Prolonged exercise, which is often used as a model of physical stress, causes acute depression of many components of the immune system(Reference Gleeson and Pyne1–Reference Pedersen and Hoffman-Goetz6). Exercise is an excellent, controllable, quantifiable and reproducible model with which to study the effects of stress, and physical stress in particular, on immunity(Reference Hoffman-Goetz and Pedersen2, Reference Pedersen, Kappel and Klokker3, Reference Pedersen and Hoffman-Goetz6). Numerous studies have investigated the effectiveness of various nutritional strategies and supplementation at minimising immune perturbations following prolonged exercise, but limited studies have investigated the effects of supplementation with bovine colostrum (COL). Bovine COL, the initial milk produced by cows in the first 48 h after parturition, contains bioactive components that play significant roles within the immune system (such as immunoglobulins and antimicrobial proteins) in addition to the macronutrients and micronutrients found in milk(Reference Kelly7, Reference Pakkanen and Aalto8). The importance of bovine COL to assist in the protection against infection for newborn calves is well known and has led to interest in the potential immunological benefits of bovine COL as a nutritional supplement in human subjects(Reference Kelly7). It has been demonstrated that bovine COL can enhance the humoral immune response to an orally administered vaccine(Reference He, Tuomola and Arvilommi9), which is also supported by its modulatory effects on cell-mediated immunity(Reference Biswas, Vecchi and Mantegani10). These results suggest that bovine COL supplementation may also be of benefit to individuals who are subjected to acutely depressed immune function (i.e. following a physical stressor such as endurance exercise). Despite this, few studies have examined the effects of bovine COL supplementation on immune function in a physical stress model. Recently, it has been demonstrated that a 12-week supplementation intervention in a group of distance runners resulted in a 79 % increase in resting salivary IgA(Reference Crooks, Wall and Cross11). A 33 % increase in salivary IgA has also been found after only 2 weeks of supplementation(Reference Mero, Kähkönen and Nykänen12). It has been proposed that an increase in resting salivary IgA may attenuate exercise-induced perturbations in salivary IgA and aid in the protection against developing upper respiratory tract infection(Reference Brinkworth and Buckley13). However, it is also important to study other components of the immune system and host defence. In a recent study, Shing et al. (Reference Shing, Peake and Suzuki14) investigated the effects of bovine COL supplementation on salivary IgA in addition to some components of innate immunity. They observed some minor effects suggestive of enhanced neutrophil function (CD89 surface receptor expression) at rest but not following exercise in the COL-supplemented group. However, leucocyte surface receptor expression has limited value as a marker of human immune modulation(Reference Albers, Antoine and Bourdet-Sicard15), and a measure of actual neutrophil function would be valuable. Furthermore, Shing et al. (Reference Shing, Peake and Suzuki14) observed limited changes in the neutrophil receptor expression markers, even in the placebo (PLA) condition, giving little scope for COL supplementation to be of benefit.
The underlying mechanism responsible for the effect of bovine COL on human immune parameters remains unclear; however, evidence from animal studies suggests that it may stimulate leucocyte capacity, specifically by modulating neutrophil phagocytosis and ‘killing’ capacity(Reference Sugisawa, Itou and Ichimura16, Reference Sugisawa, Itou and Saito17). Therefore, the aims of the present study were to determine the effects of bovine COL supplementation on blood neutrophil functional capacity and salivary lysozyme release (in addition to IgA) after a bout of endurance exercise that is known to cause significant acute depression of innate immunity.
Experimental methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Aberystwyth University Research Ethics Committee. Written informed consent was obtained from all subjects. Subjects also completed a pre-exercise screening questionnaire (physical activity readiness questionnaire) before participating in each test.
