Management Implications
The riparian, invasive species Iris pseudacorus (yellow-flag iris) causes considerable damage to riparian and wetland ecosystems by excluding native species and altering the hydrological and geomorphic characteristics of those systems. The most commonly reported management techniques for I. pseudacorus are glyphosate and imazapyr applications, with imazapyr often showing greater efficacy. While these herbicides can be effective at reducing I. pseudacorus prevalence, restrictions on imazapyr use close to irrigation diversions and the relatively low efficacy of glyphosate suggest the need to identify other options for I. pseudacorus management.
Cattle trampling coupled with inundation could be an effective option for land managers working to reduce I. pseudacorus abundance. Our research suggests that trampling should coincide with the occurrence of standing water (inundation) rather than targeting a particular stage of I. pseudacorus phenology. Simulated trampling at two different time points before flowering resulted in no differences in plant density or height between treatments; however, inundation coupled with trampling resulted in drastic reductions of both variables. Attractants, such as salt blocks, may be necessary to draw cattle into I. pseudacorus infestations. Our findings can be applied more broadly and suggest that other management techniques beyond trampling should focus on impacting portions of I. pseudacorus at and above the crown while water levels at infested sites are above the impacted portions of the plant.
Total soluble sugar concentrations present in the I. pseudacorus rhizomes were also assessed. While no treatment effects were observed, elevated sugar concentrations in the rhizomes of inundated and trampled I. pseudacorus support the need for continued research into potential carbon starvation.
Introduction
Rangeland riparian and wetland systems play a critical role in supporting ecosystem functions in what are largely arid and semiarid landscapes. These areas rely heavily on the maintenance of appropriate hydrological and geomorphic characteristics required to support desirable flora and fauna (Goodwin et al. Reference Goodwin, Hawkins and Kershner1997; Silverman et al. Reference Silverman, Brady, Donnelly, Chapman, Maestas, Wheaton, White and Naugle2019). While highly resilient, riparian and wetland systems are susceptible to disruption by stressors, such as invasive species (Zedler and Kercher Reference Zedler and Kercher2004), which can displace native flora, form monotypic stands, and alter the morphological characteristics of infested waterways (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Faltlhauser, Fuentes-Rodriguez, Martínez and Hill2020; Morgan et al. Reference Morgan, Berent and Fusaro2018; Spaak Reference Spaak2016). Riparian and wetland invasive species may also impact agricultural operations by clogging irrigation infrastructure, altering the timing and dependability of water available for irrigation, and reducing the prevalence of desirable forage on the landscape (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Faltlhauser, Fuentes-Rodriguez, Martínez and Hill2020; Jacobs et al. Reference Jacobs, Graves and Mangold2011; USDA-APHIS 2013).
Yellow-flag iris (Iris pseudacorus L.) is a perennial, emergent riparian species that produces long, blade-like leaves, showy flowers, dense rhizomes, and extensive root systems (Stone Reference Stone2009; Sutherland Reference Sutherland1990; Tu Reference Tu2003). The aesthetic appeal of the flowers has prompted I. pseudacorus to be planted as an ornamental outside its native range (Gervazoni et al. Reference Gervazoni, Sosa, Franceschini, Coetzee, Faltlhauser, Fuentes-Rodriguez, Martínez and Hill2020; USDA-APHIS 2013). While other Iris species, such as German iris (Iris germanica L.), are often used in an ornamental setting with no adverse effect, I. pseudacorus can become an aggressive invader in natural (e.g., ponds, rivers, lakes, marshes) and agricultural settings (e.g., irrigation diversions, irrigated lands) when it escapes cultivation (Alpert et al. Reference Alpert, Bone and Holzapfel2000; Tu Reference Tu2003). Currently, I. pseudacorus is found in seven Canadian provinces and territories and 48 states in the United States. The species has been listed as a noxious weed in Montana and Washington; a designated weed quarantine species in Oregon; prohibited in Massachusetts and New Hampshire; and banned in Connecticut (King County DNRP 2007; USDA-APHIS 2013; USDA-NRCS 2021).
Iris pseudacorus poses a threat to native ecosystems due to its high ecological amplitude and ability to outcompete native vegetation for resources, primarily space (Pathikonda et al. Reference Pathikonda, Acklch, Hasenstein and Mopper2008; Thomas Reference Thomas1980). This advantage can be mostly attributed to the robust rhizomatous mats interwoven between neighboring I. pseudacorus shoots, vigorous clonal expansion via rhizomes, and prolific sexual reproduction (PA DCNR n.d.; Sutherland Reference Sutherland1990; Tarasoff et al. Reference Tarasoff, Streichert, Gardner, Heise, Church and Pypker2016; Weber Reference Weber2003). The combination of clonal expansion of rhizomatous mats and high fecundity allow I. pseudacorus to monopolize available space where established, while simultaneously increasing the probability of range expansion by seed dispersal (Gaskin et al. Reference Gaskin, Pokorny and Mangold2016; Sutherland Reference Sutherland1990).
