Take-Home Points
1. Inhibition of the vesicular monoamine transporter type 2 (VMAT2) reduces dopamine storage and release.
2. Diminishing dopamine release in turn curtails the hypothetical overstimulation of supersensitive D2 dopamine receptors in the motor striatum that causes tardive dyskinesia.
3. Trimming dopamine release via VMAT2 inhibition in the motor striatum results in stronger “stop” signals and weaker “go,” signals and thus robust therapeutic effects in reducing the abnormal involuntary hyperkinetic movements of tardive dyskinesia.
Introduction
Until recently, therapeutic options for tardive dyskinesia (TD) were few and unsatisfactory. The introduction of drugs with a new mechanism of action, namely inhibition of the vesicular monoamine transporter type 2 (VMAT2), is now transforming the treatment of TD.Reference Hauser, Factor and Marder 1 , Reference Fernandez, Factor and Hauser 2 Here we review dopamine neurotransmission (see also ReferencesReference Stahl 3 – Reference Stahl 8 ) and how it is affected by TD and by various drugs to set the stage for the significance of this new breakthrough treatment mechanism.
VMAT2 and Its Inhibition by Tetrabenazine
Transporters for neurotransmitters exist not only on presynaptic nerve terminals (the well-known “reuptake pumps” targeted by most drugs for depression), but also on synaptic vesicles.Reference Stahl 3 Several types of vesicular transporters have been identified, including different ones for gamma amino butyric acid (GABA), glutamate, glycine, acetylcholine, monoamines, and others.Reference Stahl 3 The specific transporter known as VMAT2 is located on synaptic vesicles of dopamine, norepinephrine, serotonin, and histamine neurons to store these neurotransmitters until needed for release during neurotransmission.Reference Stahl 3 – Reference Jankovic 7 VMAT2 can also transport certain drugs as “false” substrates, such as amphetamine and ecstasy (MDMA; 3,4-methylenedioxymethamphetamine), which can compete for the “true,” natural neurotransmitter.Reference Stahl 3 Synaptic vesicles create low pH in their lumens (interiors) with an energy-requiring proton pump there.Reference Stahl 3 , Reference Lawal and Krantz 6 Low pH in turn serves as the driving force to sequester the neurotransmitter in the synaptic vesicle.Reference Stahl 3 , Reference Lawal and Krantz 6
There are actually two types of VMATs: VMAT1, which is localized in both the peripheral and central nervous system (CNS), and VMAT2, located only in the CNS.Reference Stahl 3 – Reference Jankovic 7 There are two known VMAT inhibitors: reserpine, which inhibits irreversibly both VMAT1 and VMAT2, and tetrabenazine, which reversibly inhibits only VMAT2. That is why reserpine-related drugs, but not tetrabenazine-related drugs, are associated with frequent peripheral side effects, such as orthostatic hypotension (reserpine was once used for hypertension), stuffy nose, itching, and gastrointestinal side effects. Although VMAT2 transports multiple neurotransmitters into synaptic vesicles, tetrabenazine preferentially affects dopamine at clinical doses.Reference Jankovic 7 When tetrabenazine blocks the transport of dopamine into presynaptic vesicles, dopamine is rapidly degraded by monoamine oxidase (MAO), leading to depletion of presynaptic dopamine proportionate to the degree of VMAT2 inhibition.Reference Stahl 3 Tetrabenazine itself is rapidly absorbed and converted into active metabolites, especially alpha and beta hydroxytetrabenazine.Reference Jankovic 7 Deuterated tetrabenazine is similarly metabolized, but more slowly; valbenazine is converted only into the alpha-hydroxy metabolite.Reference Hauser, Factor and Marder 1 , Reference Fernandez, Factor and Hauser 2 , Reference Jankovic 7
Treating Overstimulation of D2 Receptors in Tardive Dyskinesia
D2 receptors in the indirect pathway of the motor striatum hypothetically react to chronic blockade by D2 antagonists by “learning” to have tardive dyskinesia with aberrant neuronal plasticity, resulting in supersensitivity to dopamine.Reference Stahl 4 This leads to too much inhibition of “stop” signals coming from too much dopamine acting at upregulated D2 receptors in the indirect pathway (Figure 1 on the right) and unopposed “go” signals coming from the direct pathway (Figure 1 on the left), and thus involuntary hyperkinetic movements.Reference Stahl 4 If the brain has literally “learned” to have TD in an aberrant attempt to compensate for chronic D2 blockade that results in dopamine overstimulation, then TD would seem to be a disorder ideally set up to respond to interventions that lower dopamine neurotransmission. How can this be done?