Subjects
Twenty healthy recreationally active men (age 25 (sd 5) years, body mass 78·5 (sd 9·0) kg, VO2max, 52·2 (sd 7·7) ml/min per kg) participated in the present study. Subjects were randomly assigned to one of two groups, either PLA supplementation (n 10) or bovine COL supplementation (n 10). Supplementation was double blind, and subjects in the COL group received oral supplementation with bovine COL (20 g/d), whereas the PLA group received a PLA containing an isoenergetic (approximately 300 kJ per 20 g powder) and isomacronutrient mixture of milk protein concentrate (23 %) and skimmed milk powder (77 %). Approximate macronutrient composition was 52 % protein, 37 % carbohydrate (lactose) and 0·8 % fat for the bovine COL product, and 50 % protein, 41 % carbohydrate (lactose) and 0·8 % fat for the PLA. Supplements were taken daily for 28 d in accordance with the manufacturer's instructions for the COL product. COL was provided by Neovite UK, London.
Testing protocols
All subjects completed three exercise bouts; a preliminary trial (VO2max determination), a familiarisation trial and a main trial. For VO2max determination, subjects performed a continuous incremental test (30 W/min ramp rate) to volitional exhaustion on an electrically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) as previously described(Reference Davison, Allgrove and Gleeson18). Heart rate and rating of perceived exertion were recorded during this period using a telemetric device (Polar, Kempele, Finland) and the Borg Scale, respectively. This test took place at approximately day 14 of the study period (where day 0 is the day on which baseline samples were obtained: see below). The familiarisation trial was designed to familiarise subjects to the testing procedures and ensure that the correct exercise intensity had been identified from the VO2max test. This test took place on approximately day 21 and was completed at least 6 d before the main trial. The familiarisation trial was 60 min duration.
Main trials
Subjects were instructed to consume 500 ml of water 2 h pre-exercise before arrival at the laboratory on the morning of the main trial. All subjects were requested to arrive between 09.00 and 09.30 hours, after an overnight fast of at least 10 h. Subjects sat quietly in the laboratory for 20–30 min before beginning exercise for 2 h at an intensity of approximately 64 % VO2max. To ensure the relative exercise intensity was equivalent between subjects, the precise exercise intensity was determined relative to each subject's individual gas exchange threshold and their VO2max exercise intensity. The relative intensity was set at 20 % of the difference between gas exchange threshold and VO2max (20 % Δ). Expired gas was analysed during the 15th, 30th, 60th, 90th and 120th min of exercise. Heart rate and rating of perceived exertion were recorded every 15 min during exercise. Subjects were given 5 ml/kg body mass of a beverage (a low energy lemon flavoured squash with artificial sweetener, providing 21 kJ energy, 0·1 g protein, 1·1 g carbohydrate, trace fat and trace sodium per litre of solution), before exercise, followed by 2·5 ml/kg body mass every 15 min during exercise. This was equivalent to approximately 1·8 litre of beverage, on average, over the course of the 2 h trial.
Subjects completed a weighed food record diary for the 24 h period before the main trial. On the day of the main trial, they also completed a FFQ relating to the previous 7 d. The dietary records were analysed using computer dietary analysis software (CompEat, Nutrition Systems, London, UK). Subjects were all non-smokers and were required to abstain from alcohol, caffeine and strenuous activity for 48 h before the main trial. It was also stipulated that subjects should not take any other supplement (including vitamin, mineral or ‘sports’ supplements) during and for the 4 weeks before the study.
Blood samples
Blood samples were obtained by venepuncture, with minimal stasis, from an antecubital vein and collected into vacutainer tubes (4·5 ml into a K3EDTA-treated tube and 7 ml into a heparin-treated tube). An initial baseline (Baseline) sample was taken in the morning, after an overnight fast, before beginning the supplementation period. Further venous blood samples were taken immediately before beginning the 2 h exercise bout (Pre-Ex), within a few minutes of completing the exercise bout (Post-Ex) and after 1 h of recovery (1 h Post-Ex), during which subjects remained in the laboratory and were allowed to undertake restful activities such as reading or using a computer. All blood samples were obtained while subjects were in the seated position. Subjects were asked to sit without changes of posture and minimal movement for 10 min before all blood samples were drawn, except the Post-Ex sample, which was drawn as soon as possible after completing the exercise (within 3 min).
