Skin and soft-tissue infections (SSTIs) are common clinical conditions ranging from mild to life-threatening [Reference Ki and Rotstein1]. Because cultures are not performed in many cases of SSTI, the causes of these infections remain uncertain, although Staphylococcus aureus and β-haemolytic streptococci (BHS) are frequently reported as the leading pathogens [Reference Ray, Suaya and Baxter2]. Until the late 1990s, methicillin-resistant S. aureus (MRSA) was largely confined to healthcare settings and predominantly affected individuals with comorbidities [Reference Eady and Cove3]. Since then, the incidence of MRSA in the wider community has rapidly increased worldwide and is commonly associated with SSTIs as evidenced particularly by reports from the USA [Reference Edelsberg4].
Several risk factors have been reported for MRSA SSTIs, including younger age, exposure to healthcare workers, previous antibiotic use, chronic skin conditions, close contact with a person with MRSA or with a skin condition [Reference Skiest5–Reference Golding9]. Additionally, nasal carriage of MRSA has been cited as a risk factor for the development of systemic infections and represents a common reservoir during SSTI outbreaks [Reference Stevens10].
Outside of European and North American populations, little is known about the leading causal agents of SSTIs, and less so of risk factors for these infections due to MRSA in Asia. Identification of such factors may assist healthcare professionals to provide appropriate treatment for patients with suspected S. aureus SSTI. Here, we describe the results of microbiological investigations, and sociodemographic and clinical characteristics of patients treated for SSTIs at two hospital-affiliated outpatient clinics in Taiwan through a case-control study to identify potential risk factors of MRSA SSTIs.
Patients registered at Dermatology clinics in two of the 10 branches of the Taipei City Hospital (Heping and Renai) between 1 January 2012 and 31 December 2013 were investigated. Eligibility criteria for study enrolment were age ⩾20 years and presenting with a new-onset SSTI for which wound culture was planned. Patients were excluded if they were: a healthcare worker in a hospital or a long-term care facility; pregnant, hospitalized or had previous surgery, or received dialysis, endotracheal intubation, or placement of an indwelling device, or resided in a long-term care facility within 1 year before the date of culture.
Following written consent, a nasal swab and two wound swabs (for aerobic and anaerobic culture) were taken from each patient and demographic data were collected through a structured questionnaire. This recorded (1) socioeconomic status, occupation, and household composition, clinical history (hospitalization, surgical procedures, underlying medical conditions, antibiotic use), (2) dermatological conditions and treatment (skin infection history, infection characteristics location, duration, size, depth of the deepest tunnel, erythema, ulceration, and abscess, and antibiotics prescribed), (3) a pain score associated with infection, and (4) contact with persons with known MRSA infections or at greater risk of a MRSA infection, such as healthcare facility workers, day-care facility workers, and nursing home residents. The study was approved by the institutional review board of Taipei City Hospital in compliance with ethical standards of the Helsinki Declaration of 1975, as revised in 2008.
Wound and nasal swabs in Amies modified Stuart's transport medium (Copan, Italy) were plated directly on blood agar plates, Columbia-CNA agar, eosin Methylene Blue agar, and bile esculin agar (BD Diagnostic Systems, USA) and incubated for 18–24 h at 35–37°C under appropriate atmospheric conditions; anaerobic incubation was with the GasPak Anaerobic Container System (BD Diagnostic Systems). Species identification was based on colony morphology and biochemical test reactions using the BD Phoenix Automated Microbiology System, and confirmed where necessary by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry analysis (Bruker, USA). Confirmed S. aureus isolates were screened for antimicrobial susceptibility (BD Phoenix) and a standardized disk diffusion method [11] to oxacillin, penicillin, erythromycin, gentamicin, ciprofloxacin, clindamycin, co-trimoxazole, tetracycline, vancomycin and chloramphenicol.
A case-control study was conducted to identify the potential risk factors of MRSA SSTIs; cases and controls were selected from the total number of patients in the study. Cases of MRSA SSTI were defined as patients with clinically relevant and laboratory-confirmed MRSA and controls as patients with SSTIs from whom non-MRSA bacteria were isolated.