In agricultural settings, I. pseudacorus and other desirable forage species in close proximity are usually ignored by livestock (Bossuyt et al. Reference Bossuyt, De Fré and Hoffman2005). High quantities of glycosides present in the leaves and rhizomes of I. pseudacorus can have adverse effects on cattle. Consumption of leaves can cause gastroenteritis, and consumption of rhizomes has led to acute diarrhea (Sutherland Reference Sutherland1990). While ingestion of I. pseudacorus can cause irritations to livestock, there have been reported instances of seasonal cattle consumption of leaves down to the rhizomes (Jacobs et al. Reference Jacobs, Graves and Mangold2011).
Techniques employed to manage I. pseudacorus range from physical removal to herbicide treatments, with potential biological control agents being investigated (Minuti et al. Reference Minuti, Coetzee, Ngxande-Koza, Hill and Stlers2021). Because I. pseudacorus infestations are often large by the time managers attempt to address the issue, chemical treatments are the predominant management strategy, with glyphosate and imazapyr being the most commonly used herbicides (Jacobs et al. Reference Jacobs, Graves and Mangold2011; King County DNRP 2007; PA DCNR n.d.; Simon Reference Simon2008; Spaak Reference Spaak2016). Of the two, imazapyr, applied alone and in combination with glyphosate, has been shown to be more effective at reducing I. pseudacorus than glyphosate alone (DiTomaso and Kyser Reference DiTomaso and Kyser2016; Simon Reference Simon2008). It is important to note that while imazapyr is often considered the most effective herbicide for I. pseudacorus management, label restrictions limit its use near flowing water, such as irrigation infrastructures, due to the herbicide’s residual soil activity (Anonymous 2011). In either case the nonselective nature of imazapyr and glyphosate and restrictions around flowing water illustrate the importance of identifying alternative treatment options for managing I. pseudacorus in wetlands and riparian areas.
We conducted a greenhouse study and a field demonstration to evaluate the effectiveness of cattle trampling as a novel technique for I. pseudacorus management. Using cattle trampling as a management technique arose from the observation of I. pseudacorus mortality along walking paths. Subsequent research found that a onetime human trampling in June for 5 min reduced I. pseudacorus density and height by 75% and 58%, respectively (Spaak Reference Spaak2016). Additionally, its absence from locations with ample human and animal activity has previously been noted (Sutherland Reference Sutherland1990). The objective of this research was to determine the impact that inundation and timing of simulated trampling would have on the density and height of I. pseudacorus and soluble sugar concentrations in rhizomes in a greenhouse setting. A field demonstration was also conducted to translate results from the greenhouse into a natural setting.
It has been hypothesized that one aspect of the competitive advantage of I. pseudacorus in its introduced range may be the result of large quantities of storage carbohydrates (i.e., fructans) in the rhizomes that play a major role in tolerance of prolonged anoxic conditions, which primarily occur in early spring and summer (Hanhijärvi and Fagerstedt Reference Hanhijärvi and Fagerstedt1994, Reference Hanhijärvi and Fagerstedt1995; Lambers et al. Reference Lambers, Chapin and Pons2008; Schlüter and Crawford Reference Schlüter and Crawford2001; Tarasoff et al. Reference Tarasoff, Streichert, Gardner, Heise, Church and Pypker2016). Schlüter and Crawford (Reference Schlüter and Crawford2001) found that rather than downregulating metabolic activities during prolonged periods of anoxic stress, I. pseudacorus continued to break down nonsoluble carbohydrates stored in the rhizomes, presumably to maintain aboveground biomass. Hydrolysis of these molecules provides an energy source when photosynthesis is slowed or stopped by maintaining and potentially increasing the concentration of photosynthetic by-products (i.e., soluble sugars such as sucrose, fructose, and glucose). The concentrations of these sugars are highly variable, both temporally and spatially throughout a plant, and are often adjusted in response to environmental cues, whole-plant carbon balance, and stress (Pozo et al. Reference Pozo, Méndez-Espinoza and Váñez2019). We hypothesize that injury to photosynthetically active tissue could lead to increased stress and hydrolysis of storage carbohydrates, which, in turn, would increase the soluble sugar concentration in rhizomes following the injury.
Materials and Methods
Greenhouse Experiment
Collection and Growth of Experimental Plants
Mature Iris pseudacorus plants were collected in late April 2018 at green-up from a working cattle ranch in Sioux County, NE, USA (42.25°N, 103.43°W), roughly 60 km north of Mitchell and just outside the eastern border of Agate Fossil Beds National Monument. The site has an elevation of 1,372 m, a mean annual precipitation of 37 cm, and a mean annual temperature of 7.7 C (PRISM Climate Group Reference Climate Group2004). The vegetation was dominated by I. pseudacorus; however, there were several patches of common spike rush [Eleocharis palustris (L.) Roem. & Schult] and sedge (Carex L.). The soils were primarily Bigwinder fine sandy loam (coarse-loamy, mixed, superactive, calcareous, mesic Aeric Fluvaquents) with several areas being classified as part of the Las Animas–Lisco complex (USDA-NRCS 2013). Due to the soil properties and the proximity to the Niobrara River, the area is prone to frequent flooding during the spring and early summer months and is covered in ice throughout the winter from roughly November through February (USDA-NRCS 2013). The Niobrara River at this location has an average flow of 0.4 m3 s−1 (1958 to 1991; the USGS stream gauge was reactivated in February 2014 [Spaak Reference Spaak2016]). The roughly 1.5-ha stand of I. pseudacorus is located in a roughly 58-ha subirrigated meadow and upland mixed pasture and is bounded by the Niobrara River to the south, an irrigation ditch used by the ranch to the north, a perimeter of trees to the east, and a fence line to the west.