Saliva samples
Saliva samples were collected as previously described(Reference Davison, Allgrove and Gleeson18), at Baseline, Pre-Ex, Post-Ex and 1 h Post-Ex. Briefly, timed, unstimulated whole saliva was obtained while subjects were seated, with the head tilted slightly forward and making minimal orofacial movement. They were asked to swallow to empty the mouth, before whole saliva was collected by passive dribble into a pre-weighed sterile tube for an initial period of 2 min. If insufficient saliva was obtained after the initial 2 min period, the process was repeated. Subjects were not allowed to drink for at least 10 min before each sample. The tube was reweighed after collection of the sample so that saliva volume (and hence flow rate) could be estimated. Tubes were weighed to the nearest 0·1 mg (saliva density was assumed to be 1·00 g/ml) and aliquots were frozen, at − 80°C, for later analysis.
Analytical methods
Blood analysis
Haematological analysis was performed on the blood samples collected into the K3EDTA tubes using an automated haematology analyser (ABX Pentra 120, Horiba Medical, Montpellier, France). Blood Hb concentration, haematocrit, total and differential leucocyte counts were measured. Haematocrit was also determined by standard microcentrifugation (with a Hawksley microcentrifuge) for use, along with Hb concentration, to estimate post-exercise changes in blood and plasma volume (compared to the Pre-Ex sample) using the equations of Dill & Costill(Reference Dill and Costill19). Blood lactate and glucose concentrations were determined in heparinised blood using an automated analyser (YSI 2300 Stat Plus, Yellow Springs, OH, USA). Blood haematocrit analyses by microcentrifugation were carried out in triplicate.
A 1 ml aliquot of blood from the heparin tube was used for measurement of neutrophil degranulation (see below). The remaining blood was centrifuged at 1500 g for 10 min at 4°C, and aliquots of plasma were stored at − 80°C for later analysis of cortisol (K3EDTA tube) and elastase (heparin tube) concentrations. Plasma cortisol concentration was determined in duplicate using a commercially available ELISA kit (DRG, Lahn, Germany). All samples were thawed only once before analysis.
In vitro stimulated neutrophil degranulation
The neutrophil degranulation response to bacterial stimulant (840-15, Sigma, Poole, UK) was determined as previously described(Reference Davison, Gleeson and Phillips20). Neutrophil degranulation was expressed as the amount of stimulated elastase release per neutrophil. Elastase concentration was determined in duplicate using a commercially available ELISA kit (Merck Calibiochem, Darmstadt, Germany).
Salivary analysis
All samples were thawed only once before analysis. After thawing, at room temperature, samples were centrifuged at 15 000 g for 2 min to pellet debris, precipitate mucins and obtain a clear supernatant. Saliva osmolality was determined using a freezing point depression osmometer (Osmomat 030, Gonotec, GbBH, Berlin, Germany) calibrated with 300 mOsmol/kg saline solution, in accordance with the manufacturer's instructions. Accuracy was confirmed with a 50 mOsmol/kg reference solution. Saliva samples were screened for blood contamination by the measurement of salivary transferrin concentration. Blood contamination was assumed if salivary transferrin concentration exceeded 10 mg/l. In the event that blood contamination was detected, all salivary data for that subject were excluded from the analysis.
Salivary IgA, lysozyme and transferrin
Aliquots of saliva were analysed for the determination of IgA, transferrin (Salimetrics, State College, PA, USA) and lysozyme (Biomedical Technologies Inc., Stoughton, MA, USA) in duplicate using commercially available ELISA kits.
