Nutraceuticals are food-related products that confer health benefits on the consumer beyond their basic nutritional value. In the last few years, there has been a great interest in nutraceuticals and functional foods, since they open up the possibility of deterring disease (to a certain extent) as an add-on to regular diet. Although their properties are partly drug like, their origin as part of foodstuffs and their extremely low toxicity are especially attractive to the general public. Not surprisingly, the food industry has been particularly active in the search and marketing of new products of this type. However, there is an obvious risk of overselling the claimed virtues of a given nutraceutical. Current regulations in Europe (EC 432/2012) require the demonstration of specific qualities in terms of physiological benefits or disease prevention.
One of the conditions where nutraceuticals and functional foods may play a role is inflammatory bowel disease (IBD). Comprising ulcerative colitis and Crohn's disease, IBD is characterised by chronic and relapsing inflammation of the intestine, resulting in a significant deterioration of the quality of life of patients. Furthermore, the prevalence of IBD is slowly increasing( Reference Ekbom 1 , Reference Sands 2 ). IBD is considered to develop as the result of an insufficiently characterised interplay of genetic, environmental, microbial and immunological factors, involving an uncontrolled response to luminal antigens that are innocuous for the normal population. These processes have long been thought to be related to augmented adaptive immunity responses, but it has also been proposed that a defect in innate immunity may paradoxically underlie the aetiology of IBD( Reference Nenci, Becker and Wullaert 3 – Reference Qualls, Tuna and Kaplan 6 ). Whatever be the exact mechanism, IBD is regularly managed pharmacologically with drugs that down-regulate the immune system such as corticoids, infliximab, aminosalicylates and azathioprine. All these agents have a plethora of serious adverse effects that limit their application and they are not effective in all patients. Hence, the search for new treatments with a low profile of adverse effects is much warranted( Reference Sands 2 ).
Bovine glycomacropeptide (GMP), also referred to as casein macropeptide, is a sixty-four-amino acid peptide that contains varying amounts (0–5 units) of N-acetylneuraminic (sialic) acid. This peptide results from the enzymatic hydrolysis of milk κ-casein in the bovine stomach due to the action of chymosin (pepsin in humans)( Reference Brody 7 ). In addition, GMP is present in milk whey in amounts ranging from 10 to 15 % as a result of the action of the same enzyme during the cheese-making process. Therefore, there is a substantial natural exposure to this peptide. GMP has nutritional value because its amino acid profile is high in branched-chain amino acids and lacks aromatic amino acids, therefore being one of the few naturally occurring proteins safe for consumption by individuals with phenylketonuria and perhaps useful in the management of some liver diseases( Reference Nakano, Silva-Hernandez and Ikawa 8 , Reference Nakay and Modler 9 ). On the other hand, a number of biological activities have been ascribed to GMP. We have previously established that GMP has intestinal anti-inflammatory activity in experimental models of IBD( Reference Lopez-Posadas, Requena and Gonzalez 10 – Reference Daddaoua, Puerta and Zarzuelo 13 ). However, this is probably insufficient evidence to support the use of GMP as a nutraceutical, because the trinitrobenzenesulphonic acid (TNBS) and dextran sulphate sodium (DSS) rat models used previously are not strictly chronic (i.e. they heal with time) and they are not lymphocyte driven as in human disease. Some authors have advocated the use of other IBD models to achieve a better prediction of human bioactivity( Reference Koboziev, Karlsson and Zhang 14 ), such as colitis induced by the transfer of naïve T lymphocytes into immunodeficient mice, anticipating that the validation of nutritional or pharmacological treatments of IBD should include one such model. Therefore, in the present study, we set out to test the activity of GMP in lymphocyte-transfer colitis in mice. Among the parameters evaluated was the activity of myeloperoxidase (MPO), a neutrophil marker that is widely used as an index of colitis activity in preclinical models of IBD. Since tissue damage in IBD is the result of an exacerbated inflammatory response, a normalisation of this parameter is considered an improvement in disease evolution. Some other mediators related to the innate immune system such as regenerating islet-derived protein 3γ (REG3γ, an antimicrobial peptide expressed by intestinal epithelial cells), S100A8 (also a prominent neutrophil marker and a component of calprotectin), chemokine (C-X-C motif) ligand 1 (CXCL1, a chemokine with neutrophil chemoattractant activity) and IL-1β (a proinflammatory cytokine mainly produced by macrophages) were also studied. The results obtained are consistent with a beneficial effect of the peptide.