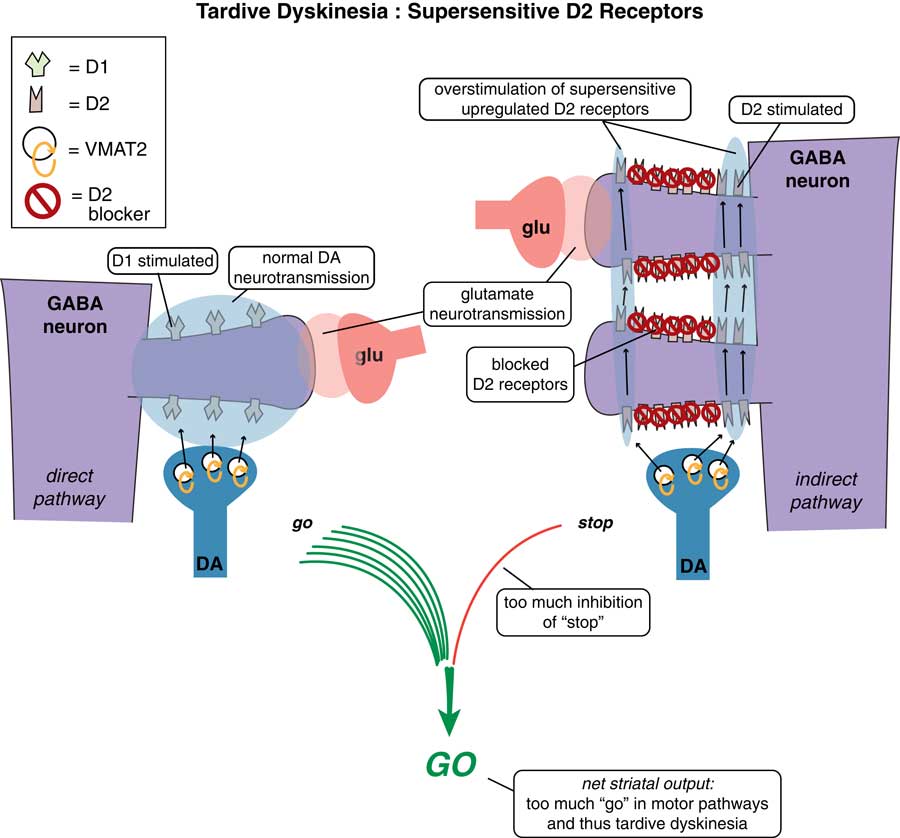
Figure 1 Tardive dyskinesia as imbalance in “stop” and “go” signals from the motor striatum. D2 receptors in the indirect pathway of the motor striatum hypothetically react to chronic blockade by D2 antagonists by “learning” to have tardive dyskinesia with aberrant neuronal plasticity, resulting in supersensitivity to dopamine. This leads to too much inhibition of “stop” signals coming from too much dopamine acting at upregulated D2 receptors in the indirect pathway (on the right), and unopposed “go” signals coming from the direct pathway (on the left), and thus involuntary hyperkinetic movements.
One way is to raise the dose of D2 antagonist to block those upregulated supersensitive D2 receptors (Figure 2). This might work in the short term in some patients at the expense of more immediate side effects and the prospects of making TD even worse down the road.Reference Caroff and Campbell 9 Another treatment possibility is to stop the offending D2 antagonist with the hope that the motor system will re-adjust back to normal and the movement disorder will reverse (Figure 3). Indeed, many patients who do not have an underlying psychotic disorder may be able to tolerate the discontinuation of their D2 antagonist. Unfortunately, it does not seem that the TD brain can “forget” its aberrant neuroplastic learning very well, and only some patients—particularly those who discontinue D2 blockade soon after the onset of their movements—will enjoy reversal of their TD.Reference Caroff and Campbell 9 In fact, most patients experience an immediate worsening of their movements when D2 blockade is eliminated, due to the completely unblocked actions of dopamine in the absence of any D2 antagonist therapy at all (Figure 3).