Data analysis
Statistical analyses were carried out using the statistical computer software package SPSS (v17.00; SPSS Inc., Chicago, IL, USA). Blood glucose concentration, immature granulocyte count, stimulated neutrophil degranulation, plasma cortisol concentration, salivary IgA concentration, IgA:osmolality ratio, IgA secretion rate and lysozyme:osmolality ratio data were normally distributed. The other variables were not normally distributed but were normalised with log transformation (neutrophil count, lymphocyte count, neutrophil:lymphocyte ratio, atypical lymphocyte count and salivary lysozyme concentration) or square root transformation (salivary lysozyme secretion rate and blood lactate concentration) before analysis. To compare the two independent groups' responses to exercise a two-way mixed model (between-groups repeated measures), ANOVA (group × time) was used. Post hoc independent t tests with the Holm–Bonferroni correction were used, where appropriate, to compare time point-specific differences between groups. When there was evidence of an interaction, the effect of time was analysed in each group independently, with one-way repeated-measures ANOVA and post hoc paired t tests with the Holm–Bonferroni correction, where appropriate. The Greenhouse–Geisser correction was applied to correct for violations of the assumption of sphericity, where necessary. All results are presented as means and standard deviations.
Results
There was no difference between groups' demographic data, dietary composition during the 48 h before the main trial (data not shown) or relative exercise intensity during the main trial. There was no differences between groups in mean heart rate (overall mean, 145 (sd 12) beats per minute), subjective rating of perceived exertion (overall, 14 (sd 2)) or mean percentage of VO2max (overall, 64·3 (sd 5·7) %) during the main trial (independent t test, P = 0·699, 0·752 and 0·882, respectively).
The dietary analysis (both the weighed food record and FFQ) also confirmed no deficiencies or excessive intake of any nutrient in all subjects. Estimates of blood volume change were similar between groups Post-Ex (4·5 (sd 1·9) % and 5·8 (sd 2·6) % decrease for PLA and COL, respectively) and 1 h Post-Ex (1·8 (sd 5·5) % and 1·1 (sd 3·2) % decrease, respectively) with no difference between groups (two-way ANOVA group × time interaction, P = 0·414). A similar pattern was evident for estimates of plasma volume change between groups: Post-Ex (3·6 (sd 1·6) % and 2·4 (sd 2·2) % decrease, respectively) and 1 h Post-Ex (1·8 (sd 2·9) % and 0·2 (sd 2·6) % decrease, respectively) with no difference between groups (two-way ANOVA group × time interaction, P = 0·138). Hence, it was not deemed necessary to correct any variables for changes in blood or plasma volume. Furthermore, adjusting for changes in blood and plasma volume did not influence the outcome of any statistical analyses (data not shown).
Blood lactate and glucose responses to exercise
Blood lactate and glucose concentrations were only measured at Pre-Ex, Post-Ex and 1 h Post-Ex. There was no difference between groups in the exercise-induced changes in blood lactate concentration (two-way ANOVA group × time interaction, P = 0·223). There was a significant time effect (P < 0·001) with a mean concentration of 0·75 (sd 0·15) mmol/l Pre-Ex, 1·32 (sd 0·39) mmol/l Post-Ex (significantly higher than Pre-Ex, P < 0·001) and 0·88 (sd 0·24) mmol/l at 1 h Post-Ex (significantly higher than Pre-Ex, P = 0·032). There was no difference between groups in the exercise-induced changes in blood glucose concentration (two-way ANOVA group × time interaction, P = 0·096). There was a significant time effect (P < 0·001) with a mean concentration of 4·4 (sd 0·4) mmol/l Pre-Ex, 4·1 (sd 0·4) mmol/l Post-Ex (significantly lower than Pre-Ex, P = 0·006) and 3·8 (sd 0·3) mmol/l at 1 h Post-Ex (significantly lower than Pre-Ex, P < 0·001).
Plasma cortisol concentration
There was no difference between groups in the exercise-induced changes in plasma cortisol concentration (two-way ANOVA group × time interaction, P = 0·297). There was a significant time effect (P = 0·001) with a mean concentration of 401 (sd 150) nmol/l at baseline, 398 (sd 189) nmol/l Pre-Ex, 500 (sd 175) nmol/l Post-Ex (significantly higher than Pre-Ex, P = 0·033) and 343 (sd 128) nmol/l at 1 h Post-Ex (not significantly different from Pre-Ex, P = 0·314).
