NCIC CTG – Contact Details
Country
Canada
Chair
Dr K. Pritchard, Toronto-Sunnybrook Regional Cancer Center, 2075 Bayview Avenue, TORONTO, ONTARIO M4N 3M5, CANADA. Tel: +1 416 480 4616 Fax: +1 416 480 6002, Email: [email protected]
NCIC CTG Office
Dr L. Shepherd, Queen's University, Cancer Research Institute, 10 Stuart Street, KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941
Website
NCIC CTG – Study Details
Title
Double-blind randomized trial of tamoxifen versus placebo in patients with node-positive or high-risk node-negative (tumor ≥ 1 cm and either higher histological grade (poorly differentiated, or SBR grade III or MSBR grade V) or lymphatic/vascular invasion or both) breast cancer who have completed CMF, CEF or AC adjuvant chemotherapy.
NCIC CTG Trial MA.12
Coordinator(s)
V. Bramwell, London Regional Cancer Centre, 790 Commissioners Road East, LONDON, ONTARIO N6A 4L6, CANADA. Tel: +1 519 685 8640 ext. 3292 Fax: +1 519 685 8624
B. Graham, NCIC CTG, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Opened in July 1993
- Target accrual: 800 patients
Objectives
- To compare the duration of overall survival among premenopausal women with axillary node-positive or high-risk node-negative breast cancer following CEF, or AC chemotherapy who will receive either tamoxifen 20 mg a day for 5 years or placebo. An additional endpoint will be disease-free survival.
- To evaluate the toxicity in patients receiving tamoxifen compared to placebo.
- To monitor FSH, LH and estradiol levels and determine if OS or DFS is affected by hormonal or menopausal status during or at completion of chemotherapy or during or after tamoxifen or placebo treatment.
Scheme

Update
- This trial was closed to accrual April 2000; 672 patients were randomized.
- All patients have now completed 5 years of blinded protocol therapy.
- Follow-up is ongoing and the second interim was performed in Spring 2006.
Related Publications
Crump M, Tu D, Shepherd L, Levine M, Bramwell V, Pritchard K. Risk of acute leukemia following epirubicin-based adjuvant chemotherapy: a report from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003; 21: 3066–3071.
Topics
None available
Keywords
None available
***************************************************
Title
A randomized trial of antiestrogen therapy versus combined antiestrogen and octreotide LAR therapy in the adjuvant treatment of breast cancer in postmenopausal women.
NCIC CTG Trial MA.14
Coordinator(s)
M. Pollak, Medical Oncology, Sir Mortimer B. Davis, Jewish General Hospital, 3755 Chemin de la Côte-Ste-Catherine, MONTREAL, QUEBEC H3T 1E2, CANADA. Tel: +1 514 340 8222 ext. 5530 Fax: +1 514 340 8302
C. Wilson, NCIC CTG, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Opened in September 1996
- Target accrual: 650 patients
Objectives
- To compare event-free, recurrence-free and overall survival.
- To compare the two treatment arms with respect to treatment toxicity and quality of life.
- To compare the two treatment arms with respect to effects of treatment on insulin-like growth factor physiology, and to study relationships between insulin-like growth factor physiology and outcome.
Scheme

Update
- This trial was closed to accrual in July 2001; 667 patients were randomized.
- All patients are now off treatment. Final analysis is expected during 2006.
Related Publications
Pollak M. Insulin resistance, estimated by serum C-peptide level, is associated with reduced event-free survival for post menopausal women in NCIC CTG MA. 14 adjuvant breast cancer trial. ASCO 2006.
Pollak M, Pritchard K, Whelan T et al. The NCIC CTG MA. 14 experience with the gallbladder toxicity of octreotide pamoate (Oncolar) in a post-menopausal patient population undergoing adjuvant treatment for state 1–3 breast cancer. Eur J Can 2002; 38(Suppl 3): S93.
Topics
None available
Keywords
None available
***************************************************
Title
A phase III randomized double blind study of letrozole versus placebo in women with primary breast cancer completing five or more years of adjuvant tamoxifen.