The significance of associations between outcome (MRSA SSTI) and the demographic and exposure variables was determined using regression diagnostics to assess collinearity among the variables. Significant variables (P < 0·15) by univariate analysis were subjected to a stepwise logistic regression analysis. All covariates were assessed as possible confounders before being excluded from further modelling. Multivariable logistic regression modelling was performed, and the reduced model included only those variables that were statistically significant at α = 0·05 or otherwise identified as possible confounders (e.g. gender). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the association of each exposure variable and MRSA SSTI. All patients’ data were extracted and entered into a standardized spreadsheet (Microsoft Excel 2007; Microsoft Corp., USA). SAS software v. 9.3 (SAS Institute, USA) was used for analyses.
In total, 100 of the 103 cases collected over a 24-month period met the study eligibility criteria. Of these 39% were female and the average age was 46·3 years. S. aureus was the most common organism and was isolated from wound samples of 39 patients; 29 (74%) of these yielded MRSA. Sixteen of the latter group also had MRSA nasal carriage compared to 5/10 patients with methicillin-susceptible isolates. Gram-negative species accounted for 21% of SSTIs and 6% grew BHS. Anaerobic bacteria were recovered from 31 patients’ wound cultures and four of these were mixed with aerobic flora. Three groups were therefore distinguished by wound culture, those yielding MRSA (29), those with other organisms (55) and those negative for identified pathogens (16).
Overall, 87% of all SSTIs were coded as cellulitis and abscess, and 59% of patients had at least one other skin disease. Furthermore, 34% reported a history of SSTI, 19% had previously received antibiotics for a SSTI, and 16% had a history of diabetes. Only 8% presented with a fever and 14% recorded a pain score of ⩾7 at examination. The head and neck were the predominant sites of SSTI for both MRSA (41·4%) and non-MRSA (38·2%) cases, and antimicrobials had been prescribed in the previous year respectively for 28% and 15% of these groups. Moreover, 24% of MRSA patients, had been exposed to a patient who had undergone surgery within a year before infection compared to 5% of the non-MRSA group. Specifically within the MRSA group (29), 69% had at least one other skin disease, and 38% had a history of SSTI (Table 1). Infection was most common in the head and neck regions (41%) and lower extremities and the majority (83%) of patients presented with abscesses, 58% of which were ⩾5 cm in diameter. Erythema was common (48%), and ulceration less so (10%); 24% of patients recorded pain scores of ⩾7.
Table 1. Characteristics of patients presenting with SSTI according to MRSA status
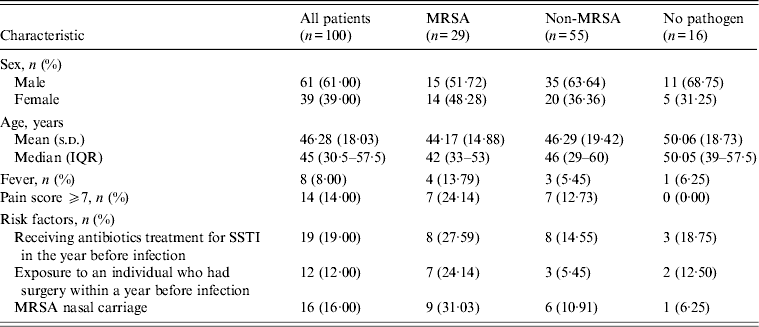
SSTI, Skin and soft-tissue infection; MRSA, methicillin-resistant Staphylococcus aureus; IQR, interquartile range.
The majority of patients (88·7% MRSA, 79·3% non-MRSA), were prescribed antimicrobials for their SSTIs. Cephalosporin (80%) was the most widely used for both groups followed by doxycycline (9%) and topical fusidic acid alone (5·5%) in patients with non-MRSA infections; clindamycin (13·8%) and amoxicillin/clavulanate (3·5%) were prescribed for patients with MRSA. Most MRSA isolates were susceptible to the antimicrobials tested ranging from 58·6% for erythromycin to full susceptibility to vancomycin. Notable resistances were erythromycin (41·3%) clindamycin (38%), chloramphenicol (20·6%), and gentamicin (17·2%); co-resistance to erythromycin and clindamycin was observed for 38% of MRSA isolates.