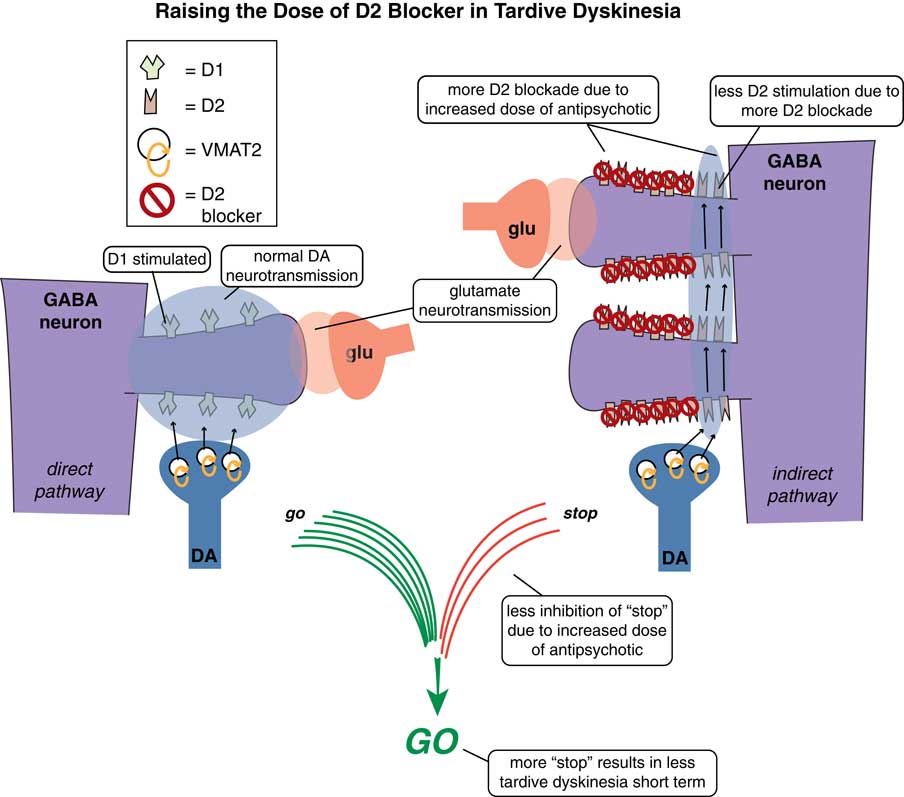
Figure 2 Raising the dose of D2 blockers in tardive dyskinesia. In an attempt to suppress unwanted hyperkinetic movements of TD, one can try to raise the dose of D2 antagonist to block some of those upregulated supersensitive D2 receptors. This might work short term in some patients but at the expense of more immediate side effects and the prospects of making TD even worse long term.