Materials and methods
Reagents
Except where indicated, all the reagents and primers were obtained from Sigma. Retrotranscription was carried out using the iScriptTM cDNA Synthesis Kit, and iTM SYBR Green Supermix was used for amplification (Bio-Rad). Antibodies were purchased from Cayman Technologies and Sigma. Mouse ELISA kits were obtained from eBioscience. DSS was obtained from ICN Biomedicals. GMP (BioPURE-GMP™) was a kind gift from Davisco Foods International, Inc. According to the manufacturer, the GMP content was 93 %, while fat and lactose contents accounted for 0·2 % and less than 1 %, respectively.
Animals
Female C57BL/6 wild-type and Rag1− / − mice were obtained from Jackson Laboratory, housed in Makrolon cages and maintained in the animal facilities of the University of Granada in air-conditioned animal quarters under a 12 h light–12 h dark cycle. The mice were given free access to tap water and food. All the animal procedures carried out in the present study were in accordance with the Directive for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes of the European Union (86/609/EEC) and were approved by the Animal Welfare Committee of the University of Granada (reference 710).
Induction of transfer colitis and experimental design
Female C57BL/6 mice were killed by cervical dislocation, and the spleens were extracted aseptically. Cell suspensions were obtained by disrupting the tissues between dissecting forceps in a medium. After centrifugation, the cells were cleared of erythrocytes by suspension in a hypotonic lysis buffer (0·15 m-NH4Cl, 10 mm-KHCO3 and 0·1 mm-Na2EDTA.2H2O, pH 7·3) for 30 min on ice. The cells were filtered using a 70 μm filter (BD Falcon™ cell strainer; Becton Dickinson) to obtain a mononuclear suspension. Mononuclear cells were washed and suspended in MACS buffer. CD4+ CD62L+ T cells were isolated from spleen cells using CD4+ CD62L+ T Cell Isolation Kit II (Miltenyi Biotec). In the first step, non-CD4+ T cells were indirectly magnetically labelled with a cocktail of biotin-conjugated antibodies and AntiBiotin MicroBeads. The labelled cells were subsequently depleted by separation over a MACS® column. In the second step, CD4+ CD62L+ T cells were directly labelled with CD62L (L-selectin) MicroBeads and isolated by positive selection from the pre-enriched CD4+ T-cell fraction. CD4+ CD62L T cells were eluted in 100 μl of sterile PBS and administered via the intraperitoneal route to C57BL/6 Rag1 − / − mice (106 cells per mouse). Rag1 − / − control mice were administered sterile PBS.
The status of the mice was monitored by general examination and specifically controlling body-weight evolution, beginning the experiment after a body-weight loss of 10 % (about 8 weeks after the transfer). Colitic mice were randomly assigned to two different groups: GMP group and transfer colitis group. The GMP group (n 7) was orally administered 15 mg/d of GMP, while the transfer colitis group (n 8) was administered a vehicle (distilled water). A non-colitic C group (Rag1 − / − mice administered 100 μl of sterile PBS) was also included in the experiment (n 6). Treatment was continued until the mice were killed after 13 d by cervical dislocation.
Induction of colitis by dextran sulphate sodium and experimental design
A total of twenty-two female C57BL/6 mice were used. Colitis was induced by adding DSS to the drinking-water for 8 d( Reference Lopez-Posadas, Requena and Gonzalez 10 , Reference Pérez-Navarro, Ballester and Zarzuelo 15 ). We selected the experimental conditions to achieve a mild-to-moderate degree of colitis by using 2 % w/v of DSS. The status of the mice was monitored by general examination and specifically by means of the disease activity index, a combined score for weight loss, diarrhoea and haematochezia, which are three main signs of pathology in this model( Reference Ito, Tanabe and Kawagishi 16 ). Food intake, water intake and body weight were measured every day.
Mice were randomly assigned to three different groups. The control (C) group (n 6) did not receive DSS and was administered water daily by means of a gastro-oesophageal catheter. The remainder of the mice drank DSS-supplemented water, and received by gavage either 15 mg/d of GMP (GMP group, n 8) or orally administered vehicle (distilled water, DSS group, n 8). Treatment was started 2 d before DSS supplementation and was continued until the mice were killed after 10 d by cervical dislocation.
