based on a child's age (Figure 1).
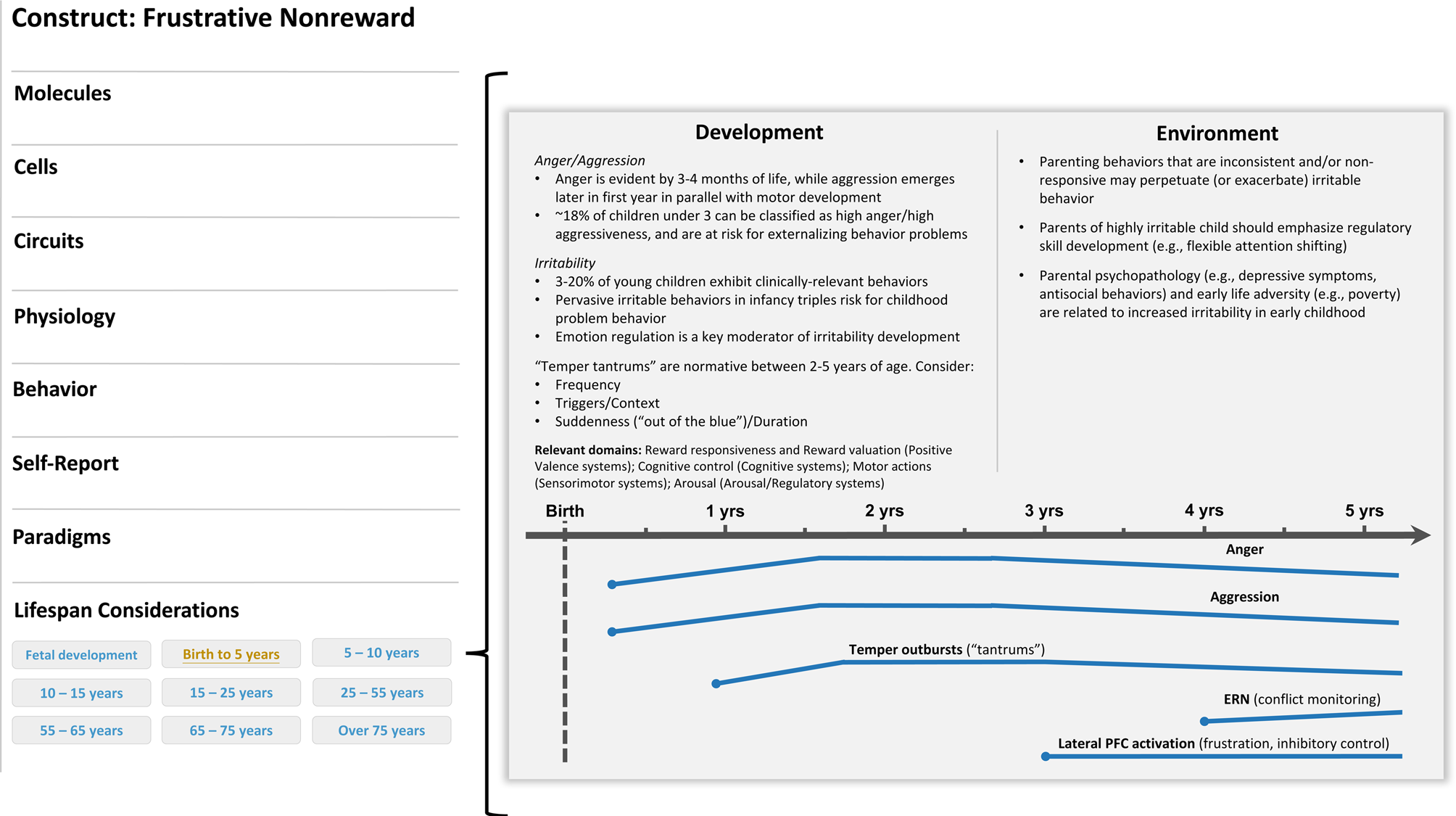
Figure 1. Illustrative example of a developmental qualifier for the frustrative nonreward (negative valence system) construct. A development subsection (“life span considerations”) could be added below the currently listed levels of analysis on the construct page (left panel). In this example, a researcher clicked on the “birth to 5 years” tab and was directed to a new screen that describes developmental considerations available for this construct in this age range. In this case, aspects of trait irritability would be described and, when applicable, visually represented across time. The point in development when a construct could be reliably measured would be indicated on the timeline with a dot. Moreover, various colors could be incorporated to indicate normative and high-risk profiles or trajectories. Relevant cross-domain considerations would also be noted.
