Osteoporosis is a major growing public health problem that affects one in three postmenopausal women and the majority of the elderly population(Reference Holroyd, Cooper and Dennison1). It is affected by genetic, endocrine, mechanical, nutritional and other lifestyle factors or health behaviours, with extensive interactions between the different factors(Reference Lane2). Nutritional factors are considered to be of particular importance to bone health because they are potentially modifiable(Reference New, Bonjour and New3). Many studies have shown that some nutrients, such as alkaline ions (K+ and Mg2+), vitamin K and vitamin C, found abundantly in fruit and vegetables, are associated with bone mass and urinary Ca excretion(Reference New, Bonjour and New3, Reference Chen and Ho4). These findings have led researchers to examine the linkages between fruit and vegetable consumption and bone health.
Many previous studies have examined the association between fruit and vegetable consumption and bone health as summarized in a recent review(Reference Chen and Ho4). New et al.(Reference New, Bolton-Smith and Grubb5) first reported the positive association of fruit and vegetable intake with bone mass in human populations. They found that bone mineral density (BMD) at the lumbar spine, femoral neck, trochanter and Ward's triangle was significantly lowered by 3·4 to 4·8 % in middle-aged (44–50 years) women who reported a low fruit intake in early adulthood compared with those who reported a medium or high intake. Similar positive associations between fruit and vegetable intake and BMD, bone mineral content (BMC) or lower fracture rates were also observed in most(Reference Tucker, Hannan and Chen6–Reference Chen, Ho and Woo10), but not all(Reference Kaptoge, Welch and McTaggart11) studies. Therefore, the hypothesis of a beneficial effect of fruit and vegetables on bone health remains unclear(Reference Hamidi, Boucher and Cheung12), and little is known about the effects of them in Asian populations who have traditionally consumed a plant-based diet.
Osteoporosis is determined by the storage of bone mass (or peak bone mass) accumulated before 30–35 years of age and the subsequent rate of bone loss. Adolescence, pregnancy and postmenopause are three important stages for bone health across the life cycle. During the 3–4 years of puberty in adolescents, BMD increases by about 50 % and BMC by about 200 %(Reference Rizzoli and Bonjour13). Excess bone loss may occur among pregnant and lactating women because of substantial Ca transfers from them to their fetus or infant(Reference Sowers14, Reference Givens and Macy15). Increased rate of bone loss occurs in women after menopause due to rapidly declining concentrations of circulating oestrogen(Reference Nilas and Christiansen16). Therefore, it is important to assess the determinants of bone mass in these life stages.
Typical Chinese diets comprise higher components of vegetables, and lower intakes of protein, than their Western counterparts(Reference Leung, Ho and Woo17, Reference Zhai and Yang18). A Chinese national survey found a higher intake of fruit and vegetables (320 g/d) among Chinese(Reference Zhai and Yang18) compared with American women at 52 years of age (3·5 (sd 1·8) servings; ∼280 g/d)(Reference Djousse, Arnett and Coon19). It is still uncertain whether greater fruit and vegetable intake is associated with better bone mass in Chinese populations(Reference Chen, Ho and Woo10). The present cross-sectional study aimed to examine the associations between fruit and vegetable consumption and bone mass in adolescents, postpartum women and postmenopausal women.
Participants and methods
Participants
Participants included in the present cross-sectional study were composed of three age groups: (i) 222 early adolescent boys and girls aged 11–14 years; (ii) 371 parturient women (within 2 weeks postpartum) aged 20–34 years; and (iii) 333 postmenopausal women aged 50–70 years. The adolescent boys and girls were recruited from Year 1 students at four typical secondary schools in Guangzhou city in November 2009. Their parents were required to fill in a form for the study registration and eligibility screening. Potential adolescents were enrolled based on the returned registration forms. The parturient women (volunteers) were recruited from in-patients in Guangdong Women and Children's Hospital and Health Institute during July 2009 to May 2010, and the postmenopausal women were enrolled from communities in urban Guangzhou by local post between July and November 2009. The girls were required to have menarche, and the postmenopausal women to have natural menopause for at least 12 months. Exclusion criteria included hormonal replacement therapy, malabsorption, lactose intolerance, chronic liver or kidney diseases, parathyroid and thyroid diseases, gastric operation or cancer, oophorectomy and/or hysterectomy.