Circulating leucocyte number and neutrophil function
There was no difference between groups in the exercise-induced changes in circulating neutrophil count, lymphocyte count, neutrophil:lymphocyte ratio, immature granulocytes and atypical lymphocytes (Table 1). Post hoc analysis revealed a significant exercise-induced increase for all of these parameters. Lymphocytes and atypical lymphocytes had returned to pre-exercise counts by 1 h Post-Ex, whereas neutrophils, neutrophil:lymphocyte ratio and immature granulocytes remained elevated (Table 1).
Table 1 Differential blood leucocyte counts and salivary parameters
(Mean values and standard deviations for the whole group†)

Pre-Ex, samples taken before 2 h exercise bout; Post-Ex, samples taken immediately after 2 h exercise bout; 1 h Post-Ex, samples taken after 1 h of post-exercise recovery; Neu:lymph ratio, neutrophil to lymphocyte ratio.
Mean values were significantly different from Pre-Ex: * P < 0·05, ** P < 0·01 (post hoc analysis time effects).
† There were no significant differences between groups (two-way ANOVA interactions, P>0·05).
There was a significant difference between groups in the exercise-induced changes in in vitro stimulated neutrophil degranulation (two-way ANOVA group × time interaction, P = 0·047). One-way ANOVA on each group showed a time effect in the PLA group (P < 0·001) and the COL group (P < 0·001). Further post hoc analysis showed that there was no difference between Pre-Ex and Baseline in stimulated elastase released per neutrophil (P = 0·12) in the PLA group. There was a significant decrease below Pre-Ex, at Post-Ex (P = 0·002) and 1 h Post-Ex (P < 0·001) in this group (Fig. 1). There was no difference between Pre-Ex and Baseline (P = 0·363) in the COL group. There was a significant decrease below Pre-Ex, at Post-Ex (P = 0·003) and 1 h Post-Ex (P < 0·001) in this group (Fig. 1). Independent t tests between groups at each time point showed no difference at Baseline (P>0·999). There was a trend for higher stimulated neutrophil degranulation in the COL group at Pre-Ex (P = 0·081), but there was no difference at Post-Ex (P = 0·963). However, stimulated neutrophil degranulation was significantly higher in the COL group at 1 h Post-Ex (P = 0·018).

Fig. 1 Neutrophil degranulation. Two-way ANOVA main effects: P = 0·077 (group); P < 0·001 (time); P = 0·047 (group × time interaction). * Mean values were significantly different from samples taken before 2 h exercise bout (Pre-Ex) (P < 0·05; post hoc analysis). † Mean values were different from placebo (PLA, □) at the same time point between the groups (P < 0·05; post hoc analysis). Post-Ex, samples taken immediately after 2 h exercise bout; 1 h Post-Ex, samples taken after 1 h of post-exercise recovery; COL, colostrum (![]() ).
).
Salivary analysis
Blood contamination was detected in the saliva samples from four subjects, two in each group leaving n 8 in each group for all saliva results.
Salivary IgA
There was no difference between groups in the exercise-induced changes in salivary IgA concentration (Table 1). There was a significant time effect (P = 0·009), but post hoc analysis could not identify any specific differences between time points. There was no difference between groups in the exercise-induced changes in salivary IgA:osmolality ratio (Table 1). There was a significant time effect (P < 0·001). Post hoc analysis showed that IgA:osmolality ratio was lower at Pre-Ex than Baseline (P = 0·027). Salivary IgA:osmolality ratio did not change Post-Ex (P = 0·156) and 1 h Post-Ex (P = 0·180) compared to Pre-Ex. There was no difference between groups in the exercise-induced changes in salivary IgA secretion rate (Table 1), and only a trend was evident for a main effect of time (P = 0·072).