Multivariable analysis identified the following significant risk factors: male gender (OR 0·40, 95% CI 0·14–1·14, P = 0·09), previous exposure to an individual who had had surgery within the last year (OR 3·63, 95% CI 1·05–12·60, P = 0·04), MRSA nasal carriage (OR 4·46, 95% CI 1·25–15·89, P = 0·02), and receiving antibiotic treatment for SSTIs in the year before infection (OR 6·02, 95% CI 1·28–28·23, P = 0·02).
The key findings of this study of community-associated SSTIs in Taiwan are the unexpectedly high frequency of MRSA in the patient population and the identification of risk factors for these infections in this group. These findings are of potential value to clinicians treating such infections in this region. The major risk factor of MRSA SSTIs identified here was exposure to an individual who underwent an operation within a year before the infection. A meta-analysis study suggested that when patients known to be colonized with nosocomial MRSA are discharged from the hospital or nursing home into the community, they can spread the pathogen to family members or other close contacts [Reference Salgado, Farr and Calfee12]. We did not screen household relatives of patients for MRSA carriage but it is likely that effective control of these community-derived MRSA infections will require similar measures to those employed for hospital-associated infections.
The current approach to treatment of SSTIs presenting in the community commonly includes the empirical use of β-lactam antibiotics. It follows that prescribing of inappropriate antibiotics to patients with MRSA may contribute to treatment failures, recurrent infections, and/or further dissemination of such strains within the population. This approach may need to be reconsidered in the light of the finding of significant numbers of patients with MRSA SSTIs in Taiwan. Although most of the MRSA isolates were susceptible to a number of other non-β-lactam antibiotics, including vancomycin, ciprofloxacin, and co-trimoxazole, we found several MRSA isolates resistant to more than one antimicrobial class. Clindamycin is frequently considered for the treatment of community-associated MRSA, but as 38% of MRSA isolates in this study were resistant to this drug, often in combination with erythromycin, this argues against its empirical use in SSTIs in Taiwan.
Our data indicate that previous antibiotic use is a significant risk factor for presentation of patients with MRSA SSTIs. This is in agreement with other studies [Reference Como-Sabetti8]; however, due to differences in the populations studied, control groups, and methods, it is difficult to compare findings. Studies from outbreak settings have found prior antimicrobial use to be associated with community-associated MRSA colonization and infection [Reference Baggett13]. It is possible that previous use of antibiotics that lack activity against MRSA might provide a positive selective pressure for MRSA strains but this appears to vary with antibiotic class and number of prescriptions [Reference Schneider-Lindner14].
We were able to identify some risk factors for acquiring a MRSA SSTI that were consistent with several recent reports [Reference Campbell6–Reference Como-Sabetti8]. However, although several variables were shown to be potential risk factors in other studies (including fever at the time of physical examination, pre-existing skin disease, and diabetes) these did not prove to be statistically significant for our study group.
Our study has some limitations, most notably a small sample size, which may have resulted in reduced statistical power. Further, we were not able to determine the genotypes (SCCmec, sequence type) of the MRSA recovered from the patient cohort and so cannot confirm the identity of the isolates in the context of the literature on community-associated MRSA. Additionally, culture results may not be representative of all SSTIs as many SSTIs cannot be cultured, particularly those which present as cellulitis without abscess. Although a clinically relevant pathogen was isolated from 44% of lesions sampled and S. aureus was predominant, the low frequency (6%) of BHS was unexpected and may reflect sampling and culture technique. It is reported that non-culturable cellulitis is more likely to be caused by BHS [Reference Jeng15] and as the majority of samples were from patients presented as ‘cellulitis with abscess’ this also might explain the low recovery of this organism.
In conclusion, our findings provide some epidemiological insights into patients’ characteristics and risk factors of MRSA SSTIs in Taiwan and may be helpful in establishing guidelines for surveillance, treatment and prevention of such infections in this population.
ACKNOWLEDGEMENTS
Funding was received from Taipei City Hospital.
DECLARATION OF INTEREST
None.