After initial screening for eligibility, we invited the potential participants to the Guangdong Women and Children's Hospital and Health Institute for face-to-face questionnaire interviews and measurements of bone mass and anthropometric indices after further confirming their eligibility. Written informed consent was obtained from all participants (or their legal guardians for the adolescents) prior to the final enrolment. The ethical committee of the School of Public Health of Sun Yat-sen University approved the study.
Data collection
Anthropometric and bone mineral status measurements
Height was measured to the nearest 0·1 cm and weight to the nearest 0·1 kg with the participant in light clothing and no shoes standing motionless and straight in the centre of the scale. BMI was calculated as weight (in kilograms) divided by the square of height (in metres).
Dual-energy X-ray absorptiometry (DPX-L instrument; GE Lunar, Waukesha, WI, USA) was used to measure BMD (g/cm2) and BMC (g) at the whole body, lumbar spine (L1–L4) and left hip (including total hip and femoral neck). The in vivo reproducibility of the machine was 1·92 %, 1·48 % and 0·68 % for the BMD tests at the femoral neck, lumbar spine and whole body, respectively.
General information and dietary assessments
Face-to-face interviews based on a structured questionnaire were used to collect general information on sociodemographic data, years since menarche (for girls) or menopause (for postmenopausal women) and physical activities. The metabolic equivalent for task (MET) was calculated for daily physical activities.
The dietary assessment of intakes of fruit, vegetables, Ca and protein was based on a quantitative FFQ that included seventy-nine food groups/items as validated in previous studies(Reference Zhang and Ho20). The mean intake of food per day, week or month was reported at the face-to-face interview, using the past 12 months (in postmenopausal women) or 3 months (in adolescents and parturient women) prior to the interview as the reference period. Food photographs in the reference portion sizes and some household measures used in the FFQ as serving size were provided for aids. Fruit intake was estimated based on ten main fruit items/groups: (i) orange, grapefruit and lemon; (ii) apple, pear, peach, pineapple and plum; (iii) banana; (iv) grapes; (v) lychee and longan; (vi) mango and persimmon; (vii) papaya; (viii) water melons and various muskmelons; (ix) durian; and (x) other fruits; Vegetable intake was estimated based on thirteen main vegetable groups/subgroups: (i) dark-green leafy vegetables (four subgroups, including nineteen common vegetables); (ii) light-green leafy vegetables (e.g. broccoli, cabbage, cauliflower, celery); (iii) onion, garlic; (iv) turnip and various vegetable melons (e.g. Chinese waxgourd, pumpkin, cucumber, towel gourd, bitter gourd, luffa-smooth loofah, eggplants); (v) tomato; (vi) peppers; (vii) carrots; (viii) starchy vegetables (e.g. yam and potato, taro, other tubers); (ix) fresh corn; (x) fresh beans; and (xi) mushrooms and edible fungi. Pickled vegetables were excluded in the calculation of vegetable intake. Dietary energy and nutrient intakes were calculated from the China Food Composition Table(Reference Yang, Wang and Pan21). The correlation coefficients for the short-term reproducibility of fruit and vegetable intakes were r = 0·65 (P < 0·0001) and r = 0·58 (P < 0·0001), respectively, in the study participants.
Statistical analyses
We examined the associations between intake of vegetables, intake of fruit and total intake of fruit and vegetables with BMD and BMC among all participants combined due to limited sample size in each age and sex group. To ensure the comparability of the values for dietary intakes of fruit and vegetables, total energy, Ca and protein, BMD and BMC, BMI and MET of physical activity, these variables were separately standardized into normal Z-scores in each subgroup of girls, boys, young women and postmenopausal women. The Z-scores were then used in the following analyses. Participants were classified into tertiles according to the Z-score of fruit or vegetable intake in each of the four subgroups. One-way ANOVA and analysis of covariance were used to compare the mean difference between Z-scores of BMD and BMC among the fruit or vegetable tertiles. In the analysis of covariance we adjusted for age (years), BMI (Z-score), dietary intakes (Z-score) of energy, protein and Ca, physical activity (Z-score of MET), use of vitamin supplements (yes/no), use of Ca supplements (yes/no), sex and menopause status (yes/no). Pair-wise comparisons were done by the Bonferroni method. Multivariate regression was conducted to assess the independent associations between total intake of fruit and vegetables and BMD and BMC in each of the sex and age groups. The enter method was used for the intake of fruit and vegetables, and the stepwise method was used for the covariates of age (years), BMI (Z-score), dietary intakes (Z-score) of energy, protein and Ca, physical activity (Z-score of MET), use of vitamin supplements (yes/no), use of Ca supplements (yes/no), sex and Tanner stage (adolescents), and years since menopause (postmenopausal women). Starchy vegetables were excluded from the calculation of total fruit and vegetable intake in the above analyses.