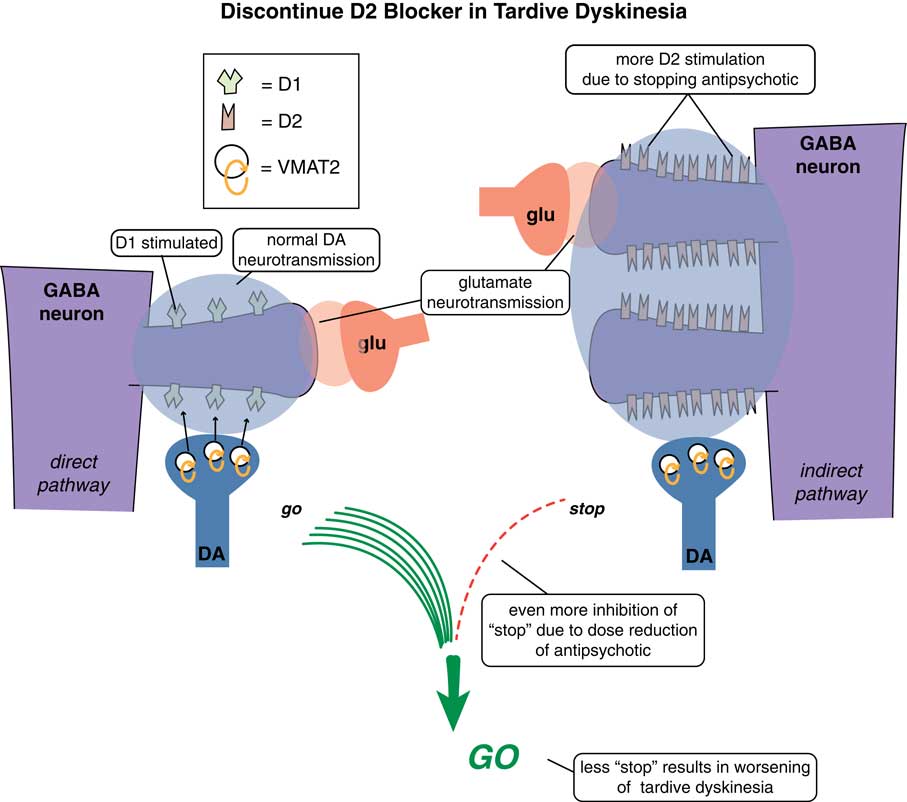
Figure 3 Discontinuing D2 blockers in tardive dyskinesia. Sometimes it is necessary or desirable to discontinue the D2 antagonist causing TD in the hope that the motor system will re-adjust back to normal and the movement disorder will reverse. Sometimes the underlying disorder will not allow that. However, if it is possible to discontinue D2 antagonist treatment, it is unfortunately uncommon that the movement disorder reverses. In fact, most patients experience an immediate worsening of their movements when D2 blockade is eliminated, due to the completely unblocked actions of dopamine in the absence of any D2 antagonist therapy at all.
VMAT2 inhibition is a mechanism that reduces dopamine stimulation without blocking D2 receptors. Thus, this action reduces the overstimulation of D2 receptors in the indirect pathway (on the right in Figure 4), resulting in less inhibition of the stop signal there. However, there is also a benefit of VMAT2 inhibition in the direct pathway where “go” signals are amplified by dopamine at D1 receptors.Reference Stahl 4 , Reference Stahl 5 Even though these D1 receptors and this direct extrapyramidal pathway may not be the site of pathology in TD,Reference Stahl 4 they do drive “go” signals for movement,Reference Lawal and Krantz 6 and lowering dopamine there by VMAT2 inhibition would thus be expected to lower the “go” signals arising from the direct pathway (Figure 4 on the left).Reference Stahl 4 Combined with more “stop” signals from the indirect pathway (Figure 4 on the right), motor output to drive abnormal involuntary hyperkinetic movements is therefore robustly reduced by this combination of effects of dopamine depletion in both pathways.
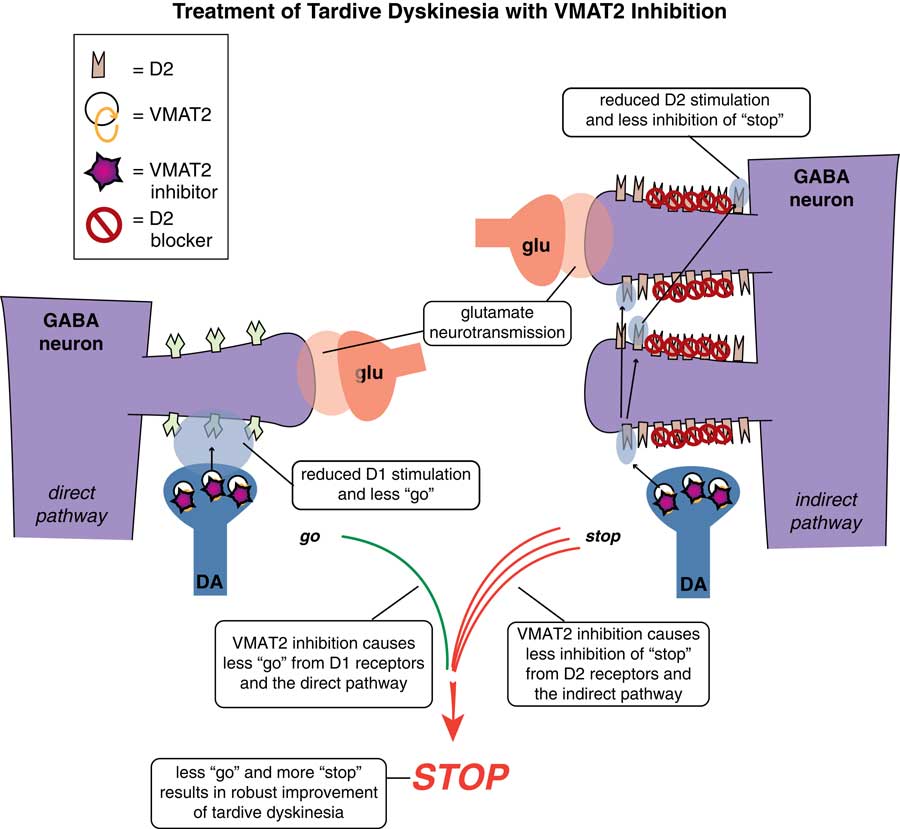