Collection involved identifying groups of about 10 I. pseudacorus shoots; digging up the shoots, roots, rhizomes, and soil (i.e., plug); placing them in individual, 11-L pots; and transporting them to the Plant Growth Facilities at Colorado State University for planting. The size of each plug was approximately 28 cm in diameter, and final planted samples included rhizomes, soil from the study site, Pro-Mix BX potting soil (Pro-Mix, 200 Kelly Road Unit E-1, Quakertown, PA, USA), and about 10 I. pseudacorus shoots. Greenhouse conditions remained constant throughout the study, with temperatures between 21 and 24 C and 16-h light/8-h dark.
Treatments
To investigate the effects of timing of simulated cattle trampling and inundation, our study was designed to be a three by two factorial with a total of six treatment groups (trampling: early trampled, late trampled, non-trampled; inundation: inundated and non-inundated). Each treatment group consisted of nine replicate pots with about 10 I. pseudacorus shoots (subsamples) per pot. Pots were randomly assigned to one of the six possible treatments. The simulated trampling treatment consisted of applying roughly 176 kPa of pressure using the blunt end of a hammer to each individual shoot as close to the crown as possible to mimic trampling by cattle (Higgins et al. Reference Higgins, Mehlhope, Moser and Wightman2017). Simulated trampling events took place during the growing season at two different times before flowering to simulate an early trampling event as well as a late trampling event. Early simulated trampling occurred 1 wk after I. pseudacorus pots were placed in the greenhouse, when shoots were roughly 20-cm tall, and late simulated trampling occurred 4 wk later, when shoots were roughly 38-cm tall.
To maintain the inundation groups and adequate water levels for non-inundated treatments, pots were placed in 13-L buckets. The water levels of inundated groups were held constant at 2.5 cm below the bucket rim (roughly 5 to 7 cm above the crowns of I. pseudacorus). Water levels in the non-inundated groups were held at 10 cm from the bottom of the bucket (roughly 7 to 9 cm below the crowns), leaving the soil surface and crowns of the plants exposed to air but providing adequate moisture to maintain growth.
Data Collection
Density and height data were collected at the start of the study (May 2018), before each simulated trampling treatment, and at the conclusion of the study (August 2018). Density was recorded for each individual pot by identifying shoots and following leaves to their bases to ensure individual plants were counted rather than individual leaves. Height was measured by selecting a live, standing leaf that appeared to represent the average leaf height inside each pot. The selected leaf was then held straight up at full height and measured from the soil to the leaf tip.
Data Collection: Soluble Sugars
Rhizome samples for soluble sugar quantification were collected at the beginning of the study for non-trampled I. pseudacorus (May 2018), immediately before all simulated trampling events (May 2018 for early trampled and June 2018 for late trampled), and at the conclusion of the study (August 2018). Following the rhizome harvest from each pot, samples were microwaved for 90 s to stop enzymatic activity and then placed in a drying oven at 55 C for 72 h. Samples were then ground to pass through a 40-mesh (425-micron) screen and placed in cold storage at −3 C until sugar extraction and quantification (Landhäusser et al. Reference Landhäusser, Chow, Dickman, Furze, Kuhlman, Schmid, Wiesenbauer, Wild, Gleixner, Hartmann, Hoch, McDowell, Richardson, Richter and Hendry2018; O’Connor et al. 2019). The soluble sugar analysis was conducted using the methods described by Landhäusser et al. (Reference Landhäusser, Chow, Dickman, Furze, Kuhlman, Schmid, Wiesenbauer, Wild, Gleixner, Hartmann, Hoch, McDowell, Richardson, Richter and Hendry2018). All plates were covered and incubated at room temperature for 60 min and absorbance values were read at 340 nm on a Model UV2600 (Shimadzu Scientific Instruments, 7102 Riverwood Drive, Columbia, MD, USA 21046) spectrophotometer.