A two-sided P value less than 0·05 was considered as statistically significant. We used winsorization to replace outliers that were at least 3 sd away from the group mean with the next most extreme value, as the few outliers might extremely affect the mean values and the associations(Reference Howell22). All data analyses were conducted using the SPSS for Windows statistical software package version 13 (SPSS Inc., Chicago, IL, USA).
Results
Participants’ characteristics and dietary intakes
Table 1 shows the general information of the participants, including age, body weight, height, BMI, age at menarche, years since menopause, physical activity, Ca and vitamin supplement use, and the summary (mean and sd) of BMD and dietary intakes of energy and Ca. Mean vegetable intake was 303, 289, 344 and 435 g/d and mean fruit intake was 185, 206, 380 and 174 g/d in boys, girls, young women and postmenopausal women, respectively. About 40 % of the fruit intake was from the group of apple, pear, peach, pineapple and plum, and 20 % from the group of orange, grapefruit and lemon. About half of all vegetables consumed were leafy vegetables, followed by various vegetable melons, carrot and radish (Table 2).
Table 1 Characteristics of the study participants, Guangdong, China, July 2009 to May 2010
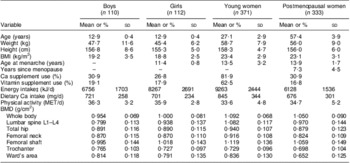
MET, metabolic equivalent for task; BMD, bone mineral density.
Data are presented as means and standard deviations or as percentages.
‡Cooking oil was not included in the calculation of energy intake due to poor accuracy.
Table 2 Intakes of vegetables and fruit (g/d), and the percentage contribution of subtypes to total intake, among the study participants, Guangdong, China, July 2009 to May 2010

‡Leafy vegetables: pak choi, choi sum, lettuce, spinach, Chinese spinach, water spinach, Chinese kale, mustard, broccoli, cabbage, cauliflower, celery, etc.
§Melons: Chinese waxgourd, pumpkin, cucumber, towel gourd, bitter gourd, luffa-smooth loofah, etc.
∥Starchy tubers: yam, potato, sweet potato, taro, lotus root, etc.
Association of fruit and vegetable intakes and bone mass in adolescents and adults
In general, ANOVA showed that fruit and vegetable intakes were significantly and positively associated with BMD and BMC at the majority of the studied bone sites. Fruit intake had a much more significant association with BMD or BMC than did vegetable intake and the total intake of fruit and vegetables. BMD Z-score increased by 0·30 (or 2·6 % of the mean), 0·25 (3·9 %), 0·27 (3·5 %) and 0·29 (4·0 %), and BMC Z-score increased by 0·44 (7·6 %), 0·31 (7·2 %), 0·44 (7·5 %) and 0·36 (5·9 %), at the total body, lumbar spine, total hip and femoral neck in participants belonging to the top tertile compared with the bottom tertile of fruit intake (all P < 0·01), respectively. Small differences in BMD Z-score (0·13–0·19) and BMC Z-score (0·10–0·18) were observed between the top and bottom tertiles of vegetable intake. The mean difference in BMD Z-score ranged from 0·13 to 0·25 and in BMC Z-score from 0·20 to 0·35 between the highest and lowest tertiles of total intake of fruit and vegetables (Table 3).
Table 3 Z-scores of BMD and BMC at various sites by fruit and vegetable intake tertiles of the study participants, Guangdong, China, July 2009 to May 2010Footnote ‡
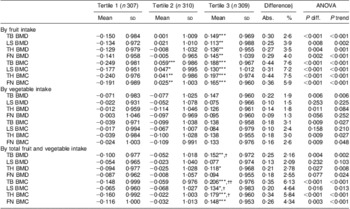
BMD, bone mineral density; BMC, bone mineral content; TB, total body; LS, lumbar spine (L1–L4); TH, left total hip; FN, femoral neck.
Mean values were significantly different from those of tertile 1: *P < 0·05, **P < 0·01, ***P < 0·001.
Mean values were significantly different from those of tertile 2: †P < 0·05, ††P < 0·01.
‡ Original BMD and BMC were converted to standard normal Z-score stratified by subgroups of girls, boys, young women and postmenopausal women.