BIG 01-97/NCIC CTG MA.17
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2-018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983. Email: [email protected]
M. Palmer NCIC CTG, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Opened in August 1998
- Target accrual: 4800 patients
Objectives
- To compare disease-free survival and overall survival.
- To compare incidence of contralateral breast cancer, and long-term clinical and laboratory safety.
- To evaluate quality of life.
Scheme

Update
- This study exceeded its accrual goal and closed in May 2002; 5187 patients were randomized.
- The trial was unblended in October 2003 after the first interim analysis because of the strong treatment effect of letrozole. Women randomized to placebo were offered letrozole at the time of unblinding.
Related Publications
See NCIC CTG MA. 17R entry
Topics
None available
Keywords
None available
***************************************************
Title
NCIC CTG MA.17 Companion study (2): The influence of letrozole on bone mineral density in women with primary breast cancer completing five or more years of adjuvant tamoxifen.
BIG 01-97/NCIC MA.17B
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2-018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983. Email: [email protected]
M. Palmer, NCIC CTG, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Opened in autumn 1999
- Target accrual: 200 patients
Endpoints
- Percentage change in BMD from baseline in the L2–L4 (PA) region of the spine and hip at 2 years and 5 years.
- Proportion of women who develop BMD below the absolute threshold for osteoporosis.
- Percentage change in bone biomarkers from baseline.
- Clinical safety of letrozole with respect to osteoporosis in the evaluation of fractures (collected as part of the core protocol).
Scheme

Update
- The trial closed in August 2002 with 226 patients accrued.
Related Publications
See NCIC CTG MA. 17R entry
Topics
None available
Keywords
None available
***************************************************
Title
NCIC CTG MA.17 Companion study (1): The influence of letrozole on serum lipid concentrations in women with primary breast cancer who have completed 5 years of adjuvant tamoxifen.
BIG 01-97/NCIC CTG MA.17L
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2-018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983, Email: [email protected]
M. Palmer, NCIC CTG, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430, Fax: +1 613 533 2941, Email: [email protected]
Summary
- Opened in August 1999
- Target accrual: 300 patients
Objective
- To evaluate the effects of letrozole on serum lipid parameters in postmenopausal women treated with letrozole or placebo following at least 5 years of adjuvant tamoxifen therapy for breast cancer.
Primary Objectives
- Mean percentage change from baseline in LDL cholesterol.
- Mean percentage change from baseline in total cholesterol.
Secondary Objectives
- Incidence of clinically relevant changes in lipid parameters.
- Incidence of requirement for prescribing of antilipidemic medication and/or diet change.
Scheme

Update
- This study reached its accrual goal of 347 patients and closed in May 2002.
Related Publications
See NCIC CTG MA. 17R entry
Topics
None available
Keywords
None available
***************************************************
Title
NCIC CTG MA.17R A double blind re-randomization to letrozole or placebo for women completing 5 years of adjuvant letrozole in the MA.17 study.
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2-018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983, Email: [email protected]
M. Palmer, NCIC CTG, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941 Email: [email protected]
Summary
- Opened in October 2004
- Target accrual: 1800 patients
Objectives
- To determine the disease-free and overall survival.
- To evaluate the incidence of contralateral breast cancer.
- To evaluate the long-term clinical and laboratory safety of letrozole.
- To evaluate overall quality of life.
- To test the hypothesis that common genetic polymorphisms for genes encoding proteins involved in pharmacokinetic and/or pharmaco-dynamic pathways for letrozole contribute to individual variation in toxicity and efficacy of letrozole therapy.
Scheme
None available
Update
- The protocol was amended in April 2005 to increase the interval of time for 6 months to 2 years between completing the 5 years of letrozole within the MA.17 study and re-randomization to MA17R.
Related Publications
Goss P, Olivotto I, Poljicak M et al. A phase III randomized double-blind study of letrozole versus placebo in women with primary breast cancer completing five or more years of adjuvant tamoxifen. Reasons for Hope Conference 1999.
Goss PE, Ingle JN, Martino S et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. New Engl J Med 2003; 349(19): 1793–1802.