Figure 4 VMAT2 inhibition in TD. This mechanism reduces dopamine stimulation without blocking D2 receptors. It will reduce overstimulation of D2 receptors in the indirect pathway (on the right), resulting in less inhibition of the stop signal there. It will also inhibit VMAT2 and reduce dopamine release in the direct pathway where “go” signals are amplified by dopamine at D1 receptors (on the left). Even though these D1 receptors and this direct extrapyramidal pathway may not be the site of pathology in TD, this pathway does drive “go” signals for movement, so lowering dopamine there by VMAT2 inhibition lowers “go” signals arising from the direct pathway (on the left). Combined with more “stop” signals from the indirect pathway (on the right), motor output to drive abnormal involuntary hyperkinetic movements can be robustly reduced by this combination of effects of dopamine depletion in both pathways.
So, it appears that VMAT2 inhibition can “train” motor pathways of TD to compensate for their abnormal “learning” after chronic D2 receptor blockade.Reference Hauser, Factor and Marder 1 , Reference Fernandez, Factor and Hauser 2 , Reference Stahl 4 , Reference Jankovic 7 Whether this will be disease modifying in the long run, and whether it will reverse rather than only treat movements symptomatically, must be determined by long-term studies of VMAT2 inhibition in TD.
Variable in Treating TD with VMAT2 Inhibition
Treating TD is not going to be simple (Figure 5). That is, there will be a balancing act between the need to continue D2 blockade, which caused TD in the first place (Figure 1), to increase its dose (Figure 2), or whether the D2 blocker can be discontinued (Figure 3). All these choices will affect the impact of dopamine stimulation on the symptoms of TD differently, and thus will affect the dose of VMAT2 inhibitor and degree of VMAT2 inhibition needed in a given patient.