Assessment of colonic damage
The entire colon was removed, gently flushed with saline and placed on an ice-cold plate, cleaned of fat and mesentery, and blotted on filter paper. Each specimen was weighed, and its length was measured under a constant load (2 g). The large intestine was longitudinally opened and scored for visible damage by a blinded observer. Transfer colitis was scored on a scale of 0–15 according to the following criteria: adhesions (0–3); obstruction (0–2); hyperaemia (0–3); thickness (0–5); ulceration (0–2). DSS colitis was scored on a scale of 0–13 according to the following criteria: adhesions (0–3); hyperaemia (0–3); fibrosis (rigidity, 0–3); deformation (0–2); thickening (0–2). A small segment was dissected from the intestine and used for RNA isolation. The colon was subsequently cut longitudinally into several pieces for the determination of biochemical parameters. The fragments were immediately frozen in liquid N2 and kept at − 80°C until use. Formalin-fixed colonic tissue was cut and stained with haematoxylin and eosin. The activities of MPO and alkaline phosphatase (AP) were measured spectrophotometrically as described previously( Reference Krawisz, Sharon and Stenson 17 , Reference Lopez-Posadas, Gonzalez and Ballester 18 ), and they are expressed as mU/mg protein. In addition, the sensitivity to the AP inhibitor levamisole was assessed, and it is expressed as a percentage of inhibition.
Analysis of gene expression by RT-PCR analysis
Total RNA was obtained by the TRIzol method (Invitrogen), and 1 μg was retrotranscribed and specific RNA sequences were amplified with a Stratagene MX3005P real-time PCR device (Agilent) using the following primers: 18S sense: ACA CGG ACA GGA TTG ACA GAT TG, 18S antisense: GCC AGA GTC TCG TTC GTT ATC G; S100A8 sense: GCC CTC TAC AAG AAT GAC TTC AAG, S100A8 antisense: ATC ACC ATC GCA AGG AAC TCC; IL-1β sense: AAG GGC TGC TTC CAA ACC TTT GAC, IL-1β antisense: TGC CTG AAG CTC TTG TTG ATG TGC; TNF-α sense: CGT GGA ACT GGC AGA AGA GG, TNF-α antisense: CAG GAA TGA GAA GAG GCT GAG AC; interferon (IFN)-γ sense: GCT CTG AGA CAA TGA ACG CTA CAC, IFN-γ antisense: TTC TTC CAC ATC TAT GCC ACT TGA G; CXCL1 sense: CCG AAG TCA TAG CCA CAC TCA AG, CXCL1 antisense: ACC AGA CAG GTG CCA TCA GAC; REG3γ sense: CAG AGG TGG ATG GGA GTG GAG, REG3γ antisense: CAC AGT GAT TGC CTG AGG AAG AAG AG.
Secretion of cytokines by mesenteric lymph node cells
Mesenteric lymph nodes (MLN) were extracted from the mice using a sterile technique and dissected mechanically. Cells were washed once with fresh medium and were filtered using a 70 μm filter (BD Falcon™ cell strainer, Reference 352350; Becton Dickinson) to obtain a mononuclear suspension, mostly of T cells. The cells were incubated in RPMI 1640 medium containing fetal bovine serum (10 %), 2 mm-l-glutamine, 100 U/ml penicillin, 0·1 mg/ml streptomycin, 2·5 mg/ml amphotericin B and 0·05 mm-mercaptoethanol. The cells were cultured at 106 cells/ml and stimulated with concanavalin A at a final concentration of 5 μg/ml. Concanavalin A is a polyclonal T-cell stimulant that evokes a surge in the secretion of cytokines by MLN cells, which is typically enhanced in colitic animals. Cell-culture medium was collected after 48 h and assayed for cytokine content by commercial ELISA. The cytokines assayed were IL-6, IL-10, IL-17, IFN-γ and TNF-α. Plates (Nunc™ Inmuno plate) were read at 450 nm using a plate reader (Tecan, model Sunrise-basic).