§ Difference between tertile 3 and tertile 1: Abs., absolute mean difference (tertile 3 – tertile 1); %, relative difference compared with the mean BMD
or BMC, % = [(Abs. × sd)/Mean] × 100 %, where Mean and sd are the mean and sd of BMD or BMC.
After adjustment for potential confounding factors, such as age, BMI, dietary intakes of energy, protein and Ca, physical activity, use of vitamin supplements, use of Ca supplements and menopause status, significant associations between fruit intake and BMD/BMC remained, although the associations were slightly attenuated. The P values for trend ranged between <0·001 and 0·002. Mean differences in Z-score ranged between 0·22 and 0·25 (BMD) and 0·25 and 0·34 (BMC) between the top and bottom tertiles of fruit intake (all P < 0·05). However, only a marginally significant association between vegetable intake and total body BMD (P = 0·030) was observed. There was no significant association of vegetable intake with either BMD or BMC at any other bone site. The effect of total intake of fruit and vegetables on BMD and BMC was in the range between those of fruit and vegetable intake separately (Table 4).
Table 4 Covariable-adjusted of BMD and BMC at various sites by fruit and vegetable intake tertiles of the study participants, Guangdong, China, July 2009 to May 2010Footnote ‡
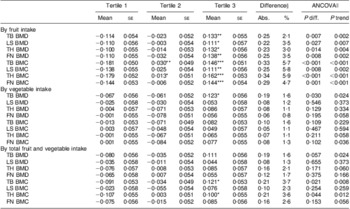
BMD, bone mineral density; BMC, bone mineral content; ANCOVA, analysis of covariance; TB, total body; LS, lumbar spine (L1–L4); TH, left total hip; FN, femoral neck.
Mean values were significantly different from those of tertile 1: *P < 0·05, **P < 0·01, ***P < 0·001.
‡ Original BMD and BMC were converted to standard normal Z-score stratified by subgroups of girls, boys, young women and postmenopausal women.
§ Difference between tertile 3 and tertile 1: Abs., absolute mean difference (tertile 3 – tertile 1); %, relative difference compared with the mean BMD
or BMC, % = [(Abs. × sd)/Mean] × 100 %, where Mean and sd are the mean and sd of BMD or BMC.
∥ Covariates adjusted for in the multivariate model: age (years), BMI (Z-score), dietary intakes (Z-score) of energy, protein and Ca, physical activity (Z-score of metabolic equivalent for task), use of vitamin supplements (yes/no), use of Ca supplements (yes/no), sex and Tanner stage (adolescents), menopause status (yes/no) and years since menopause (menopausal women).
Association of fruit and vegetable intakes and bone mass in subgroups
To explore the potential sensitive populations, we conducted subgroup-stratified analyses by age and sex groups. Among the four age and sex groups, boys had the strongest associations of total intake of fruit and vegetables with BMD and BMC, followed by girls and postmenopausal women. Multivariate regression analyses showed a marginally significant positive association of total intake of fruit and vegetables with BMD at the hip site in boys, at the trochanter in girls and at the whole body in postmenopausal women (P values: 0·014–0·081), but no significant association was observed at all bone sites except for the whole body BMC (P = 0·043) in young women (Table 5).
Table 5 Regression coefficients of the association between total fruit and vegetable intake and bone mass in subgroups by sex and age, Guangdong, China, July 2009 to May 2010
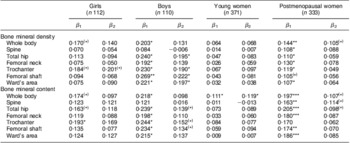
β 1 and β 2, univariate and multivariate regression coefficients, unit: Z-score/Z-score.
Covariates adjusted for in multivariate model: age (years), BMI (Z-score), dietary intakes (Z-score) of energy, protein and Ca, physical activity (Z-score of metabolic equivalent for task), use of vitamin supplements (yes/no), use of Ca supplements (yes/no), sex and Tanner stage (adolescents), and years since menopause (postmenopausal women).
Significance of the regression coefficient: (*)P < 0·1, *P < 0·05, **P < 0·01, ***P < 0·001.
Sensitivity analysis
Almost the same results were observed when the original values of the outliers were included (data not shown).