Data Analysis
Visual assessments of quantile-quantile plots and subsequent Shapiro-Wilk tests indicated that density and height data violated the assumption of normally distributed errors. Square-root and log transformations were performed, but neither resulted in meeting this assumption. As a result, these data were analyzed in R v. 4.0.4 (R Core Team 2020) using nonparametric Kruskal-Wallis tests to determine whether density and height of I. pseudacorus differed across the six treatments (combinations of timing of trampling and inundation). A post hoc Dunn’s test using a Holm adjustment for multiple comparisons (dunn.test package; Dinno Reference Dinno2017) was used to determine differences among treatment groups. Data for individual sugar fractions (e.g., glucose, fructose, and sucrose) also violated the assumption of normally distributed errors, so individual Kruskal-Wallis analyses for each were performed to determine whether treatments affected specific osmolytes. A Kruskal-Wallis analysis was also performed to determine differences in total soluble sugar concentrations among treatment groups. The density, height, and total sugars of pretreatment samples were not statistically different, confirming that treatment groups were initially similar, so analyses of posttreatment data were deemed appropriate to assess treatment effects. Before analysis, data points outside the interquartile range were assessed as potential outliers; Grubbs’s test was used to verify outliers, which were then removed. All analyses were tested at a significance level of α = 0.05.
Field Demonstration
The field demonstration took place in the same 1.5-ha meadow where I. pseudacorus were collected for use in the greenhouse study. The demonstration consisted of non-trampled plots inside constructed exclosures and trampled plots outside the exclosures (Figure 1). Seven 7.5-m2 circular exclosures were built and randomly located in the study area. Each exclosure consisted of two welded-wire cattle panels and five T-posts. Sample units in trampled plots were paired with sample units inside non-trampled exclosures to ensure initial plant compositions in trampled and non-trampled plots were similar (Figure 1). Sampling in trampled plots occurred between 1.5 and 4 m away from exclosures to prevent confounding effects from human trampling that occurred directly adjacent to exclosures during construction and subsequent data collection.
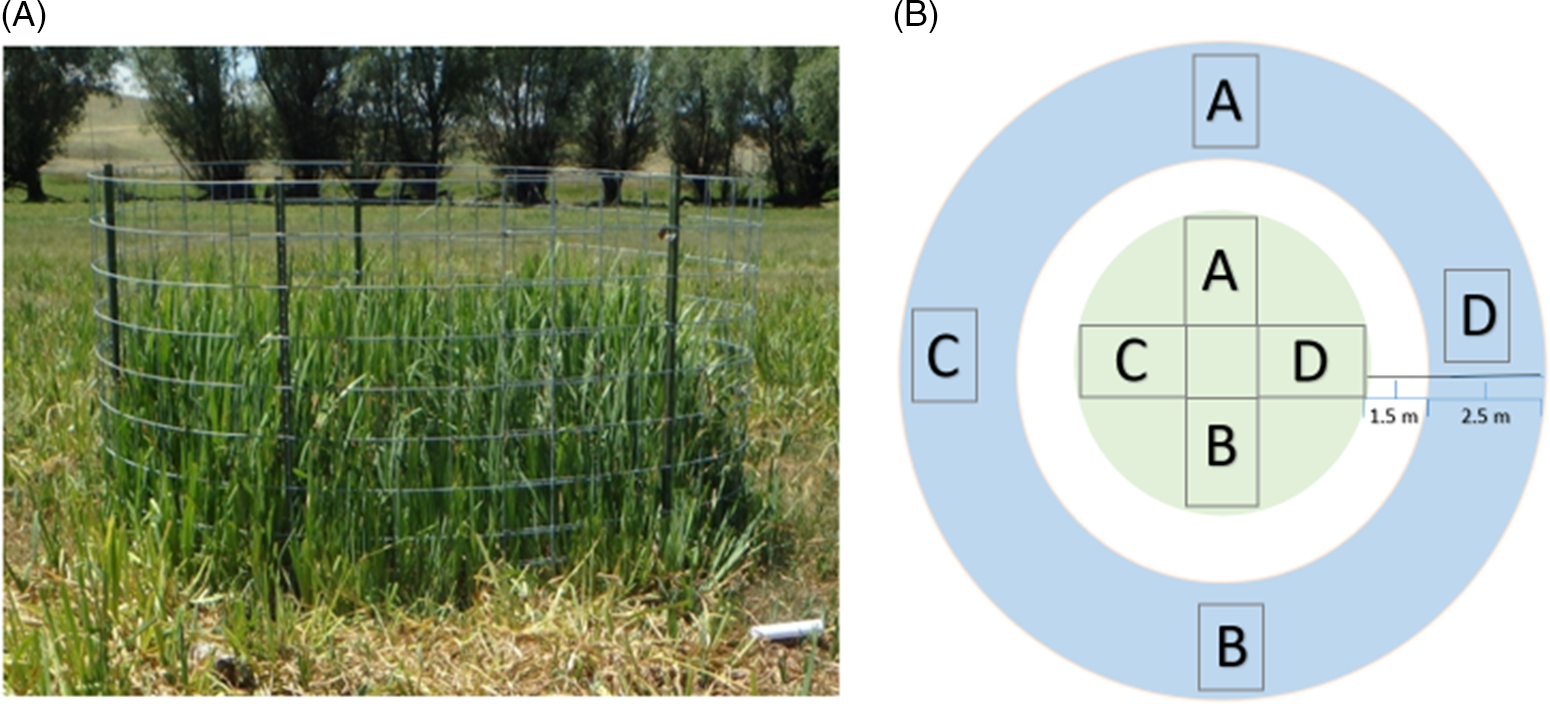
Figure 1. Field demonstration plot layout with (A) photo showing the structure of exclosures used for non-trampled plots and (B) diagram detailing non-trampled plots inside exclosures (green circle) and paired trampled plots (blue circle). The sampled 0.5-m2 quadrats are represented by the rectangles labeled A–D (subsamples).