Epithelial cell experiments
Using GMP and intestinal epithelial cells, three different in vitro experiments were carried out. First, the effect of GMP (0·01–1 g/l) on the secretion of IL-8 by confluent HT29 cell monolayers was assessed by ELISA (R&D Systems). Second, the effect of GMP on bacterial invasion in IEC18 cells was studied. Escherichia coli K12 and LF82 strains (kindly provided by Dr Arlette Darfeuille Michaud) expressing green fluorescent protein were used. Bacteria were grown routinely in lysogeny broth supplemented with 20 μg/ml of gentamicin overnight at 37°C with shaking. For every experiment, bacteria were freshly grown overnight. The invasion assays were carried out in IEC18 cell monolayers cultured in twelve-well plates. GMP (5–20 g/l) was added to the medium 24 h before and during the invasion assays. Each monolayer was infected with 1 ml of the cell-culture medium without antibiotics at a multiplicity of infection of 100 bacteria per epithelial cell. After 4 h of incubation, infected monolayers were washed three times with Hanks balanced salt solution (HBSS) and fresh cell-culture medium containing 100 mg/l of kanamycin, 500 mg/l of streptomycin and 500 000 UI/l of penicillin was added to kill extracellular bacteria. After incubation for an additional hour, monolayers were washed three times again with HBSS. The cells were collected by trypsinisation and analysed by flow cytometry (FACSCalibur, BD), and results are expressed as a percentage of green-positive cells, each positive event representing a cell containing green fluorescent protein-expressing bacteria.
Third, the effect of GMP on wound was assessed. Rat epithelial IEC18 cells were grown to confluence in twenty-four-well dishes and were mechanically wounded by parallel scratches using a sterile pipet tip. Medium present on the wounded monolayers was replaced with a normal medium or a normal medium containing GMP 1 g/ml and incubated for 24 h. Photomicrographs of the lineal wounds were taken using the 4 × objective of an Olympus IX71S8F-3 microscope at the moment of wounding and at 6, 11 and 24 h, and the area of each wound was quantified with the Image J software (National Institutes of Health).
IEC18 (ECACC 88011801) and HT29 (ECACC 91072201) cells were supplied by the Cell Culture Facility of the University of Granada and were grown in Dulbecco's modified Eagle's Medium supplemented with 10 % fetal bovine serum (Boehringer Mannheim), 2 mm-l-glutamine, 100 mg/l streptomycin, 100 000 UI/l penicillin and 2·5 mg/l amphotericin B in a humidified 5 % CO2 atmosphere at 37°C.
Statistical analysis
In all the experiments, samples were run in triplicate, and results are expressed as means with their standard errors. Differences among the means were tested for statistical significance by one-way ANOVA and a posteriori Fisher least significant difference tests on preselected pairs. All the analyses were carried out with the SigmaStat 3.5 program (Jandel Corporation). Differences were considered significant at P <0·05.
Results
Lymphocyte-transfer colitis
Rag1 − / − mice were monitored for 8 weeks after lymphocyte transfer for body-weight evolution and overall status. The mice exhibited body-weight loss (10 % average) and were randomised for treatment with GMP or vehicle. Hence, this is a post-treatment protocol. The vehicle-administered mice exhibited a relatively stable body weight, which was slightly increased after 13 d (Table 1). Food intake and water intake were essentially normal (data not shown). The colon appeared thickened, with hyperaemia but with no overt signs of ulceration, obstruction or adhesion (Table 1). The resulting damage score was significantly increased. At the microscopic level, intense mucosal infiltration, crypt elongation and occasional epithelial erosions were observed (data not shown). The ileum was also thickened (29·6 (se 4·9) v. 21·9 (se 4·9) mg/cm; P <0·05) and there was splenomegaly (5·4 (se 3·5) v. 1·3 (se 0·1), spleen:body weight ratio × 1000, P <0·05).
Table 1 Macroscopic damage parameter values of transfer colitic mice and body weight (Mean values with their standard errors)

GMP, glycomacropeptide.
* Mean values were significantly different from those of the control group (P< 0·05).
At the biochemical level, colitis was characterised by a 2-fold increase in the activity of MPO and a 4-fold increase in that of AP (Figs. 1 and 2(a)). The sensitivity to the specific AP inhibitor levamisole was augmented (Fig. 2(b)). The mRNA levels of REG3γ, S100A8, CXCL1, IL-1β, TNF-α and IFN-γ were significantly increased (Fig. 3). MLN were enriched in CD4+ IFN-γ+ cells (Fig. 4), and the MLN cells exhibited an augmented production of cytokines.
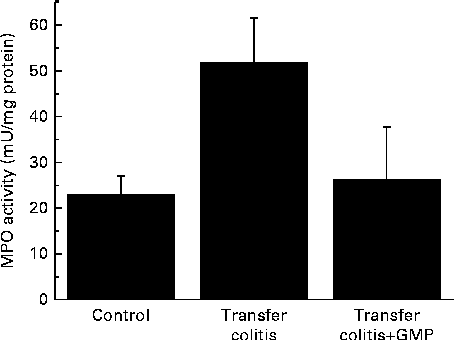
Fig. 1 Colonic myeloperoxidase (MPO) activity in mice with lymphocyte-driven colitis. Chronic colitis was induced in mice and they were then treated with glycomacropeptide (GMP; 15 mg/kg) or vehicle for 13 d. MPO activity was measured spectrophotometrically. Values are means, with standard errors represented by vertical bars.