Irritability is a dimensional phenotype conceptualized as the propensity to react to a blocked goal with anger and agitation. In early childhood, irritability is often accompanied by elevated aggression or temper outbursts, which can be maladaptive if inconsistent with context (i.e., “out of the blue”) or persistent (e.g., Denham et al., Reference Denham, Bassett, Way, Mincic, Zinsser and Graling2012). The roots of both anger and aggression can be traced to infancy. Anger is evident in the first months of life, while aggression tends to emerge later in infancy in parallel with motor development; both constructs are thought to peak in toddlerhood and decrease thereafter in conjunction with the development of self-regulatory skills (Braungart-Rieker et al., Reference Braungart-Rieker, Hill-Soderlund and Karrass2010; Gartstein & Hancock, Reference Gartstein and Hancock2019; Hay et al., Reference Hay, Waters, Perra, Swift, Kairis, Phillips and van Goozen2014; Lorber, Del Vecchio, & Slep, Reference Lorber, Del Vecchio and Slep2015). Importantly, the type, frequency, and intensity of the behaviors tend to manifest differently over the first 5 years of life, both within and between individuals (e.g., Denham, Lehman, & Moser, Reference Denham, Lehman and Moser1995; Eisenberg et al., Reference Eisenberg, Fabes, Shepard, Murphy, Guthrie, Jones and Maszk1997; Liu et al., Reference Liu, Moore, Beekman, Pérez-Edgar, Leve, Shaw and Neiderhiser2018). Capturing these variations may help to identify patterns associated with later psychopathology (e.g., Damme, Wakschlag, Briggs-Gowan, Norton, & Mittal, Reference Damme, Wakschlag, Briggs-Gowan, Norton and Mittal2021). Using a person-centered approach, Perra, Paine, and Hay (Reference Perra, Paine and Hay2020) found that, from 6 to 36 months of age, three subgroups of infants could be identified based on their levels of anger/aggressiveness. One group (18% of the sample) showed exceptionally high levels of anger and physical force at each time point, and were at elevated risk for externalizing psychopathology at age 7.
High levels of irritability in infancy have been shown to predict behavior problems in childhood (Winsper & Wolke, Reference Winsper and Wolke2014), while childhood irritability has been linked to more functional impairment and internalizing symptoms later in development (e.g., Copeland, Angold, Costello, & Egger, Reference Copeland, Angold, Costello and Egger2013; Dougherty et al., Reference Dougherty, Smith, Bufferd, Stringaris, Leibenluft, Carlson and Klein2013a, Reference Dougherty, Tolep, Bufferd, Olino, Dyson, Traditi and Klein2013b, Reference Dougherty, Smith, Bufferd, Kessel, Carlson and Klein2015; Stringaris & Goodman, Reference Stringaris and Goodman2009). Irritability tends to peak by preschool age, then decreases and remains stable in later childhood and through adulthood (e.g., Caprara, Paciello, Gerbino, & Cugini, Reference Caprara, Paciello, Gerbino and Cugini2007; Wakschlag et al., Reference Wakschlag, Choi, Carter, Hullsiek, Burns, McCarthy and Briggs-Gowan2012). Importantly, high levels of irritability in early life underpin multiple psychological disorders, including disruptive mood dysregulation disorder, oppositional defiant disorder, and attention deficit/hyperactivity disorder (Beauchaine et al., Reference Beauchaine, Zisner and Sauder2017). Recent findings from Damme and colleagues (Damme et al., Reference Damme, Wakschlag, Briggs-Gowan, Norton and Mittal2021) suggest that the course of irritability over early development may also influence manifest psychopathology in late childhood. In other words, children with high and stable levels of irritability in preschool (M age = 4.17 years) and elementary school (M age = 6.95 years) were reported to show more externalizing behavior in pre-adolescence. In contrast, children with high but decreasing levels of irritability across these time points displayed more internalizing behavior in pre-adolescence (Damme et al., Reference Damme, Wakschlag, Briggs-Gowan, Norton and Mittal2021). Together, these findings suggest that clinical prediction may be improved when the developmental course of irritability is taken into consideration. Since trait irritability is dimensional, early emerging, transdiagnostic, and cuts across multiple domains of functioning (i.e., cognition, emotionality, behavior), this phenotype is well positioned to inform the RDoC matrix.
The neural and physiological mechanisms that contribute to irritability in early childhood have been described in detail elsewhere (Beauchaine et al., Reference Beauchaine, Zisner and Sauder2017; Brotman, Kircanski, & Leibenluft, Reference Brotman, Kircanski and Leibenluft2017). In brief, high levels of irritability have been associated with psychobiological disruptions in Cognition×Emotion interactions, namely deficits in attention, threat detection, and reward processing, driven by aberrant activity in a range of frontal and subcortical regions (Brotman et al., Reference Brotman, Kircanski and Leibenluft2017; Leibenluft, Reference Leibenluft2017; Wakschlag et al., Reference Wakschlag, Perlman, Blair, Leibenluft, Briggs-Gowan and Pine2018). Specific links between irritability and disrupted attentional processing, error monitoring, and inhibitory control have been observed. For example, high levels of irritability in infancy have been associated with poor behavioral inhibitory control, but only among infants with left frontal asymmetry, a putative indicator of approach-motivated tendencies measured via electroencephalography (EEG) (He et al., Reference He, Degnan, McDermott, Henderson, Hane, Xu and Fox2010). In addition, children who are highly irritable tend to show deficits in rapid attention capture (N1), conflict monitoring (N2), and allocation of attentional resources (P3), assessed via event-related potentials (ERPs), relative to their peers (Deveney et al., Reference Deveney, Briggs-Gowan, Pagliaccio, Estabrook, Zobel, Burns and Wakschlag2019; Rich et al., Reference Rich, Schmajuk, Pérez-Edgar, Fox, Pine and Leibenluft2007). These children also show abnormal engagement of the anterior cingulate cortex (Perlman et al., Reference Perlman, Jones, Wakschlag, Axelson, Birmaher and Phillips2015; Rich et al., Reference Rich, Carver, Holroyd, Rosen, Mendoza, Cornwell and Leibenluft2011) and medial frontal gyrus (Adleman et al., Reference Adleman, Kayser, Dickstein, Blair, Pine and Leibenluft2011), areas critical for performance monitoring and attentional control. Collectively, results to date point to a pattern of cognitive dysfunction that may abet frustration and thwart effective self-regulation among children with high levels of irritability.