During the first year of the demonstration in 2017, 140 cow–calf pairs were present in the pasture between late June and late July. In August 2017, 10 bulls were also present in the pasture; however, they appeared to congregate outside the I. pseudacorus infestation. In 2018, 140 cow–calf pairs were present in the pasture from early June until late July and for another 10 days in early September. To encourage cattle use in the study site, six salt blocks were placed in the meadow among the plots after water levels declined.
All data were collected from four 0.5-m2 quadrats (subsamples) in each trampled and non-trampled plot (Figure 1). Initial density and height measurements for all plots were taken in June 2017 before cattle turnout. For both trampled and non-trampled plots, shoot density was recorded inside each of the 0.5-m2 quadrats. Shoots were identified by following leaves to their bases to ensure individual plant counts as opposed to just counting leaves. For both trampled and non-trampled plots, height was measured by selecting a live, standing leaf that appeared to represent the average leaf height inside each 0.5-m2 subsample. The selected leaf was then held straight up at full height and measured from the soil to the leaf tip. Density and height measurements were taken again in June 2018 to quantify trampling impacts 1 yr after treatment. The final data collection occurred in June 2019 to quantify additional impacts following 2 yr of cattle trampling.
A repeated-measures ANOVA using the lmerTest package (Kuznetsova et al. Reference Kuznetsova, Brockhoff and Christensen2017) in R v. 4.0.4 (R Core Team 2020) was used to determine trampling effects on I. pseudacorus density and height. Factors considered in the model were treatment (trampled and non-trampled), year, and interactions as fixed effects; year as the repeated measure; and plot as a random factor. A post hoc pairwise comparison using Tukey’s honestly significant difference was performed where there were significant F-tests from the ANOVA, and all main effects, interactions, and pairwise comparisons were tested at a significance level of α = 0.05.
Results and Discussion
Greenhouse Experiment
Density and Height
Iris pseudacorus density and height were both affected by treatment (P < 0.0001). Interestingly, the effects of trampling were only evident in inundated treatments (Figure 2). Iris pseudacorus density and height in non-inundated treatments were similar regardless of trampling treatment (P = 1.0). In the inundated treatment groups, Iris pseudacorus density of the early and late trampled treatments were similar to one another (P = 1.0) but considerably lower than the non-trampled treatment (P = 0.00057 and P = 0.0009, respectively). Similarly, in the inundated treatments, Iris pseudacorus height for the early and late trampled treatments were similar to one another (P = 1.0) but much lower than the non-trampled treatment (P = 0.00028 and P = 0.00046, respectively).
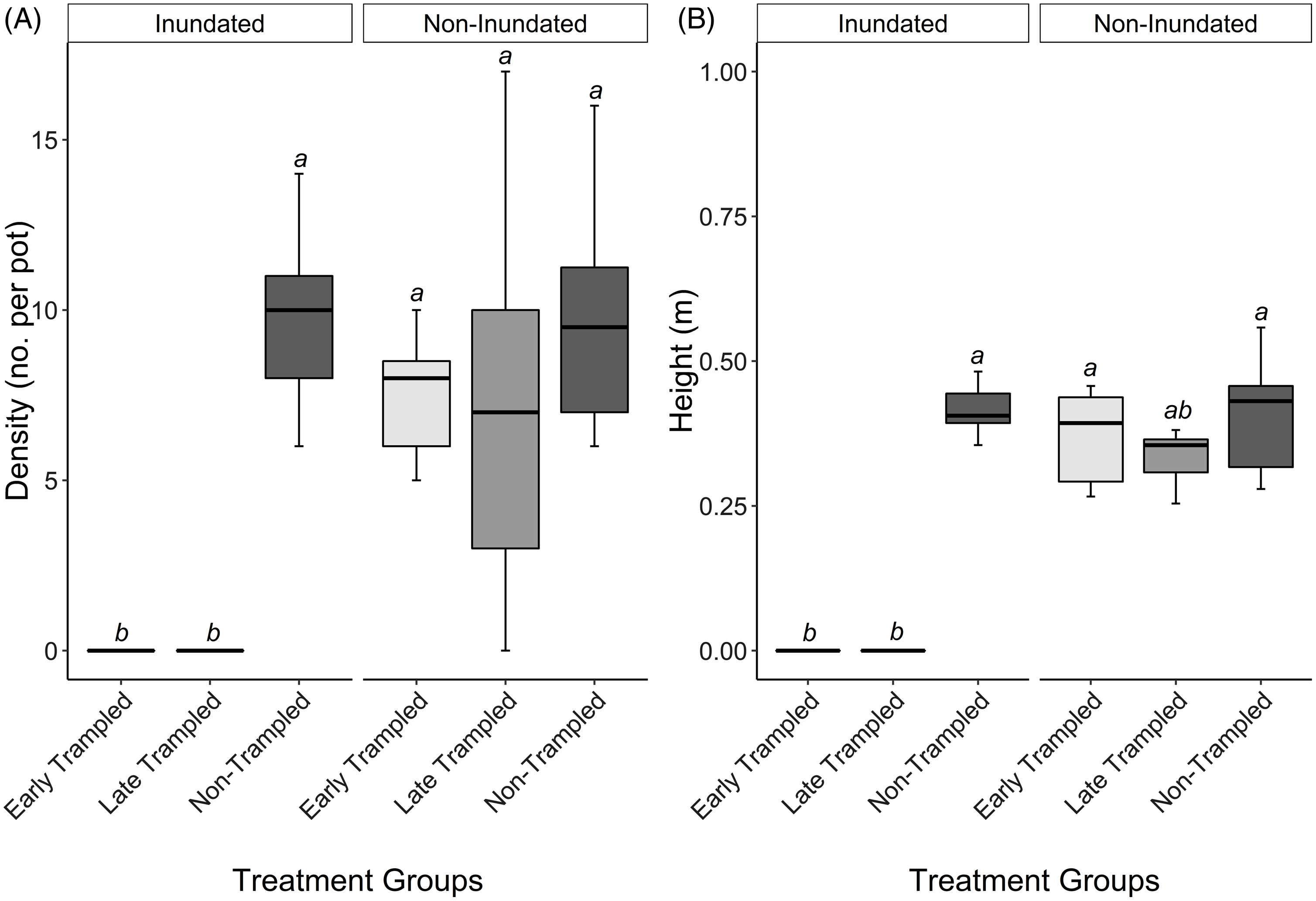