Fig. 2 Colonic alkaline phosphatase (AP) activity in mice with lymphocyte-driven colitis. Chronic colitis was induced in mice and they were then treated with glycomacropeptide (GMP; 15 mg/kg) or vehicle for 13 d. AP activity was measured spectrophotometrically. (a) AP activity, (b) Inhibition of AP activity by levamisole in vitro. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05). ![]() , Levamisole 0·1 mm; ■, levamisole 1 mm;
, Levamisole 0·1 mm; ■, levamisole 1 mm; ![]() , levamisole 10 mm.
, levamisole 10 mm.

Fig. 3 Colonic expression of inflammatory markers in mice with lymphocyte-driven colitis. Chronic colitis was induced in mice and they were then treated with glycomacropeptide (GMP; 15 mg/kg) or vehicle for 13 d. The mRNA levels were measured by RT-PCR. (a) REG3γ, (b) S100A8, (c) chemokine (C-X-C motif) ligand 1 (CXCL1), (d) IL-1β, (e) TNF-α, (f) interferon-γ (IFN-γ). Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05).

Fig. 4 Cytokine secretion by mesenteric lymph node cells ex vivo in mice with lymphocyte-driven colitis. Chronic colitis was induced in mice and they were then treated with glycomacropeptide (GMP; 15 mg/kg) or vehicle for 13 d. Mesenteric lymph node (MLN) cells were isolated and cultured, with or without concanavalin A, and the levels of cytokines in the supernatant were measured by ELISA. (a) Percentage of CD4+ IFN-γ+ cells (assessed by flow cytometry), (b) IFN-γ, (c) TNF-α, (d) IL-6, (e) IL-10, (f) IL-17. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05). † Mean value was significantly different from that of the transfer colitis group (P< 0·05). ■, Basal; ![]() , concanavalin A.
, concanavalin A.
GMP resulted in higher body-weight gain (despite a similar food intake) and a reduction of the colonic damage score and ileal (26·5 (se 4·3) v. 29·6 (se 4·9) mg/cm; P>0·05) and colonic weight:length ratio (Table 1), although these did not reach statistical significance. Histological analysis indicated that the features of the treated mice were similar to those of the control mice (data not shown). The activity of MPO was virtually normalised (Fig. 1), while that of AP was reduced by 17 %, but without reaching significance (Fig. 2(a)). The sensitivity of AP to levamisole was unaffected (Fig. 2(b)). The colonic expression of REG3γ, S100A8, CXCL1 and IL-1β was unaffected by GMP. Conversely, the colonic expression of TNF-α and especially IFN-γ was up-regulated (Fig. 3). GMP treatment reduced the percentage of CD4+ IFN-γ+ cells in MLN (Fig. 4(a)). The basal production of IL-6 by MLN cells obtained from the GMP-treated mice ex vivo was markedly increased (Fig. 4(d)). The basal production of IL-17, IFN-γ and TNF-α displayed a similar but much weaker trend, significant only for IL-17. However, concanavalin A-evoked production was similar in all the cases (Fig. 4). There was no effect on spleen size (data not shown).
Dextran sulphate sodium colitis
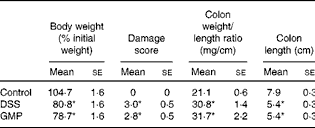
The administration of drinking-water supplemented with 2 % DSS to the mice resulted in diarrhoea, haematochezia and body-weight loss (Fig. 5), with an increased disease activity index (data not shown). The colon was thickened and shortened, resulting in an increased weight:length ratio (Table 2). The mucosa exhibited intense hyperaemia and fibrosis, which gave rise to a substantially augmented damage score (Table 2). The colitic mice also had splenomegalia (2·8 (se 0·15) v. 4·0 (se 0·32), spleen:body weight ratio × 1000, P <0·05, control v. DSS). The activity of colonic MPO was greatly increased (Fig. 6), while that of AP was augmented approximately 3-fold (Fig. 7(a)). The sensitivity of AP to levamisole was not significantly affected (Fig. 7(b)). The basal production of cytokines by MLN cells ex vivo was negligible (data not shown). Upon concanavalin A stimulation, there was a surge in the release of IL-17 and IL-10, while the release of IL-6, TNF-α and IFN-γ exhibited only a non-significant trend to increase (Fig. 8).