This pattern of cognitive dysfunction may also have an impact on emotional processing for these young children. Indeed, children high in irritability show biased attention toward and away from threat, impaired emotion labeling, and a lower threshold for interpreting a facial expression as angry (Brotman et al., Reference Brotman, Kircanski and Leibenluft2017; Hommer et al., Reference Hommer, Meyer, Stoddard, Connolly, Mogg, Bradley and Brotman2014; Salum et al., Reference Salum, Mogg, Bradley, Stringaris, Gadelha, Pan and Leibenluft2017; Stoddard et al., Reference Stoddard, Sharif-Askary, Harkins, Frank, Brotman, Penton-Voak and Leibenluft2016), all similar to affect-biased attention patterns implicated in anxiety (e.g., Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & Van Ijzendoorn, Reference Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg and Van Ijzendoorn2007). Neuroimaging studies indicate that greater irritability is associated with aberrant amygdala activation during emotional face processing, although some studies show hypoactivation (Brotman et al., Reference Brotman, Rich, Guyer, Lunsford, Horsey, Reising and Leibenluft2010) while others show hyperactivation (Thomas et al., Reference Thomas, Kim, Bones, Hinton, Milch, Reynolds and Leibenluft2013). These inconsistent findings may reflect non-linear brain–behavior relationships (Northoff & Tumati, Reference Northoff and Tumati2019). For example, Grabell and colleagues (Reference Grabell, Li, Barker, Wakschlag, Huppert and Perlman2018) found that lateral prefrontal cortex activation during frustration was positively correlated with irritability within the typical range, but negatively correlated for those with clinical levels of irritability. This finding highlights the necessity to examine irritability dimensionally to clarify unique neural mechanisms associated with lower or higher severity. A child's age and developmental status may also play a role in this brain–behavior relationship. Tseng and colleagues (Reference Tseng, Deveney, Stoddard, Kircanski, Frackman, Yi and Leibenluft2019), for example, showed that after being frustrated, children high in irritability exhibit abnormal frontal and striatal engagement during a subsequent nonfrustrating attention-orienting task. This pattern of neural activation suggests that children prone to irritability may have difficulty shifting processing responses with shifts in context or events. Thus, prior frustrating events may spill over into subsequent behavior. Importantly, this association was stronger for children compared to adolescents, indicating that attentional impairment specific to emotional contexts may vary with age, and potentially with an increasing ability to regulate and engage in flexible behavior.
Highly irritable children also show disrupted reward learning, with difficulty detecting contingencies between responses and rewards, as well as impaired behavioral adaptation when contingencies change (e.g., Adleman et al., Reference Adleman, Kayser, Dickstein, Blair, Pine and Leibenluft2011; Dickstein et al., Reference Dickstein, Finger, Brotman, Rich, Pine, Blair and Leibenluft2010). This effect is thought to be attributable to dopaminergic dysfunction in mesolimbic circuitry of the brain (Schultz, Reference Schultz2002; Tobler, Fiorillo, & Schultz, Reference Tobler, Fiorillo and Schultz2005), such that mismatches between reward predictions and outcomes are not adequately encoded. Evidence suggests that children high in irritability show elevated sensitivity to rewards, indexed by an exaggerated reward positivity, an ERP associated with medial prefrontal cortex and ventral striatum activation (Kessel et al., Reference Kessel, Dougherty, Kujawa, Hajcak, Carlson and Klein2016). These children also show greater frustration accompanied by ventral striatum deactivation when an expected reward is denied (e.g., Deveney et al., Reference Deveney, Connolly, Haring, Bones, Reynolds, Kim and Leibenluft2013). Similarly, another study of school-aged children showed heightened anterior cingulate cortex activation when a reward was anticipated, and greater posterior cingulate engagement, a region involved in shifting the focus of attention from internal to external, when progress toward a reward was blocked (Perlman et al., Reference Perlman, Jones, Wakschlag, Axelson, Birmaher and Phillips2015). These patterns suggest that highly irritable children may experience anticipated reward as more salient, and barriers toward reward as particularly aversive and/or unexpected.
Importantly, irritability during the first few years of life predicts greater reward sensitivity and proneness to frustration (e.g., Kessel et al., Reference Kessel, Dougherty, Kujawa, Hajcak, Carlson and Klein2016) as well as reduced inhibitory control (He et al., Reference He, Degnan, McDermott, Henderson, Hane, Xu and Fox2010) later in childhood. Abnormalities in connectivity and reward-related activation of the amygdala and frontal regions, circuitry involved in emotion regulation and cognitive control, have been shown to differ based on the developmental timing of the emergence of high irritability (Dougherty et al., Reference Dougherty, Schwartz, Kryza-Lacombe, Weisberg, Spechler and Wiggins2018). That is, irritability-linked frontal–amygdala connections were found to be more lateralized among highly irritable preschoolers, and more bilaterally distributed among irritable school-aged children. This suggests that early abberant neural activation may become more entrenched as regulatory circuitry matures, highlighting the importance of identifying high-risk phenotypes that emerge early in life.