Goss P, Ingle JN, Martino S et al. Updated analysis of the NCIC CTG MA.17 randomized placebo controlled trial of letrozole after five years of tamoxifen in postmenopausal women with early stage breast cancer. Proc Am Soc Clin Oncol 2004; 23: 87.
Goss PE, Ingle JN, Martino S et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor positive breast cancer: updated findings from NCIC CTG MA.17. J Nat Cancer Inst 2005; 97: 1262–1271.
Goss PE, Ingle J, Tu D, Shepherd L, Pater J. NCIC CTG MA17: Disease free survival according to estrogen receptor and progesterone receptor status of the primary tumor. San Antonio Breast Cancer Symposium 2005
Goss PE, Ingle JN, Palmer MJ, Shepherd LE, Tu D. Updated analysis of NCIC CTG MA.17 (letrozole vs placebo to letrozole vs placebo) post unblinding. San Antonio Breast Cancer Symposium 2005.
Ingle J. NCIC CTG MA.17: Intent to treat analysis of randomized patients after a median follow-up of 54 months. ASCO 2006.
Ingle JN, Goss PE, Tu D. Analysis of duration of letrozole extended adjuvant therapy as measured by hazard ratios of disease recurrence over time for patients on NCIC CTG MA.17. San Antonio Breast Cancer Symposium 2005.
Ingle JN, Tu D, Pater JL et al. Duration of letrozole treatment and outcomes in the placebo-controlled NCIC CTG MA.17 extended adjuvant therapy trial (Online Publication). Breast Cancer Res and Treat 2006.
Luk C, Goss P, Pritchard K et al. Determinants of preferences for starting extended adjuvant letrozole in postmenopausal women following five years of tamoxifen. Proc Am Soc Clin Oncol 2005; 23(16S Part 1), 39s.
Maunsell E, Au H, Palmer M et al. Health-related quality of life in breast cancer survivors after 5 years of adjuvant tamoxifen. Qual Life Res 2005; 14(9): 2092.
Meng D, Chapman J-A, Shepherd L et al. Competing causes of death in MA.17, a placebo controlled trial of letrozole as extended adjuvant therapy for breast cancer patients. Presented: ENAR Int Biometric Soc 2006.
Moy B. NCIC CTG MA.17: Tolerability of letrozole among ethnic minority women. ASCO 2006.
Palmer M, Parulekar W, Bertrim C et al. Challenges in implementing a study involving re-randomization to an ongoing multinational, intergroup phase III trial (accepted for presentation). Society for Clinical Trials 2006.
Perez EA, Josse RG, Pritchard KI et al. Effect of letrozole versus placebo on bone mineral density in women completing >5 years of adjuvant tamoxifen: NCIC CTG MA.17b. Presented: 27th Annual San Antonio Breast Cancer Symposium 2004.
Robert NJ. Updated analysis of NCIC CTG MA.17 (letrozole vs placebo to letrozole vs placebo) post unblinding. ASCO 2006.
Wasan KM, Goss PE, Pritchard PH, et al. The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L). Ann Oncol 2005; 16: 707–715.
Whelan T, Goss P, Ingle J et al. Assessment of quality of life in MA.17, a randomized placebo-controlled trial of letrozole in postmenopausal women following five years of tamoxifen. Proc Am Soc Clin Oncol 2004; 23: 6.
Whelan TJ, Goss PE, Ingle JN et al. Assessment of quality of life in MA.17: A randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 2005; 23: 6931–6940.
Topics
None available
Keywords
None available
***************************************************
Title
A phase III study of regional radiation therapy in early breast cancer.
NCIC CTG trial MA.20
Coordinator(s)
T. Whelan, Hamilton Regional Cancer Centre, 699 Concession St., HAMILTON, ONTARIO L8V 5C2, CANADA. Tel: +1 905 387 9711 ext. 64501 Fax: +1 905 575 6308
S. McGill, NCIC CTG, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Opened December 1999
- Target accrual: 1822 patients
Objectives
- To improve the outcome of women with early breast cancer treated with breast conserving therapy – BCT (lumpectomy and breast irradiation) – and adjuvant systemic therapy – AST (chemotherapy and/or hormonal therapy).