Figure 2. Iris pseudacorus (A) density (I. pseudacorus per pot) and (B) height (in meters) as affected by simulated cattle trampling and inundation. Effects of treatment on density and height (note that y-axis scales differ) were compared across all six treatment combinations. Results are presented as box plots to show the data spread and reflect differences in medians (bold horizontal lines). For each panel, treatment groups with a letter in common are not different (Dunn’s test with Holm adjustment; α = 0.05; n = 9).
The large variance in density recorded for the non-inundated/late trampled group is likely the result of a lag effect in recovery time. While the non-inundated/early trampled group had 2 mo post-trample to recover, the non-inundated/late trampled group only had 1 mo. Heights for the inundated/early trampled group and inundated/late trampled group were also significantly lower than all treatment groups that were non-inundated, apart from the non-inundated/late trampled group (P = 0.18; Figure 2). Again, this is likely due to a lag effect of recovery time.
While both the density and height of the early and late trampled plants under inundated conditions were statistically different from most other treatment groups, they were statistically similar to each other. Conversely, the density and height of early and late trampled plants under non-inundated conditions did not differ from either non-trampled control. These results indicate a significant interaction between trampling and inundation, but no impact of either trampling or inundation alone. Interestingly, time of trampling was not a critical factor under either inundated or non-inundated conditions (P = 1). This suggests inundation is a greater driver of the observed plant responses to simulated trampling than I. pseudacorus phenology at the time of treatment.
Limitations on I. pseudacorus aboveground biomass vigor due to inundation have been reported in other studies. Thomas (Reference Thomas1980) used elevation as a proxy for inundation length at a site adjacent to the Potomac River, assuming that locations at higher elevations would be inundated for a shorter period compared with locations at lower elevations. A positive relationship between biomass and elevation was observed, and elevation explained 47% of biomass variation. Of all factors investigated (e.g., light, vegetation structure, soil color as an indicator for oxidation, and presence of soil hardpan), length of inundation was found to be the most limiting factor for I. pseudacorus growth (Thomas Reference Thomas1980). Additionally, Tarasoff et al. (Reference Tarasoff, Streichert, Gardner, Heise, Church and Pypker2016) report that cutting I. pseudacorus leaves while plants were continuously inundated resulted in no aboveground biomass regrowth and rhizome decomposition 1-yr posttreatment; however, shorter periods of inundation did not significantly impact either variable 1-yr posttreatment. This is further supported by later research showing that I. pseudacorus regrowth and rhizome mortality were significantly impacted by completely inundated conditions, regardless of water depth, and that the duration of inundation was the driving factor in the relationship (Tarasoff and Gillies Reference Tarasoff and Gillies2021). These results, combined with our findings, provide growing evidence to support removal of aboveground biomass during inundated conditions as a viable strategy for I. pseudacorus management.