Together, this pattern of neural, cognitive, and affective activation that associates with trait irritability in early childhood may contribute to increased reward (and novelty) motivation, as well as dysregulated, reactive, and impulsive behavior (Beauchaine et al., Reference Beauchaine, Zisner and Sauder2017; Wakschlag et al., Reference Wakschlag, Perlman, Blair, Leibenluft, Briggs-Gowan and Pine2018). It is important, however, to consider the environment in which this activation may occur. Sensitive caregiving, for example, has been shown to protect against increases in anger and aggressive behavior over time, whereas a mother's history of antisocial behavior had the opposite effect (Perra et al., Reference Perra, Paine and Hay2020; see also Hay, Pawlby, Waters, Perra, & Sharp, Reference Hay, Pawlby, Waters, Perra and Sharp2010, Reference Hay, Mundy, Carta, Roberts, Carta, Waters and van Goozen2011, Reference Hay, Waters, Perra, Swift, Kairis, Phillips and van Goozen2014; NICHD Early Child Care Research Network, 2004; Reuben et al., Reference Reuben, Shaw, Neiderhiser, Natsuaki, Reiss and Leve2016; Robinson et al., Reference Robinson, Mattes, Oddy, Pennell, van Eekelen, McLean and Newnham2011). Bidirectional associations between child irritability and negative parenting behaviors (i.e., non-responsiveness and inconsistent discipline) have also been identified (e.g., Lengua, Reference Lengua2006; van den Boom, Reference van den Boom1989). Further, infant irritable distress has been shown to predict greater behavioral problems in toddlerhood, but only among those whose parents guided their attention toward the source of frustration (Crockenberg, Leerkes, & Bárrig Jó, Reference Crockenberg, Leerkes and Bárrig Jó2008). Thus, irritable children may benefit from parents able to orient their child's attention away from aversive stimuli, setting the stage for adaptive self-regulation. Critically, broad social contextual factors must also be considered when examining the link between irritability and caregiving behaviors. Socioeconomic status moderates the association between parental support and child negative emotionality such that stronger effects are detected among families with low as opposed to high socioeconomic status (Paulussen-Hoogeboom, Stams, Hermanns, & Peetsma, Reference Paulussen-Hoogeboom, Stams, Hermanns and Peetsma2007). Irritability is also correlated with parental anxiety and depression (Dougherty et al., Reference Dougherty, Smith, Bufferd, Stringaris, Leibenluft, Carlson and Klein2013a, Reference Dougherty, Tolep, Bufferd, Olino, Dyson, Traditi and Klein2013b), and adversity in early life, including maltreatment and poverty (Pagliaccio, Pine, Barch, Luby, & Leibenluft, Reference Pagliaccio, Pine, Barch, Luby and Leibenluft2018).
Behavioral inhibition: A trait antecedent of acute threat
Acute threat (“fear”) is defined as a response to perceived danger that motivates defensive actions across systems to protect oneself (NIMH, 2018). Underpinned by overlapping neural mechanisms, behavioral inhibition is a possible antecedent to the acute threat construct evident in early childhood (Figure 2). Aspects of behavioral inhibition may also inform development of other RDoC constructs, chiefly the potential threat (“anxiety”) construct (negative valence systems). As noted above, some RDoC constructs lack specificity in their description of dysfunction, and at times overlap conceptually as well as across levels of analysis. For simplicity, we will only consider the link between behavioral inhibition and the acute threat construct. Nevertheless, the putative link between behavioral inhibition and the potential threat construct underscores the transdiagnostic utility of early emerging temperament traits, as well as the fact that matching temperament traits to RDoC constructs in a one-to-one manner is likely to be an oversimplification of the trait-based approach to psychopathology risk.