- To determine if regional radiation therapy (to the ipsilateral supraclavicular, axillary and internal mammary nodes) in addition to breast irradiation prolongs survival in women with early breast cancer compared with breast irradiation alone.
- To compare disease-free survival in these two treatment approaches.
- To compare isolated local regional disease-free survival in these two treatment approaches.
- To compare distant disease-free survival in these two treatment approaches.
- To evaluate the toxicity effects of these two treatment approaches.
- To evaluate the quality of life associated with these two treatment approaches.
- To determine the cosmetic outcomes of these two treatment approaches.
Scheme

Update
- The study was activated in December 1999 and as of February 2006, 1563 patients have been accrued.
- This trial is now on CTSU menu and has been endorsed by NSABP, SWOG, NCCTG and TROG (Australia).
Related Publications
Goffin J, Savage C, Tu D, Shepherd L, Whelan T, Olivotto I. The difference between study recommendations, stated policy, and actual practice in a clinical trial. Ann Oncol 2004; 15: 1267–1273.
Olivotto I, Chua B, Elliott EA et al. A clinical trial of breast radiation therapy versus breast plus regional radiation therapy in early-stage breast cancer: the MA20 trial. Clin Breast Cancer 2003; 4(5): 361–363.
Topics
None available
Keywords
None available
***************************************************
Title
A phase III adjuvant trial of sequenced EC + GCSF Taxol versus sequenced AC → Taxol versus CEF as therapy for premenopausal women and early postmenopausal women who have had potentially curative surgery for node positive or high-risk node negative breast cancer.
NCIC CTG Trial MA.21
Coordinator(s)
M. Levine, Hamilton Regional Cancer Centre, 699 Concession St., HAMILTON, ONTARIO L8V 5C2, CANADA. Tel: +1 905 387 9711 Fax: +1 905 575 6308
M. Burnell, St Johns Regional Cancer Center, STJOHNS, NEW BRUNSWICK, CANADA
P. O'Brien, NCIC Clinical Trials Group, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941 Email: [email protected]
Summary
- Opened December 2000
- Target accrual: 2100 patients
Objectives
- To compare disease-free survival and overall survival among women with high-risk operable breast cancer following surgical resection of all known disease who are randomized to receive as adjuvant therapy either cyclophosphamide, epirubicin and 5-fluorouracil (CEF), or epirubicin and cyclophosphamide with GCSF followed by taxol (EC + GCSF/T), or adriamycin and cyclophosphamide followed by Taxol (AC/T).
- To compare the rates of toxicities among the patients who receive either CEF, EC + GCSF/T or AC/T.
- To compare the quality of life among patients who receive either CEF, EC + GCSF/T, or AC/T.
Scheme
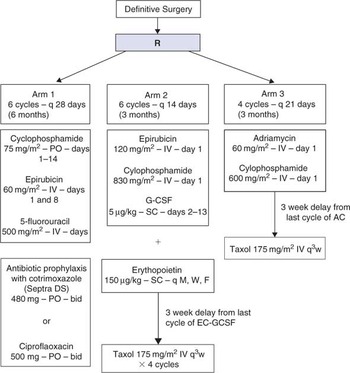
Update
- Study closed to accrual in April 2005 with a total of 2104 randomized patients. All patients have now completed treatment. An interim analysis is planned for Spring 2006.
Related Publications
None available
Topics
None available
Keywords
None available
***************************************************
Title
A phase I/II study of increasing doses of epirubicin and docetaxel + pegfilgrastim for locally advanced or inflammatory breast cancer.
NCIC CTG Trial MA.22
Coordinator(s)
M. Trudeau, Toronto Sunnybrook Regional Cancer Centre, 2075 Bayview Avenue, TORONTO, ONTARIO M4N 3M5, CANADA. Tel: +1 416 480 5145 Fax: +1 416 480 6002, Email: [email protected]
P. O'Brien, NCIC Clinical Trials Group, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Study opened February 2003
- Target accrual: 46
Objectives
- To determine the maximum tolerated doses (MTDs) and the recommended phase II doses of docetaxel given in combination with epirubicin with pegfilgrastim support in phase I dose escalation studies (either q 21 days or q 14 days) as neoadjuvant therapy in locally advanced or inflammatory breast cancer.