Discussion
In the present cross-sectional study containing adolescents, young women and postmenopausal women, we found a significant positive association between fruit intake and BMD and BMC at all studied bone sites and between vegetable intake and total body BMD. A previous study estimated that a 1 sd decrease in total hip BMD was associated with an 85 % (95 % CI 70, 101 %) increase in the risk of total osteoporotic hip fractures(Reference Leslie, Lix and Tsang23). According to this estimation, an increase in BMD of 0·22 sd at the total hip would result in a 19 % decrease in the risk of hip fracture in the top compared with the bottom tertile of fruit intake in our population. These results suggest that greater intake of fruit and vegetables may be beneficial to improve bone mass.
Several small studies have examined the association between fruit and vegetable intake and bone measurements and yielded weak positive or null effects. Prynne et al.(Reference Prynne, Mishra and O'Connell7) found that greater fruit and vegetable intake was associated with higher BMD and BMC at the whole body and spine, and BMC at the hip, in both 111 boys (mean age 16·8 years) and 101 girls (mean age 17·4 years). A similar positive association of fruit and vegetable consumption with heel BMD was found by McGartland et al.(Reference McGartland, Robson and Murray24) in 378 girls (but not in 324 boys) aged 12 years. In a 7-year follow-up study, Vatanparast et al.(Reference Vatanparast, Baxter-Jones and Faulkner9) found that every additional serving of fruit and vegetables was associated with an increase of 5·4 (se 1·3) g in total body BMC accrual in eighty-five boys aged 8–20 years, but no significant effect was observed in sixty-seven girls of the same age. Tylavsky et al.(Reference Tylavsky, Holliday and Danish8) found that fruit and vegetable intake was a significant independent predictor of bone area but not BMD or BMC in fifty-six girls aged 8–13 years. Our findings are consistent with the previous results. However, due to the limitations of study design and small sample size in previous studies, more prospective large studies are needed to confirm the effect in adolescents in future.
New et al.(Reference New, Bolton-Smith and Grubb5) first reported the positive association of fruit and vegetable consumption with bone mass in a cross-sectional study in 1997. Similar positive associations between fruit and vegetable intake and BMD, BMC or low fracture rate were also observed in elderly men and women of the Framingham cohort(Reference Tucker, Hannan and Chen6), in middle-aged postmenopausal women in Hong Kong(Reference Chen, Ho and Woo10), in a population-based survey of mainland Chinese men and women(Reference Zalloua, Hsu and Terwedow25), and in old women in the UK(Reference Prynne, Mishra and O'Connell7). However, inconsistent results (null effect) were also found in several studies or sub-populations(Reference Tucker, Hannan and Chen6, Reference Prynne, Mishra and O'Connell7, Reference Kaptoge, Welch and McTaggart11).
To our knowledge, the present study is the first one assessing the association of fruit and vegetable intakes with bone measurements in postpartum women. Only a marginally significant association was found for total body BMC in the multivariate regression analyses in this group. Up to date, very limited data have been published on young women. Prynne et al.(Reference Prynne, Mishra and O'Connell7) reported non-significant associations between vegetable and fruit intake and BMD or BMC at the total body, lumbar spine, total hip and neck in women aged 23–37 years. It is unclear whether the null association with BMD was due to the specificity of age, pregnancy, greater changes in dietary intakes due to pregnancy, or just because of the weak or null effect of fruit and vegetables in this population.
In general, the above studies showed a weak beneficial effect of higher intake of fruit and vegetables on bone measurements. In contrast to the previous results, the present study observed significant positive associations between fruit intake and BMD and BMC at several bone sites even adjustment for potential confounders in adolescents, young and postmenopausal women combined, and in some of the studied bone sites among the four subgroups. The reasons for the inconsistent results found in different studies remain unclear. The weak effect of fruit and vegetables, small study size, large random error in the assessment of long-term intake of fruit and vegetables, and other environmental and genetic heterogeneity in different populations might partially explain the inconsistent results. However, due to the limitations of study design and small sample size in previous studies, more prospective large studies are needed to confirm the effect in future.