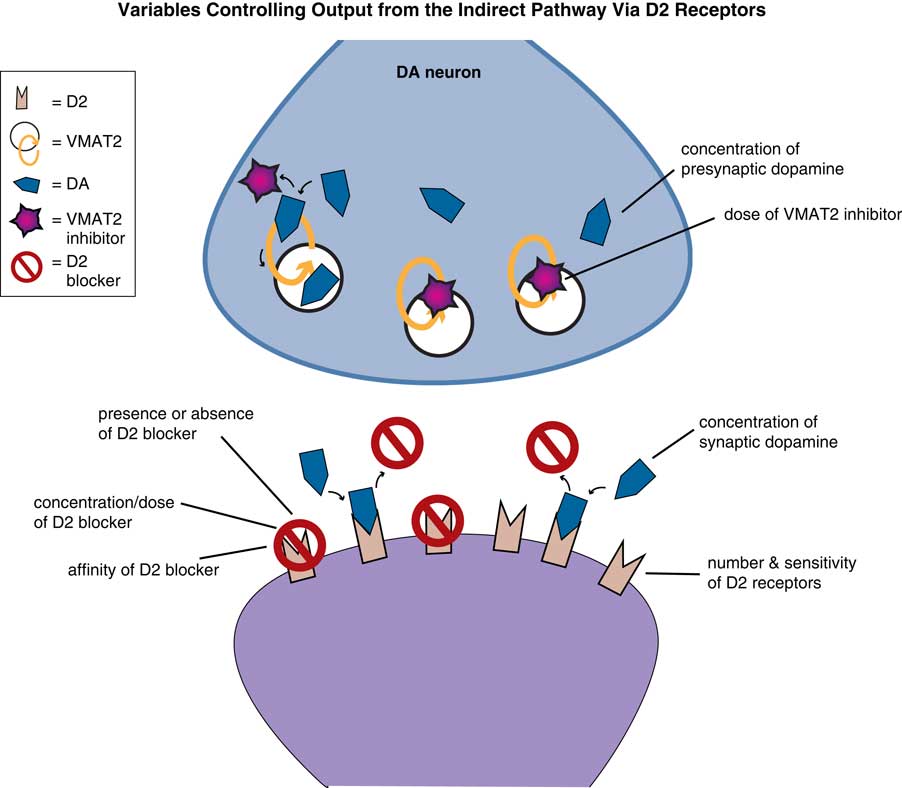
Figure 5 Variables in treating TD with VMAT2 inhibition. Treating TD with VMAT2 inhibitors requires balancing many issues: whether D2 blockade can be increased, decreased, or switched to another D2 blocker. All these choices will affect the impact of dopamine stimulation on the symptoms of TD differently, and thus will affect the dose of VMAT2 inhibitor and degree of VMAT2 inhibition needed in a given patient. In addition, VMAT2 inhibition is reversible, so the amount of inhibition in a given patient at a given dose will be dependent upon the concentration of dopamine in the presynaptic neuron, which can be affected by drugs (such as amphetamine and MAO inhibitors) and disease state (such as stress, psychosis, depression). The amount of VMAT2 inhibition and reduction of dopamine stimulation required for a given patient also depends upon several variables on the postsynaptic neurons receiving dopamine stimulation. That is, the number and sensitivity (and possible reversibility) of supersensitive D2 receptors will vary from one patient to another, with time, and in different areas of the motor striatum, depending upon which body parts are most affected. The degree of dopamine reduction caused by VMAT2 inhibition that is needed by a given patient will also vary with which specific D2 blocker is given, at which dose, and with how much dopamine is being released into the synapse. Changes in psychosis status, mood, and other factors will change dopamine levels in the synapse and therefore the amount of VMAT2 inhibition required. Since dopamine can compete with any D2 blocker, the effects of any D2 blocker depend upon the affinity of the drug for the D2 receptor and the dose of drug.
However, even this is not the whole story. Since VMAT2 inhibition is reversible, the amount of inhibition in a given patient at a given dose will be dependent upon the concentration of dopamine in the presynaptic neuron (Figure 5).Reference Stahl 3 Dopamine levels can be affected by drugs (such as amphetamine and MAO inhibitors) and disease state (such as stress, psychosis, and depression).Reference Stahl 3 The degree of VMAT2 inhibition required for a given patient also depends upon several variables on the postsynaptic neurons receiving dopamine stimulation (Figure 5). That is, the number and sensitivity (and possible reversibility) of supersensitive D2 receptors will vary from one patient to another, with time, and in different areas of the motor striatum, depending upon which body parts are most affected. The degree of dopamine reduction caused by VMAT2 inhibition that is needed by a given patient will also vary with which specific D2 blocker is given,Reference Stahl 8 and at which dose, and with how much dopamine is being released into the synapse. Changes in psychosis status, mood, and other factors will change dopamine levels in the synapse and therefore the amount of VMAT2 inhibition required. That is, dopamine can compete with any D2 blocker administered, but this depends quite widely on which blocker is given (affinities for the D2 receptors have a broad range), and at which dose.Reference Stahl 8 Thus, the skilled clinician must balance these factors when administering a VMAT2 inhibitor to attain best results in the individual patient. When done well, there are very good prospects for robust improvements in TD in many patients.