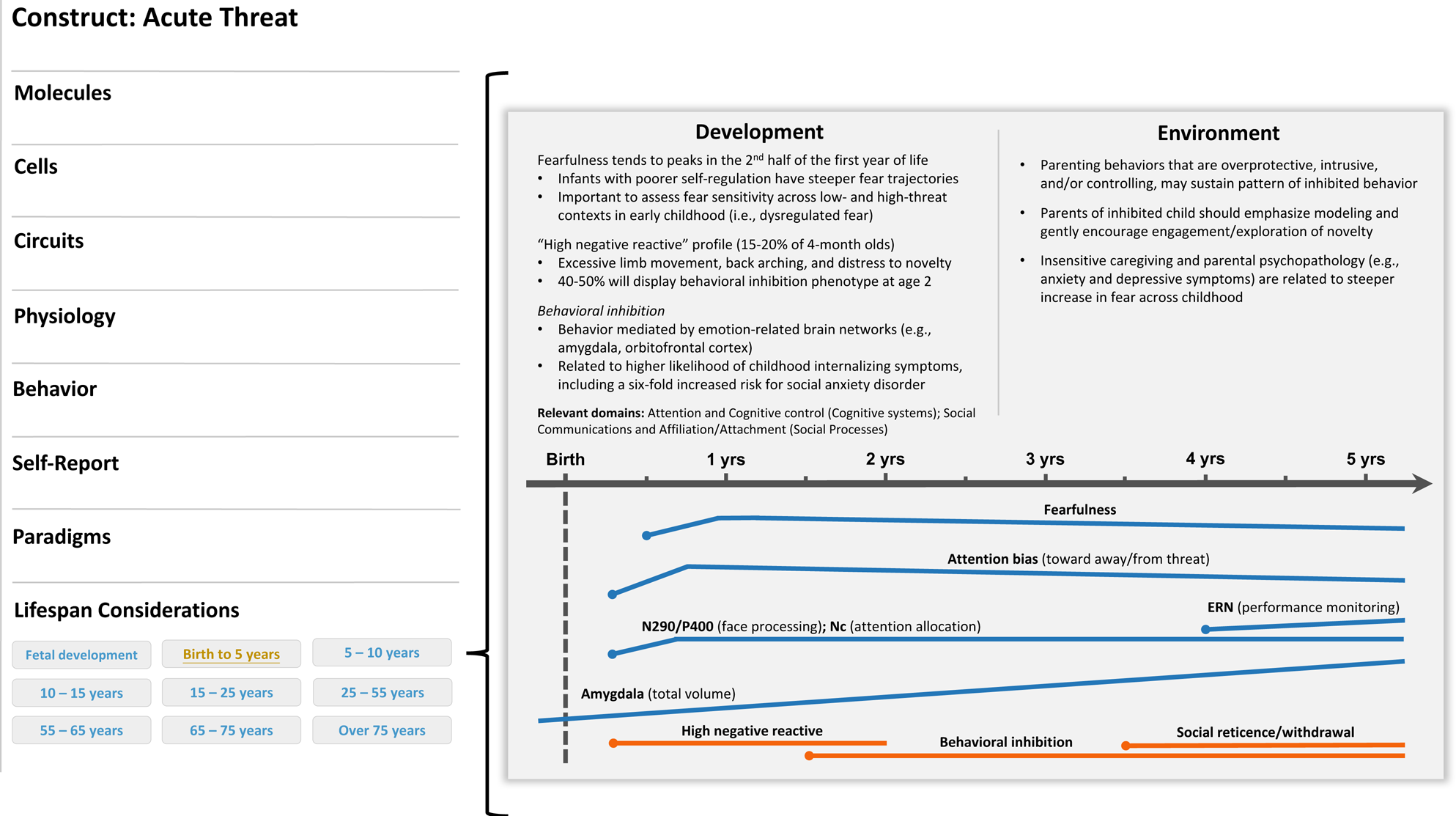
Figure 2. Illustrative example of a developmental qualifier for the acute threat (negative valence system) construct. A development subsection (“life span considerations”) could be added below the currently listed levels of analysis on the construct page (left panel). In this example, a researcher clicked on the “birth to 5 years” tab and was directed to a new screen that describes developmental considerations available for this construct in this age range. In this case, aspects of behavioral inhibition would be described and, when applicable, visually represented across time. The point in development when a construct could be reliably measured would be indicated on the timeline with a dot. Moreover, various colors could be incorporated to indicate normative and high-risk profiles or trajectories. Relevant cross-domain considerations would also be noted.
Infants who are more likely to be behaviorally inhibited in toddlerhood tend to display high levels of limb movement and distress in response to low-threat yet novel stimuli (e.g., a moving mobile), a phenotype referred to as “high negative reactive” (Fox et al., Reference Fox, Henderson, Rubin, Calkins and Schmidt2001; Garcia-Coll et al., Reference Garcia-Coll, Kagan and Reznick1984). Just under half of high-negative-reactive 4-month-old infants will go on to display the behavioral inhibition phenotype (Kagan, Reference Kagan2018). As these children age, the stimuli that evoke a distress response tend to move from undifferentiated novelty to social interactions or social cues, although the putative mechanisms underlying behavioral inhibition are thought to be constant over time. Social reticence, anxiety, and depression are common outcomes for children who were behaviorally inhibited as toddlers (Clauss & Blackford, Reference Clauss and Blackford2012; Klein & Mumper, Reference Klein, Mumper, Pérez-Edgar and Fox2018). Most worthy of note, meta-analytic data indicate that over 40% of behaviorally inhibited young children had a social anxiety disorder as adolescents (Clauss & Blackford, Reference Clauss and Blackford2012), lending support to the clinical utility of this early emerging phenotype.
Heterogeneity in this behavioral phenotype may be parsed further to improve the prediction of high-risk trajectories. To this end, Buss and colleagues have demonstrated the utility of considering context inappropriate fear reactivity as an indicator of elevated psychopathology risk above and beyond inhibited behavior (Buss, Reference Buss2011; Buss et al., Reference Buss, Davis, Ram and Coccia2018). A core assumption of this approach is that adaptive (or maladaptive) behavior is a function of a young child's appraisal of context-dependent threat, whether it be real or perceived. Assessment of fear reactivity across situations that varied from low- to high-threat is crucial, as toddlers who display dysregulated fear in low-threat situations cannot be distinguished from their inhibited peers in high-threat contexts.