Fig. 5 Body-weight evolution in mice with dextran sulphate sodium (DSS, ![]() ) colitis. Colitis was induced in mice with 2 % DSS in drinking-water. Glycomacropeptide (GMP; 15 mg/kg) or vehicle was administered 2 d before DSS treatment and for an additional 7 d. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05).
) colitis. Colitis was induced in mice with 2 % DSS in drinking-water. Glycomacropeptide (GMP; 15 mg/kg) or vehicle was administered 2 d before DSS treatment and for an additional 7 d. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05). ![]() , Control;
, Control; ![]() , DSS+GMP.
, DSS+GMP.
Table 2 Macroscopic damage parameter values of dextran sulphate sodium (DSS) colitic mice and body weight (Mean values with their standard errors)
GMP, glycomacropeptide.
* Mean values were significantly different from those of the control group (P< 0·05).
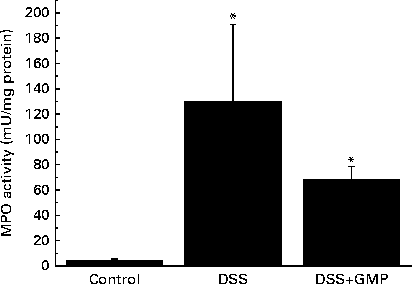
Fig. 6 Colonic myeloperoxidase (MPO) activity in mice with dextran sulphate sodium (DSS) colitis. Colitis was induced in mice with 2 % DSS in drinking-water. Glycomacropeptide (GMP; 15 mg/kg) or vehicle was administered 2 d before DSS treatment and for an additional 7 d. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05).

Fig. 7 Alkaline phosphatase (AP) activity in mice with dextran sulphate sodium (DSS) colitis. Colitis was induced in mice with 2 % DSS in drinking-water. Glycomacropeptide (GMP; 15 mg/kg) or vehicle was administered 2 d before DSS treatment and for an additional 7 d. (a) AP activity, (b) Inhibition of AP activity by levamisole in vitro. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05). ![]() , Levamisole 0·1 mm; ■, levamisole 1 mm;
, Levamisole 0·1 mm; ■, levamisole 1 mm; ![]() , levamisole 10 mm.
, levamisole 10 mm.

Fig. 8 Cytokine secretion by mesenteric lymph node cells ex vivo in mice with dextran sulphate sodium (DSS) colitis. Colitis was induced in mice with 2 % DSS in drinking-water. Glycomacropeptide (GMP; 15 mg/kg) or vehicle was administered 2 d before DSS treatment and for an additional 7 d. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different from those of the control group (P <0·05). † Mean value was significantly different from that of the transfer colitis group (P< 0·05). ■, Concanavalin A 5 μg/ml. IFN, interferon.
Pre-treatment with GMP reduced the activity of colonic MPO by about 50 % (Fig. 6), but failed to alter weight gain/disease activity index, colonic weight:length ratio and damage score, AP activity or splenomegalia (Table 2 and Figs. 5–7). Interestingly, GMP augmented the production of IL-10 by MLN cells ex vivo (Fig. 8), while it was essentially neutral towards other cytokines. It also exhibited a clear trend to increase the production of IL-6 (Fig. 8(a)), although without reaching statistical significance.
Effect of glycomacropeptide on intestinal epithelial cells
The difference observed between the effect of GMP in lymphocyte-transfer colitis and that of GMP in DSS-induced colitis prompted us to assess the effects of the peptide on intestinal epithelial cells. As shown in Fig. 9, there was no effect of GMP on epithelial IL-8 secretion, wound repair or bacterial invasion.

Fig. 9 Effect of glycomacropeptide (GMP) in intestinal epithelial cells. (a) Effect on the secretion of IL-8 by HT29 cells (GMP 0·01–1 g/l), (b) Effect on bacterial invasion in IEC18 cells. Cells containing green fluorescent protein (GFP)-expressing bacteria are detected as GFP+ events and represented as a percentage of total cells, (c) Effect on wound healing in IEC18 cells. There was no significant effect of GMP in any case. ■, Control; ![]() , GMP 1 g/l.
, GMP 1 g/l.