The mechanisms whereby vegetables and fruit affect bone health have been explored in previous publications and focused mainly on two aspects. The first one, called the acid–base hypothesis, postulates that acid load is, in part, buffered by bone mineral, leading to bone dissolution and reduced bone density(Reference Barzel26, Reference Green and Kleeman27). Diets high in acid-forming components (including several amino acids in protein foods, P and Cl) and low in base-forming components (K, Ca, Mg and vitamin C) lead to a higher dietary acid load(Reference Bushinsky28). Vegetables and fruit, as a good source of alkaline-forming components, could neutralize the calciuric effects of acids derived from the diet(Reference Buclin, Cosma and Appenzeller29, Reference Tucker, Hannan and Kiel30). Moreover, some literature documents that the above alkaline-forming cations have an independent impact on improving Ca balance(Reference Lemann, Pleuss and Gray31, Reference Sebastian, Harris and Ottaway32) and bone health(Reference New, Bolton-Smith and Grubb5, Reference Tucker, Hannan and Chen6, Reference Macdonald, New and Golden33). The second aspect is that vegetables and fruit might affect bone health via the roles of antioxidant vitamins, such as vitamin C(Reference Morton, Barrett-Connor and Schneider34, Reference Hall and Greendale35) and vitamin K(Reference Binkley and Suttie36, Reference Booth, Broe and Gagnon37). However, after adjusting for the potential influence of these nutrients, vitamin D and fibre, McGartland et al.(Reference McGartland, Robson and Murray24) proved that the positive association between fruit intake and heel BMD in the 12-year-old girls remained. It is possible that the observed association between fruit and BMD might be related to another mechanism other than the two mentioned. Other compounds found in vegetables and fruit, such as phyto-oestrogens and phytochemicals, might be taken into account(Reference Tobe, Muraki and Kitamura38). However, these mechanisms cannot explain the more pronounced favourable effect of fruit than of vegetables on bone mass observed in the present and previous studies(Reference Prynne, Mishra and O'Connell7, Reference Chen, Ho and Woo10). It is well established that Na plays a key role in Ca metabolism(Reference Ho, Chen and Woo39, Reference Teucher, Dainty and Spinks40). Vegetables are consumed mainly in cooked form in Chinese populations. Therefore, the higher intake of Na consumed with vegetables might counteract the favourable effect of vegetables as compared with fruit, which tend to be consumed fresh.
Study validity and limitations
There were several potential limitations in the present study. First, the participants were volunteers, not a nationally representative sample. We could not exclude potential volunteer bias. Next, the study was cross-sectional in design. The validity of the causal relationship between fruit and vegetable consumption and bone mass depends mainly on the stability of fruit and vegetable intakes. Since it is unlikely to increase consumption of fruit and vegetables due to good bone mass, any changes in fruit and vegetable intake would attenuate the association. Therefore, we might not overestimate the strength of the association. Furthermore, we could not adjust for the influence of vitamin D because of very poor accuracy in the assessment of vitamin D intake using the FFQ, due to limited inclusion of vitamin D-enriched food items. However, plenty sunlight in Guangzhou in southern China would decrease the contribution of vitamin D from the diet. Also, we could not precisely adjust for supplemental Ca because most participants could not accurately report the dosage. Another limitation was the relatively small sample size in each sub-population: although we had a total of 926 participants, we did not have sufficient power to detect weak associations in the subgroups.
Nevertheless, the exposures we examined here – dietary factors – permitted elucidation of the associations of fruit and vegetable consumption with bone health owing to their relatively stable nature, particularly in postmenopausal women, as discussed previously(Reference Chen, Ho and Woo10). Dietary intake was assessed using a validated FFQ with good validity and reliability in the assessment of habitual intake of fruit and vegetables in this population(Reference Zhang and Ho20), and we adjusted for a number of important covariates in the multivariate analysis.
Conclusions
Fruit and vegetable consumption was positively associated with bone mass in adolescents, young women and postmenopausal women combined. More benefits were observed in boys and postmenopausal women than in girls and young women. Fruits had a much stronger association with bone mass than vegetables. Our findings add to the existing evidence that fruit and vegetables may have a bone sparing effect.
Acknowledgements
The study was jointly supported by the 11th Five Year Key Programs for Science and Technology Development of China (no. 2008BAI58B02) and the National Natural Science Foundation of China (no. 30872100, 81072299). There are no conflicts of interest. Y.-X.S. and Y.-M.C. obtained the grants, had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Y.-M.C., Y.-X.S. and Z.-W.H. were responsible for the study concept and design; J.-J.L., R.-Q.W., X.-M.M., Z.-Q.Z. and Z.L. acquired the data; Y.-M.C., J.-J.L. and Y.-X.S. analysed and interpreted the data; J.-J.L. and Y.-M.C. drafted the manuscript; Y.-M.C., Y.-X.S. and Z.-W.Z. critically revised the manuscript for important intellectual content.