Few temperament traits have been as extensively examined across levels of analysis as behavioral inhibition (Buss & Qu, Reference Buss, Qu, Pérez-Edgar and Fox2018; Clauss et al., Reference Clauss, Avery and Blackford2015). Behaviorally inhibited individuals show structural neural differences compared to their peers, including larger amygdala, caudate, and orbitofrontal cortex volume (Clauss et al., Reference Clauss, Seay, Vanderklok, Avery, Cao, Cowan and Blackford2014; Hill, Tessner, Wang, Carter, & McDermott, Reference Hill, Tessner, Wang, Carter and McDermott2010), increased thickness in the ventromedial prefrontal cortex (Schwartz et al., Reference Schwartz, Kunwar, Greve, Moran, Viner, Covino and Wallace2010), and decreased thickness in the dorsal anterior cingulate and lateral orbitofrontal cortices (Schwartz et al., Reference Schwartz, Kunwar, Greve, Moran, Viner, Covino and Wallace2010; Sylvester et al., Reference Sylvester, Barch, Harms, Belden, Oakberg, Gold and Pine2016). In addition, burgeoning research into intrinsic functional connectivity indicates that behaviorally inhibited adults (Blackford et al., Reference Blackford, Clauss, Avery, Cowan, Benningfield and Vanderklok2014; Roy et al., Reference Roy, Benson, Degnan, Pérez-Edgar, Pine, Fox and Ernst2014) and children (Taber-Thomas, Morales, Hillary, & Pérez-Edgar, Reference Taber-Thomas, Morales, Hillary and Pérez-Edgar2016) have increased connectivity in the salience network (e.g., dorsal anterior cingulate) and decreased amygdala connectivity with the anterior cingulate. Together, this pattern of activation points to dysfunction in both “bottom-up” (e.g., vigilance to potential threat) and “top-down” (e.g., regulation of amygdala reactivity) processing (Blackford, Clauss, & Benningfield, Reference Blackford, Clauss, Benningfield, Pérez-Edgar and Fox2018). The pattern of activation differs when contextual or temporal factors are considered; we refer readers to Jarcho and Guyer (Reference Jarcho, Guyer, Pérez-Edgar and Fox2018), who provide an illustrative example demonstrating how neurocognitive processing (e.g., salience network, mentalizing network) differ based on the environmental demands of a social interaction for a behaviorally inhibited child (e.g., decision to attend a party, engaging with unfamiliar peers).
Young children who are behaviorally inhibited exhibit distinct patterns of evoked neural activity associated with attentional control and conflict monitoring (e.g., Brooker & Buss, Reference Brooker and Buss2014; Lamm et al., Reference Lamm, Walker, Degnan, Henderson, Pine, Mcdermott and Fox2014; McDermott et al., Reference McDermott, Pérez-Edgar, Henderson, Chronis-Tuscano, Pine and Fox2009; Thai, Taber-Thomas, & Pérez-Edgar, Reference Thai, Taber-Thomas and Pérez-Edgar2016), operationalized by two ERPs, the N2 and the error-related negativity. Physiologically, behaviorally inhibited young children show a pattern of right-biased activation of the frontal lobe (e.g., Calkins, Fox, & Marshall, Reference Calkins, Fox and Marshall1996; Fox et al., Reference Fox, Henderson, Rubin, Calkins and Schmidt2001), larger pupil dilation (Kagan et al., Reference Kagan, Reznick and Snidman1987), higher skin-conductance levels (Gilissen, Koolstra, van Ijzendoorn, Bakermans-Kranenburg, & van der Veer, Reference Gilissen, Koolstra, van Ijzendoorn, Bakermans-Kranenburg and van der Veer2007; Scarpa, Raine, Venables, & Mednick, Reference Scarpa, Raine, Venables and Mednick1997), higher baseline cortisol levels (Essex, Klein, Slattery, Goldsmith, & Kalin, Reference Essex, Klein, Slattery, Goldsmith and Kalin2010; Pérez-Edgar, Schmidt, Henderson, Schulkin, & Fox, Reference Pérez-Edgar, Schmidt, Henderson, Schulkin and Fox2008; Smider et al., Reference Smider, Essex, Kalin, Buss, Klein, Davidson and Goldsmith2007), and greater reductions in parasympathetic-mediated cardiac physiology (Brooker et al., Reference Brooker, Buss, Lemery-Chalfant, Aksan, Davidson and Goldsmith2013; Buss et al., Reference Buss, Davis, Ram and Coccia2018). Physiological results have been mixed, however, particularly with regard to parasympathetic activity (see Buss & Qu, Reference Buss, Qu, Pérez-Edgar and Fox2018 for detailed discussion).
Although a reliable predictor of psychopathology, it is important to remember that most behaviorally inhibited toddlers do not show clinically salient behavior problems in childhood, suggesting that internal and/or external factors may moderate trait expression over time. Attention is one particularly potent internal factor that affects the emergence and maintenance of behavioral inhibition, underscoring the importance of cross-domain considerations for trait development (Bell & Wolfe, Reference Bell and Wolfe2004; Degnan & Fox, Reference Degnan and Fox2007). Affect-biased attention is a putative domain-general mechanism that describes the automatic process through which a child attends to motivationally salient stimuli. Attention to motivating aspects of the environment may be particularly relevant for maintaining psychopathology risk among behaviorally inhibited young children as it shapes the child's subjective sense of the environment. We refer the reader to Morales, Fu, and Pérez-Edgar (Reference Morales, Fu and Pérez-Edgar2016) for a detailed discussion of affect-biased attention and socioemotional development. In brief, emerging evidence suggests that affect-based attention may moderate the link between behavioral inhibition and later socioemotional functioning, with stable patterns of attentional bias toward or away from salient stimuli (i.e., not reflecting change in context) serving to sustain a young child's behavioral tendencies over time.