Discussion
The main goal of the present study was to validate the intestinal anti-inflammatory activity of GMP using a truly chronic, lymphocyte-driven model of IBD( Reference Koboziev, Karlsson and Zhang 14 , Reference Ostanin, Bao and Koboziev 19 ). This model is characterised by progressive expansion of the transferred T-lymphocyte population, with a predominance of T helper type 1/T helper type 17 cells and a paucity of T regulatory cells, resulting in intestinal inflammation, which mainly affects the colon. The process is chronic and may remain relatively stable for weeks or deteriorate slowly until animal death (spontaneous or by euthanasia due to ethical reasons). Despite the obvious advantages of using a model more closely resembling human IBD( Reference Koboziev, Karlsson and Zhang 14 , Reference Ostanin, Bao and Koboziev 19 ), it poses practical problems for the investigation of bioactive products, compared with chemical models. In particular, there is an extended lag period until the development of disease, with a significant variation among mice even within a single experiment. The onset of the disease is insidious, making it difficult to judge when the animal is ‘ready’ for testing. In the present study, we transferred naïve T lymphocytes into a relatively large number of Rag1 − / − mice and grouped them for further experimentation based on body-weight evolution and other signs of disease such as reduced movement, huddling or diarrhoea (other groups were used for unrelated treatments). Thus, the mice used for this experiment exhibited similar characteristics before randomisation to the administration of GMP orally or vehicle. It is important to note that the use of this model represents a leap forward in the state of the art in the development of nutraceuticals for intestinal inflammatory conditions.
GMP was administered at a dose of 15 mg/d, which is equivalent to that used successfully as a pre-treatment in rats (500 mg/kg), based on body surface. This dose is roughly equivalent to 5 g for an adult human (again on a body surface basis), an amount that cannot be easily achieved by milk consumption, but is easily attainable by consumption of a functional food or a drug. Because of the reasons stated above, the protocol applied in this case is a post-treatment, which is expected to produce less pronounced effects in general. Another possible source of variation is the use of a different species (mouse v. rat). In these conditions, GMP exerted significant protective effects. A positive response was anticipated before killing the animals based on a higher body-weight gain (albeit non-significant), which was achieved despite the food intake being similar to that of the control mice. Body-weight loss in acute and semi-chronic models of colitis is mainly a consequence of anorexia, which is part of the acute response. In this model, food intake was within normal boundaries in both the control and GMP-treated mice, suggesting that differences are due to absorptive problems and/or enhanced catabolism.
The large intestine exhibited signs of a relatively mild colitis compared with the harsh impact of TNBS or DSS colitis, with loss of visible vascular patterns (oedema), hyperaemia and thickening, but no necrosis, major adhesions or strictures. This was better evidenced at the histological level, where intense infiltration and crypt enlargement but with only minor areas of epithelial erosion and no overt ulceration were observed. Intestinal thickening was a major sign of colitis, and it was improved by GMP treatment in both the colon and ileum, but without reaching statistical significance. Thus, GMP appears to ameliorate oedema and crypt elongation, but this effect probably requires a bigger group size to be picked up during statistical analysis. The effect of GMP was better evidenced by biochemical analysis. Thus, the activity of colonic MPO was increased 2-fold in the colitic group, consistent with a relatively mild degree of inflammation, and this was fully normalised by GMP treatment, indicating the inhibition of neutrophil infiltration. On the other hand, GMP exerted only a non-significant effect on the activity of colonic AP. The activity of AP is increased in colitis as a result of the combination of an infiltration of leucocytes and a change of isoform expressed at the epithelial level( Reference Lopez-Posadas, Gonzalez and Ballester 18 ). Thus, colonic inflammation is characterised not only by an augmented activity of AP, but also by a higher inhibition by the specific inhibitor levamisole in vitro, as has been observed in the present study. The fact that the activity of MPO was reduced by GMP while that of AP was not suggests that it failed to modulate the epithelial component, in line with its documented lack of activity in epithelial cells. In fact, the in vitro experiments carried out in the present study indicate that GMP is inactive in modulating epithelial repair, antibacterial defence or cytokine secretion, in line with this hypothesis. However, we cannot completely exclude the possibility of epithelial actions because of the limitations imposed by the use of intestinal epithelial cell lines. Thus, it remains possible that GMP exerts significant effects on the epithelium in vivo. This is very difficult to assess directly, because primary enterocytes generally have an extremely limited lifespan.