Salivary lysozyme
Two-way ANOVA showed a significant group × time interaction for salivary lysozyme concentration (P = 0·044, Table 2). One-way ANOVA on each group showed a time effect in the PLA group (P = 0·018) but not in the COL group (P = 0·146). Further post hoc analysis showed that there was no difference between Pre-Ex and Baseline (P = 0·126) in the PLA group. There was a significant decrease below Pre-Ex, at Post-Ex (P = 0·014) but not 1 h Post-Ex (P = 0·075) in this group (Table 2). Independent t tests between groups at each time point showed a trend for higher salivary lysozyme at Post-Ex (P = 0·065) in the COL group. There was no difference between groups in the exercise-induced changes in salivary lysozyme:osmolality ratio (Table 1), with a significant time effect evident (P = 0·003). Post hoc analysis showed no difference in lysozyme:osmolality ratio between Baseline and Pre-Ex (P = 0·141) and a decreased below Pre-Ex at Post-Ex (P = 0·004) and 1 h Post-Ex (P = 0·013). Two-way ANOVA showed a significant group × time interaction for salivary lysozyme secretion rate (P = 0·002, Table 2). Post hoc analysis showed that there was no difference in the resting measures before and after the supplementation period. One-way ANOVA on each group showed a time effect in the PLA group (P = 0·004) but not in the COL group (P = 0·373). Further post hoc analysis showed that there was no difference between Pre-Ex and Baseline (P = 0·122) in the PLA group, but a significant decrease below Pre-Ex and at Post-Ex (P = 0·036), which recovered by 1 h Post-Ex (P = 0·301).
Table 2 Salivary lysozyme responses
(Mean values and standard deviations)
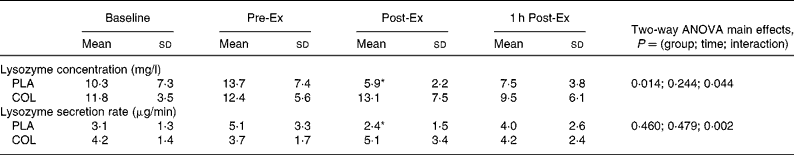
Pre-Ex, samples taken before 2 h exercise bout; Post-Ex, samples taken immediately after 2 h exercise bout; 1 h Post-Ex, samples taken after 1 h of post-exercise recovery; PLA, placebo; COL, colostrum.
Post hoc analysis for time effects within the PLA group: mean values were significantly different from Pre-Ex within the PLA group (*P < 0·05). There were no differences in the COL group. Independent t tests did not reveal any between-group differences at specific time points.
Discussion
The main findings of the present study are that 4 weeks of bovine COL supplementation improved the speed of recovery of neutrophil function after exercise-induced immunodepression and reduced exercise-induced alterations in salivary lysozyme concentration and secretion rate. This is the first study to demonstrate that COL supplementation enhances these innate immune parameters in the recovery period after prolonged endurance exercise that causes acute immunodepression. While some previous studies have shown beneficial effects on resting salivary IgA(Reference Crooks, Wall and Cross11, Reference Mero, Kähkönen and Nykänen12) and upper respiratory tract infection incidence(Reference Brinkworth and Buckley13) in athletes, none have investigated the effects on neutrophil function and innate salivary defences such as lysozyme. The present findings suggest that bovine COL may confer some benefits to innate host defence following prolonged exercise, but there were no effects on salivary IgA.
Shing et al. (Reference Shing, Peake and Suzuki14) observed an enhancement effect of bovine COL on neutrophil surface receptor expression (CD89) at rest, which agrees with the trend for higher resting (Pre-Ex) neutrophil function in the present study. However, in the Shing et al. (Reference Shing, Peake and Suzuki14) study, there were no differences in neutrophil CD11b, CD18 and CD35 expression. Although enhanced CD89 expression may represent enhanced microbicidal activity, no previous study has investigated a functional measure of neutrophil killing capacity (e.g. stimulated degranulation) in response to exercise. Furthermore, in the Shing et al. (Reference Shing, Peake and Suzuki14) study, there were no differences in CD89 expression after exercise, possibly due to the relatively small changes that were actually observed acutely after exercise, even in the PLA condition. In the present study, when a significant decrease in neutrophil function was evident, neutrophil-stimulated degranulation was significantly higher at 1 h Post-Ex in the COL group. We believe that this is the first study to demonstrate that COL supplementation enhances neutrophil function in the recovery period after prolonged endurance exercise.