- To evaluate the toxicity of the combination as neoadjuvant therapy in locally advanced or inflammatory breast cancer when given in the recommended phase II doses.
- To evaluate the clinical and pathological response rate and duration of response of docetaxel and epirubicin as neoadjuvant therapy in locally advanced or inflammatory breast cancer when given in the recommended phase II doses.
- To conduct tissue studies through microarray analysis to determine drug sensitivity/resistance to epirubicin and docetaxel, and through immunohistochemistry to evaluate prognostic and predictive markers.
Scheme

Update
- This protocol was amended in January 2005 to include a dose dense regimen. Schedule A is 6 cycles of q 21 day intervals and Schedule B is 8 cycles of q 14 day intervals.
- Phase IA was closed to accrual in September 2004 with the MTD at dose level 4 (120 mg/m2 epirubicin + 75 mg/m2 docetaxel q 21 days). Phase IIA Schedule A opened to accrual in September 2004. 30 Patients were registered at this dose level and schedule. Registration is now on hold pending review of response data.
- Phase IB was opened in November 2005 and four patients have been registered to the first dose level (50 mg/m2 epirubicin + 50 mg/m2 docetaxel q 14 days). Phase IIB will open once the MTD and recommended phase II doses are determined in the phase IB portion of the study.
Related Publications
None available
Topics
None available
Keywords
None available
***************************************************
Title
A randomized phase III trial of exemestane versus anastrozole with or without celecoxib in postmenopausal women with receptor positive primary breast cancer.
NCIC CTG Trial MA.27
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2-018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983, Email: [email protected]
C. Elliot, NCIC Clinical Trials Group Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Opened in April 2003 in Canada and June 2003 in the US (through CTSU)
- Planned sample size 6840
Primary Endpoint
- Event-free survival
Secondary Endpoints
- Overall survival
- Time to distant recurrence
- Incidence of contralateral breast cancer
- Long-term clinical and laboratory safety
Patient Population
- Postmenopausal women who have histologically or cytologically confirmed, receptor-positive, adequately excised, primary breast cancer.
Scheme

Update
- Three companion studies have been developed for this trial: Quality of Life (led by ECOG), Bone Mineral Density (led by NCIC CTG) and Breast Density (led by NCCTG). Accrual has been much faster than anticipated; consequently accrual was put on hold temporarily to allow time for activation and participation in the companion studies.
Related Publications
None available
Topics
None available
Keywords
None available
***************************************************
Title
A randomized feasibility study of letrozole in postmenopausal women at increased risk for development of breast cancer as evidenced by high breast density.
NCIC CTG Trial MAP.1
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2-018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983 Email: [email protected]
D. Johnston, NCIC Clinical Trials Group Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941 Email: [email protected]
Summary
- Study opened in December 2005
- Target accrual: 120
Primary Objective
- To determine the proportion of women with breast density ≥ grade 4 who have a decrease in breast density of at least one grade after treatment with letrozole 2.5 mg daily for 1 year.
Secondary Objectives
- To determine if the decrease in breast density grade is sustained 1 year after cessation of therapy.
- To determine if there is a correlation between the plasma estrogen profile (El, E1S, E2) and breast density grade at baseline (before start of treatment).
- To determine the percentage of subjects with breast tissue hyperplasia and atypical hyperplasia before and after therapy as assessed by histopathological examination of breast tissue biopsies in those women consenting to undergo biopsies.
- To assess changes in estrogen profile from baseline and at 1 year and 1 year after cessation of therapy.
- To assess changes in predetermined specific parameters of safety at the end of 1 year of therapy compared to baseline evaluation, including:
– Bone metabolism as assessed by BMD changes at lumbar spine and hip and bone biomarkers.