Other biochemical inflammatory markers indicative of ongoing inflammation are the up-regulated parameters measured by RT-PCR in colonic tissue and the cytokines released by MLN cells ex vivo ( Reference Hirano and Kudo 20 ). The effects of GMP at this level are complex, but they are generally consistent with the dual action previously observed with this peptide, boosting innate immunity but blocking adaptive immunity. In this regard, it is interesting that GMP is associated with an increase in the production of IL-6. We( Reference Requena, Daddaoua and Guadix 21 ) and others( Reference Fomon 22 ) have noted the stimulatory effect of GMP on macrophages, in terms of cytokine production and phagocytic activity. GMP may interfere with IL-1β receptor binding( Reference Monnai and Otani 23 ). Conversely, GMP has been described to inhibit the proliferation of splenocytes and Peyer's patch cells( Reference Otani and Hata 24 ). We have recently observed that GMP inhibits the release of IFN-γ by rat splenocytes by blocking STAT4 activation (F Sánchez de Medina and O Martínez-Augustin, unpublished results). However, the production of IgG by mouse B lymphocytes seems to be increased by GMP( Reference Monnai, Horimoto and Otani 25 ). Therefore, it is possible that GMP acts by modulating the functions of lymphocytes and macrophages so as to enhance intestinal barrier function and dampen colitis. At any rate, the mechanism of the action of GMP must involve the MLN cell population or monocytes and lymphocytes in the lamina propria, since it has no effect on epithelial cells. IL-6 may be a common factor in both the models, as has been mentioned above.
It is interesting to compare these data with those obtained in the mouse DSS model. We carried out the present study originally as a preliminary stage to examine the involvement of macrophages v. lymphocytes in the effects of GMP, namely applying the DSS model to Rag1 − / − mice. The present results indicate that although GMP exerts a beneficial effect in regular C57BL/6 mice, the magnitude of the effect is certainly diminished compared with that observed in rat colitis or ileitis. Clinical improvement was evidenced by the amelioration of the activity of colonic MPO and increased production of IL-10 by MLN cells ex vivo. Taken together, our findings indicate that GMP is active in several different models of intestinal inflammation, but the results differ depending on animal species and experimental model, with rats being more sensitive than mice and TNBS being more effective than DSS. DSS is thought to elicit intestinal inflammation by slowly altering the epithelial integrity, augmenting permeability and ultimately resulting in an immune reaction against luminal antigens( Reference Cooper, Murthy and Shah 26 ). In contrast, TNBS acts as a hapten by a delayed hypersensitivity mechanism involving the reaction with mucosal proteins( Reference Morris, Beck and Herridge 27 ). Oxidative stress may also play a role( Reference Grisham, Volkmer and Tso 28 ). The epithelium, therefore, plays a more important role in DSS colitis than in TNBS colitis, and since, as noted, GMP has no effect on the epithelium, it is logical that less pronounced effects are observed in the former model. Another example of this disparate behaviour is the effect of the flavonoid luteolin in DSS and IL-10− / − colitis in mice, being deleterious in the former and protective in the latter( Reference Karrasch, Kim and Jang 29 ), a discrepancy explained by the inhibitory action of the flavonoid on the NF-κB pathway in the epithelium, which may compromise the defence of the mucosa towards epithelial disruption by DSS.
In conclusion, our data validate the intestinal anti-inflammatory activity of GMP in chronic, lymphocyte-driven colitis, considered one of the best models of colitis because of its resemblance to human IBD.
Acknowledgements
The authors are grateful to Dr Mercedes González for her assistance and also to Dr Arlette Darfeuille Michaud for providing the bacterial strains. They are also thankful to Davisco Foods International, Inc. for providing GMP.
The present study was supported by grants from Fundación Ramón Areces and by the Instituto de Salud Carlos III (PI051625 and PI051651) and the Ministry of Economy and Competitivity (SAF2008-01432, AGL2008-04332, SAF2011-22922 and SAF2011-22812) and by funds from Junta de Andalucía (CTS6736 and CTS164). None of the funders had any role in the design and analysis of the study or in the writing of this article. CIBERehd is funded by the Instituto de Salud Carlos III. M. O.-G., P. R., B. O. and C. A. are funded by the Ministry of Education.
The authors' contributions are as follows: M. O.-G., F. C.-C., P. R., B. O., I. R.-C. and C. A. carried out the experiments; M. D. S., A. Z., F. S. d. M. and O. M.-A. designed the experiments; F. S. d. M. and O. M.-A. wrote the manuscript. All authors contributed to the discussion and overall structure of the manuscript.
None of the authors has any conflicts of interest to declare.