Although research has typically focused on attentional performance during a single task (e.g., Bar-Haim et al., Reference Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg and Van Ijzendoorn2007; Pérez-Edgar et al., Reference Pérez-Edgar, Reeb-Sutherland, McDermott, White, Henderson, Degnan and Fox2011), emerging evidence suggests that stability of attention across multiple tasks is related to temperament traits, such as fearfulness, in early childhood (Fu & Pérez-Edgar, Reference Fu and Pérez-Edgar2019). With this in mind, Vallorani and colleagues (Reference Vallorani, Fu, Morales, LoBue, Buss and Pérez-Edgar2021) examined affect-based attention across multiple tasks in infancy (4- to 24-month-olds). Using a variable-centered method (factor analysis), the authors identified two factors that described affect-based attention: (a) an “engagement” factor characterized by increased dwell time to affective faces in the dot-probe and overlap tasks, along with decreased latency to affective faces in the vigilance task, and (b) a “disengagement” factor characterized by increased latency to probe and dwell time in the dot-probe and overlap tasks, respectively. Higher levels of maternal anxiety were related to less engagement with faces among infants. Using a person-centered method (latent profile analysis) these authors identified two homogeneous groups of infants: (a) a “vigilant” group characterized by more engagement with faces and a better ability to disengage, and (b) an “avoidant” group characterized by less engagement with faces and a poorer ability to disengage. Further, infants of mothers who reported more anxiety were more likely to be in the “vigilant” group if they also exhibited high levels of negative affect.
It is worth noting that the developmental mechanism through which the interplay between attention bias and negative affect in infancy influences the emergence of behavioral inhibition is an active area of debate. Field and Lester (Reference Field and Lester2010) outlined three hypothetical models aimed at describing this attention–affect association, namely, the integral bias model, the moderation model, and the acquisition model. On the one hand, the integral bias model suggests that specific characteristics of the individual (e.g., temperament) determine the presentation and maintanence of attentional biases, not development. The moderation model, on the other, suggests that attentional biases are maintained over time, but only for individuals with specific characteristics, such as behavioral inhibition. For individuals without these characteristics, it is predicted that attentional biases will decrease over time. Lastly, the acquisition model suggests that specific developmental experiences will cause normative attentional biases to increase over time. Our research team is currently collecting data to test these proposed models (Burris et al., Reference Burris, Oleas, Reider, Buss, Pérez-Edgar and LoBue2019; Reference Pérez-Edgar, LoBue, Buss, Field, Reider, Burris and Metcalfin press).
In terms of the caregiving context, infants of mothers who are less sensitive in a dyadic interaction, or who report more depressive symptoms, show a steeper increase in fear over early childhood (Braungart-Rieker et al., Reference Braungart-Rieker, Hill-Soderlund and Karrass2010; Gartstein et al., Reference Gartstein, Bridgett, Rothbart, Robertson, Iddins, Ramsay and Schlect2010). Sensitive caregiving for an inhibited child involves a greater emphasis on modeling and gently encouraging engagement with novelty, compared to their less inhibited peers (Hane, Cheah, Rubin, & Fox, Reference Hane, Cheah, Rubin and Fox2008; Kiel, Premo, & Buss, Reference Kiel, Premo and Buss2016). It has been found that inhibited toddlers with intrusive, controlling, or overprotective mothers (e.g., excessive limits on independence and exploration) exhibit higher levels of social reticence at preschool age (Degnan, Henderson, Fox, & Rubin, Reference Degnan, Henderson, Fox and Rubin2008; Kiel et al., Reference Kiel, Premo and Buss2016; Mount, Crockenberg, Jó, & Wagar, Reference Mount, Crockenberg, Jó and Wagar2010; Rubin, Burgess, & Hastings, Reference Rubin, Burgess and Hastings2002; Rubin, Cheah, & Fox, Reference Rubin, Cheah and Fox2001; Rubin, Hastings, Stewart, Henderson, & Chen, Reference Rubin, Hastings, Stewart, Henderson and Chen1997).
Future Directions
We emphasized trait expression from birth to preschool age given that multiple overlapping neural, cognitive, emotional, motivational, and behavioral systems are being consolidated as a function of varied maturational and environmental influences. With this in mind, we offer three necessary next steps to infuse development (and temperament) into RDoC.
One future direction for RDoC involves the inclusion of measurement tools and guidelines that are appropriate for use with infants and young children. The RDoC matrix only lists a few paradigms suitable for children under the age of 5, such as the laboratory temperament assessment battery (LabTAB) (Goldsmith & Rothbart, Reference Goldsmith and Rothbart1996), the still-face paradigm (Tronick, Als, Adamson, Wise, & Brazelton, Reference Tronick, Als, Adamson, Wise and Brazelton1978), and the strange situation (Ainsworth, Blehar, Waters, & Wall, Reference Ainsworth, Blehar, Waters and Wall1978). Developmental scientists, however, utilize a rich repertoire of behavioral tasks to understand early-life cognitive and emotional functioning. For example, LoBue and colleagues (LoBue & Deloache, Reference LoBue and Deloache2008) provide support for a touch-screen detection paradigm that assesses attentional biases in young children. This method could be used with older individuals and/or in conjunction with methods from other units of analysis (e.g., electroencephalogram), thus exemplifying how a single paradigm can be utilized to measure a dynamic construct related to psychopathology risk from early childhood onward.