Soluble Sugars
There were no statistical differences among total soluble sugar concentrations (P = 0.1209; Figure 3) or when comparing concentrations for individual sugar fractions (0.067 ≤ P ≤ 0.5039). Although not statistically significant, there are several apparent trends that may provide additional insight into the density and height results presented earlier. The inundated/early trampled group had the highest (numerical) concentration of soluble sugars, which could be a lag effect. Early trampled samples had 2 mo of recovery time posttreatment before rhizome harvest, while the late trampled samples had only 1 mo for recovery. Without the production of new leaf material, the rhizomes in the inundated/early trampled group would have remained in an anoxic state and continued with anaerobic respiration. While anoxic environments may lead to decreases in metabolic activity and often dormancy, there is evidence that I. pseudacorus does not downregulate its metabolic activity under anoxic stress and continues with glycolysis and ethanol fermentation (Hanhijärvi and Fagerstedt Reference Hanhijärvi and Fagerstedt1994; Schlüter and Crawford Reference Schlüter and Crawford2001). One individual pot was identified as an outlier and removed from this analysis. This one individual in the inundated/late trampled treatment group regrew following simulated trampling and had leaf height and (numerical) soluble sugar concentrations similar to non-inundated treatments. Interestingly, a leaf of this individual rested on the water surface following simulated trampling, leading to a reduction in length of inundation period, potentially allowing for continued gas exchange and aerobic respiration, which could explain its ability to regrow compared with other inundated samples.

Figure 3. Iris pseudacorus soluble sugar fractions as affected by simulated cattle trampling and inundation. Effects of treatment on glucose, fructose, and sucrose concentrations, as well as total soluble sugar concentrations, were compared across all six treatment combinations. Results are presented as box plots to show the data spread and reflect differences in medians (bold horizontal lines). No significant differences were observed (Kruskal-Wallis test; α = 0.05; n = 4).
It is unknown whether storage carbohydrate (i.e., fructan) levels in the rhizomes of inundated groups following treatment could be sufficient to support regrowth the following growing season; however, the continued presence of and often apparent increase in free sugars suggest there was at least enough stored carbon at the conclusion of the study for continued plant function. Stored carbon in the inundated/early trampled treatment group may have continued to be depleted over time and led to eventual cell death (Tarasoff et al. Reference Tarasoff, Streichert, Gardner, Heise, Church and Pypker2016). To obtain a more holistic picture of carbon starvation as a potential mechanism driving decreased growth capacity of I. pseudacorus under prolonged inundation, continued research into the concentrations of fructans and starch is required.
Field Demonstration
The effects of trampling on Iris pseudacorus densities sampled between 2017 and 2019 varied by year (P = 0.0038). Iris pseudacorus density increased steadily in non-trampled plots over the course of the demonstration (2017 to 2019). There was no effect of trampling on density 1 yr after treatment (P = 0.7791); however, in 2019, after 2 yr, trampling reduced I. pseudacorus density (P = 0.0101; Figure 4).
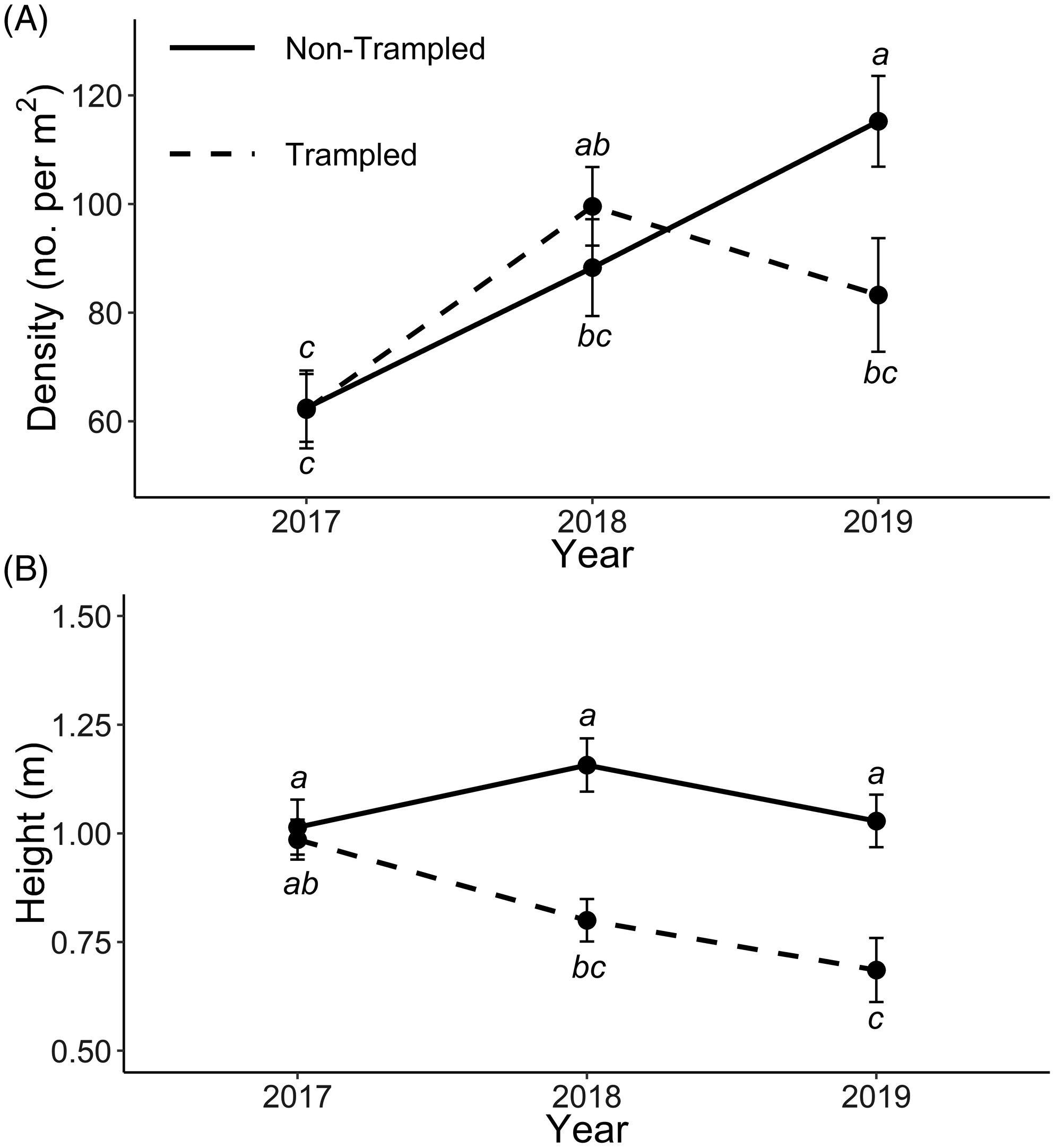
Figure 4. Iris pseudacorus (A) density (no. m−2, shown as mean ± SE) and (B) height (in meters, shown as mean ± SE) from 2017 to 2019 as affected by cattle trampling in wetlands along the Niobrara River, NE, USA. Means with a letter in common are not different (Tukey’s HSD; α = 0.05; n = 7).