Many previous studies have examined the effect of prolonged exercise on neutrophils, demonstrating increased circulating neutrophil counts, depressed phagocytic activity and killing functions, such as oxidative burst and degranulation(Reference Davison, Gleeson and Phillips20–Reference Scharhag, Meyer and Gabriel32). The findings of the present study are in line with these findings and confirm that a significant physiological stress was evident in both groups. There were significant exercise-induced increases is blood neutrophils, lymphocytes, neutrophil:lymphocyte ratio, immature granulocytes, atypical lymphocytes, plasma cortisol and blood lactate concentration, and a decrease in blood glucose, with no differences between the PLA and COL groups in the nature of these exercise-induced changes. An increase in atypical lymphocytes may also be indicative of a physiological stress response(Reference Simon33). This also confirms that participants were exposed to a matched physiological stressor and relative exercise stress. The magnitude of exercise-induced immunodepression is often proportional to the changes in circulating leucocytes, and the neutrophil to lymphocyte ratio, in particular, has been suggested as a marker of physiological stress to the immune system(Reference Lancaster, Jentjens and Moseley34, Reference Nieman, Nehlsen-Cannarella and Fagoaga35). An increased release of immature cells from the bone marrow has also been suggested to contribute to decreased mean circulating neutrophil function post-exercise(Reference Davison, Gleeson and Phillips20, Reference Davison and Gleeson22–Reference Davison and Gleeson24, Reference Berkow and Dodson36). The increase in immature granulocytes observed in the present study provides support for this.
The fact that neutrophil degranulation was higher in the COL group at 1 h Post-Ex suggests that ‘recovery’ of this particular immune function may be speeded with COL supplementation. Inadequate recovery of the immune system (i.e. cumulative immunodepression(Reference Lancaster, Halson and Khan37)) can lead to significantly compromised immunity and increased risk in athletes. Li & Cheng(Reference Li and Cheng27) showed that a similar bout of exercise (2 h cycling at approximately 60 % VO2max) caused a significant decrease in neutrophil functions (degranulation and oxidative burst), which persisted for at least 6 h post-exercise. It is possible, therefore, that bovine COL supplementation can reduce this recovery period, but further study, with a more prolonged recovery period, is required in order to fully determine the extent to which COL enhances the recovery from exercise-induced immunodepression. As there was no difference between groups in any of the leucocytosis responses, it is likely that the effects of COL are related to direct effects on leucocytes, as observed in vitro by Sugisawa et al. (Reference Sugisawa, Itou and Ichimura16, Reference Sugisawa, Itou and Saito17). It is worthy of note that Robson et al. (Reference Robson, Bouic and Myburgh31) observed enhanced neutrophil oxidative burst capacity, under resting conditions, in a group of athletes after 21 d of daily supplementation with a multivitamin and mineral supplement. The multi-nutrient supplement was administered to ensure that the recommended daily allowances were achieved and prevented any micronutrient deficiencies. It is possible, therefore, that the effects in the present study were related to micronutrients present in bovine COL. Bovine COL also contains antioxidants (such as vitamins A and E)(Reference Sugisawa, Itou and Ichimura16), and it is a limitation of the present study that plasma antioxidant status was not assessed. However, findings from our previous studies(Reference Davison, Gleeson and Phillips20, Reference Davison and Gleeson23) suggest that this is an unlikely mechanism for the effects observed with bovine COL supplementation in the present study. For example, we have previously demonstrated that 2–4 weeks of dietary antioxidant supplementation(Reference Davison, Gleeson and Phillips20, Reference Davison and Gleeson23) does not influence neutrophil degranulation at rest or in response to exercise similar to that employed in the present study. The lack of difference, in the present study, between groups in the cortisol response (which may be influenced by antioxidant status(Reference Davison, Gleeson and Phillips20, Reference Davison and Gleeson22, Reference Davison and Gleeson23, Reference Peake28)) also provides support for this argument. Furthermore, we have previously shown that supplementation of antioxidants for a 4-week period has little effect on overall plasma antioxidant capacity, presumably because antioxidant ingestion over such a time period results in down-regulation of natural endogenous antioxidant defences in response and/or adaptation to the higher intake of exogenous antioxidant compounds, so that total antioxidant capacity is maintained fairly constant(Reference Davison and Gleeson23). These findings, taken together with the fact that there were no deficiencies in the normal diet of all subjects, and no differences between groups in the cortisol, glucose or leucocytosis responses, suggest that the effects are most likely due to other (bioactive) components present in COL. Strong evidence for this is provided by the work of Sugisawa et al. (Reference Sugisawa, Itou and Saito17), who showed that low molecular weight ( < 10 kDa) components of COL directly prime and enhance neutrophil functions. Sugisawa et al. (Reference Sugisawa, Itou and Saito17) were not able to identify a specific compound, although suggested the proteose peptones as potential candidates. As an explanation for the present findings, this remains speculative and further research is required to determine whether this is the mechanism by which oral supplementation with bovine COL enhances innate immunity after physical stress in human subjects. It is also worthy of note that, in the present study, we only assessed one aspect of neutrophil function. In order to determine the mechanisms of action, future studies should also examine other aspects of neutrophil function, such as chemotactic, phagocytic and oxidative burst responses for example.