Measures that are valid, reliable, and can describe functioning across the life span will advance developmental research in the RDoC era. This is a challenging task given that trait expression often changes over time. Nevertheless, methodological approaches that identify risk traits or phenotypes and adapt mixed-method measurement instruments for various age groups across the life span will provide greater insight into how RDoC constructs develop. The Patient-Reported Outcome Measurement Information System, for example, identified risk traits (e.g., anger/irritability) and then adapted the measure in a developmentally sensitive manner for adults, children (5–17 years), and young children (1–5 years). As a result, researchers and clinicians may now administer a developmentally sensitive instrument to evaluate risk for psychological (and physical) health and dysfunction across the life span (Blackwell et al., Reference Blackwell, Wakschlag, Krogh-Jespersen, Buss, Luby, Bevans and Cella2020).
In relation to this challenge, the National Institutes of Health Toolbox is an existing database that outlines developmentally appropriate paradigms, and could seamlessly be integrated into the RDoC matrix with the addition of a “life span considerations” subsection. Temperament researchers and developmental psychopathologists have also sought to establish measures of temperament traits and clinical symptoms that can be considered from birth through adulthood (e.g., Achenbach, Reference Achenbach2009; see Fu & Pérez-Edgar, Reference Fu, Pérez-Edgar and Wright2015 for discussion of methodological approaches in temperament research). Paired with biological and behavioral (e.g., LabTAB) measures, these questionnaires may also prove useful in assessing psychopathology risk across the life span. If development is to be infused into RDoC, developmentally sensitive and psychometrically sound measures and procedures ought to be described in the RDoC matrix, possibly in the proposed “life span considerations” subsection.
Another future direction for both RDoC and early childhood temperament research may be to investigate the mechanisms underlying prenatal contributions to individual differences in cognitive and behavioral dysfunction, a critical next step for understanding intergenerational transmission of psychopathology risk. Although the neural, physiological, and behavioral correlates of early temperament have been well documented, the prenatal origins of these neurobehavioral differences remain largely unknown. Explanations that invoke only genetic or postnatal socialization pathways may not sufficiently explain the origins of temperament, as they fail to appreciate the ontogenesis of biological systems relevant to trait expression, particularly during periods of heightened susceptibility to environmental influences. Burgeoning evidence supports the influence of pre- and perinatal experiences as under-appreciated, experience-dependent contributors to individual differences in the biological underpinnings of temperament (see Van den Bergh et al., Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer and Schwab2017 for review). For example, a recent study from Qiu and colleagues (Reference Qiu, Anh, Li, Chen, Rifkin-Graboi, Broekman and Meaney2015) found that 6-month-old infants whose mothers reported higher levels of depressive symptoms while pregnant showed greater functional connectivity between the left amygdala and brain networks related to perception and regulation of emotions (e.g., anterior cingulate, insula, orbitofrontal cortex, temporal cortex). Elucidating the pre- and perinatal contributions (and associated mechanisms) to temperament in early childhood may inform RDoC on putative pathways underlying the intergenerational transmission of psychopathology risk.
Lastly, future research should consider how information on individual differences in early childhood temperament can bridge the gap with pediatric clinical practice in the RDoC era. Fortunately, clinical neurodevelopmental models that utilize information on temperamental tendencies already exist. Wakschlag and colleagues (Reference Wakschlag, Perlman, Blair, Leibenluft, Briggs-Gowan and Pine2018), for example, outline a developmental specification framework, in which a child's psychopathology risk is characterized as a function of neurodevelopmental predispositions (i.e., temperament traits) and skill acquisition (e.g., social perspective taking) that are evident in early childhood. This characterization provides researchers and clinicians with foundational information from which abnormal deviations in behavior may be determined and linked to dysfunction in cognitive and affective systems. This information may then be used to determine the clinical course, correlates, and prognosis (see Wakschlag et al., Reference Wakschlag, Perlman, Blair, Leibenluft, Briggs-Gowan and Pine2018 for example application with early childhood irritability and callousness). The inclusion of temperament in this neurodevelopmental model and, as we propose, in the RDoC matrix may provide a bridge between RDoC and front-line treatments for pediatric samples. This approach may ultimately guide clinicians toward malleable points of intervention to prevent the onset of psychopathology in later childhood via the identification of dysfunction in early cognitive and affective systems.
Conclusions
RDoC is an innovative approach designed to explore dimensions of human behavior that move beyond the limits of psychiatric categories in the hope of aligning the identification of psychological health and dysfunction with clinical neuroscience. To advance this goal, we propose the incorporation of core principles of temperament theories into a new “life span considerations” subsection as one option for infusing development into the RDoC matrix. Temperament traits represent transdiagnostic vulnerabilities that transact with environmental influences to shape functional development for a young child. This proposal necessitates that researchers embrace complexity to understand the early life origins of psychopathology risk. In doing so, researchers and clinicians may ultimately have the tools necessary to support emotional development and reduce a young child's likelihood of psychological dysfunction beginning in the first years of life.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421000511.
Author Contributions
All authors contributed to conceptualization of the manuscript. B. D. Ostlund and S. Myruski drafted the manuscript, with critical revisions provided by K. Buss and K. E. Pérez-Edgar. All authors approved the final version of the manuscript.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.