Iris pseudacorus plant heights sampled between 2017 and 2019 varied by year and trampling treatment, simultaneously (P = 0.00093). Plant height remained consistent throughout the demonstration in non-trampled plots and was not statistically different across years (Figure 4). Plant height in trampled plots steadily decreased with time, and by 2019, after 2 yr of trampling, leaf height was lower than at the start of the study in 2017 (P = 0.0005). Significant within-year differences between trampled and non-trampled plots were also observed following both the first (P < 0.0001) and second years of treatment (P = 0.0001). These data support the hypothesis that cattle trampling could reduce I. pseudacorus density and height. Despite the significant decrease observed in plant height following 1 and 2 yr of trampling, significant differences in density only occurred after the second year.
Physical management treatments, such as cutting or mowing, applied early in the growing season seem to have the greatest impact for this species (Simon Reference Simon2008; Spaak Reference Spaak2016). This could be due to the limited time for plants to replenish root carbohydrates that are heavily utilized earlier in developmental stages (Whitehead Reference Whitehead1971). While cattle were placed in the meadow in late June in 2017, it was not until mid- to late July (following placement of salt blocks) that most of the trampling impacts occurred. In 2018, cattle were placed in the meadow in early June, and the highest concentration of cattle occurred in early July. This slight change in timing of trampling could have contributed to the more pronounced decrease in density observed in 2019.
The difference in timing of trampling between 2017 and 2018 also resulted in differences in whether or not I. pseudacorus were inundated while being trampled by cattle. In 2017, when cattle were placed in the meadow later in the growing season, trampling occurred during dry conditions. In 2018, cattle were turned out earlier, and as a result, trampling partly occurred while the I. pseudacorus were still inundated. The impact to aboveground biomass from trampling while partly inundated could have reduced the ability for gas exchange and led to a reduction in available resources required to produce leaves of the same height and density in trampled plots following the 2018 treatment. This is supported by other research in which aboveground biomass injury (i.e., aggressive cutting of I. pseudacorus) during inundated conditions led to reductions in regrowth (Tarasoff et al. Reference Tarasoff, Streichert, Gardner, Heise, Church and Pypker2016).
Alternatively, the results from the field demonstration may speak to a need for multiyear trampling treatments. While the height of trampled I. pseudacorus decreased steadily throughout the course of the demonstration, the same is not true for I. pseudacorus density. The density data collected in 2018 could be capturing a potential compensatory growth response of I. pseudacorus to trampling and herbivory (Bazzaz Reference Bazzaz1996; Schmid et al. Reference Schmid, Puttick, Burgess and Bazzaz1988), and the observed decrease in density in 2019 may be dependent on the cumulative impact of 2 yr of trampling. These results suggest a potential need for a multiyear commitment were cattle trampling to be used as a treatment option for I. pseudacorus.
Acknowledgments
The authors would like to thank the Panhandle Research Integration and Discovery Education group (PRIDE WMA) for funding this research. A special thanks goes to James Hill and the staff at Agate Fossil Beds National Monument, Jordan Spaak, Juliet Siebel, Ryan Schroeder, Travis Banet, and Jake Courkamp for their help in the field and lab. We also thank Ryan Wersal and two anonymous reviewers for their detailed and thoughtful feedback. No conflicts of interest have been declared.