In the present study, there were no differences between groups for any of the salivary IgA parameters. Most notably, the supplementation did not cause an increase in resting IgA concentration or release, which does not agree with previous research(Reference Crooks, Wall and Cross11, Reference Mero, Kähkönen and Nykänen12). However, in the Crooks et al. (Reference Crooks, Wall and Cross11) study, supplementation comprised of 26 g of bovine COL per day for 12 weeks. In the Mero et al. (Reference Mero, Kähkönen and Nykänen12) study, supplementation comprised of 20 g of a bovine COL product per day for 2 weeks. However, this was divided into four equal doses spread throughout the day. Taken together, these findings suggest that a period of supplementation of longer than 4 weeks and/or dividing the dose throughout the day is necessary to cause significant changes in resting salivary IgA. Indeed, Crooks et al. (Reference Crooks, Wall and Cross11) did not observe any differences between groups after 4 and 8 weeks of supplementation and a difference was only evident after 12 weeks.
Salivary lysozyme concentration and secretion rate were significantly reduced in the PLA group post-exercise, and had ‘recovered’ after 1 h of rest. We are not aware of any other study that has demonstrated an acute decrease of salivary lysozyme after prolonged exercise of this nature, and this may account for the observations of West et al. (Reference West, Pyne and Kyd38) of chronically lowered salivary lysozyme in trained rowers. In the present study, the acute ‘open window’ of decreased salivary lysozyme, evident in the PLA group, was not evident in the COL group. It has been suggested that enhanced salivary IgA, when consuming bovine COL, may be responsible for protecting athletes from upper respiratory tract infection (self-reported symptoms)(Reference Brinkworth and Buckley13). However, the present findings suggest that enhanced components of the innate immune system (neutrophil function and salivary lysozyme) may also contribute to host protection. This is in line with the suggestion of Shing et al. (Reference Shing, Peake and Suzuki14), but the nature of the relationships between infection risk and aspects of mucosal immunity other than IgA (such as antimicrobial peptides) requires further study(Reference Davison, Allgrove and Gleeson18).
In conclusion, 4 weeks of daily supplementation with bovine COL enhances the recovery of neutrophil function and prevents the decrease in salivary lysozyme release, but not IgA, under conditions of exercise-induced immunodepression. This is the first study to demonstrate that COL supplementation enhances innate immune parameters in the recovery period after prolonged endurance exercise that causes significant immunodepression, and supplementation may confer some benefits to host defence.
Acknowledgements
The author contributions were as follows: G. D. was responsible for the conception of the study, and G. D. and B. C. D. produced the original study design, performed the laboratory investigations and biochemical analyses. G. D. undertook the statistical data analysis and interpretation. G. D. and B. C. D. wrote, read and approved the final manuscript. None of the authors had a conflict of interest. The authors would like to thank Ruth Hughes for management of the supplementation blinding procedures. Sources of support: The present study received partial financial support from Neovite UK, London, who also donated the bovine COL.