– Lipid metabolism as assessed by lipid profile:
O Total cholesterol level.
O Total cholesterol/HDL ratio.
O Triglycerides.
O Modification in the percentage of subjects with:
[squf ] Low risk: (plasma total cholesterol <5.2 mmol/L and total cholesterol/HDL ratio <5 and plasma triglycerides <2.3 mmol/L).
[squf ] Mild risk: (plasma total cholesterol 5.2–7.8 mmol/L and total cholesterol/HDL ratio <5 and plasma triglycerides <2.3 mmol/L).
[squf ] Moderate risk: (plasma total cholesterol 5.2–7.8 mmol/L and total cholesterol/HDL ratio from 5–6.5 and/or plasma triglycerides >2.3 mmol/L and <4.6 mmol/L).
[squf ] High risk: (plasma total cholesterol >7.8 mmol/L and total cholesterol/HDL ratio > 6.5 and plasma triglycerides >2.3 mmol/L) of cardiovascular disease.
- Modification in percentage of subjects taking lipid lowering medication.
- To determine if modifications of these predetermined specific parameters of safety are sustained 1 year after cessation of therapy.
- To assess the general safety of the utilization of letrozole in healthy postmenopausal women for 1 year.
- To compare the effects on menopause-specific quality of life.
Scheme
None available
Update
- Accrual continues.
Related Publications
None available
Topics
None available
Keywords
None available
***************************************************
Title
A randomized study of the effect of exemestane (Aromasin) versus placebo on breast density in postmenopausal women at increased risk for development of breast cancer.
NCIC CTG Trial: MAP.2
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2–018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983 Email: [email protected]
D. Johnston, NCIC Clinical Trials Group, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Study opened in June 2001
- Target accrual: 120
Objectives
- To determine whether, in postmenopausal women with spontaneous breast density, treatment with exemestane leads to a decrease in breast density of at least one grade after 1 year of treatment as compared to placebo.
- To determine if the decrease in breast density grade is sustained in a year after cessation of therapy.
- To determine the correlation between the grade of breast density and bone density at baseline and on study.
- To assess overall safety (bone and lipid metabolism).
- To compare menopause-specific quality of life.
Scheme
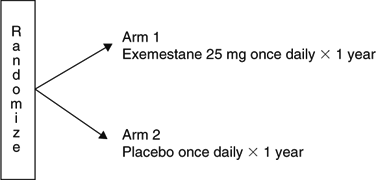
Update
- Accrual continues. It is anticipated that the target accrual will be reached in 2006.
Related Publications
None available
Topics
None available
Keywords
None available
***************************************************
Title
A phase III randomized study of exemestane versus placebo in postmenopausal women at increased risk of developing breast cancer.
NCIC CTG Trial: MAP.3
Coordinator(s)
Dr P. Goss, The Toronto Hospital, (General Division), 200 Elizabeth St., M/LW 2-018, TORONTO, ONTARIO M5G 2C4, CANADA. Tel: +1 416 946 4534 Fax: +1 416 946 2983, Email: [email protected]
D. Johnston, NCIC Clinical Trials Group, Queen's University, 10 Stuart St., KINGSTON, ONTARIO K7L 3N6, CANADA. Tel: +1 613 533 6430 Fax: +1 613 533 2941, Email: [email protected]
Summary
- Study opened in February 2004
- Target accrual: 4560
Objectives
- To determine if exemestane reduces the incidence of invasive breast cancer compared with placebo.
- To determine if exemestane reduces the incidence of invasive and non-invasive (DCIS) breast cancer compared with placebo.
- To compare the incidences of all clinical fracture.
- To determine whether there are differences between exemestane and placebo with respect to the incidence of clinically relevant cardiac events.
- To assess the impact, in comparison to placebo of exemestane on menopausal symptoms and quality of life.
- To determine whether there are significant differences in adverse events among the treatment arms.
- To assess quality of life.
Scheme
None available
Update
- 50 North American and 30 Spanish centers are participating and accrual is improving. New sites in geographical areas not yet covered are being solicited.
Related Publications
None available
Topics
None available
Keywords
None available


