Total daily energy expenditure (TDEE) consists of three components: expenditure for maintenance processes, usually called resting energy expenditure (REE); expenditure for the processing of ingested food or diet-induced energy expenditure; energy cost of physical activity or activity-induced energy expenditure (AEE). The first two components, REE and diet-induced energy expenditure, are generally measured under controlled conditions with a ventilated hood or in a respiration chamber( Reference Westerterp, Lovegrove, Hodson and Sharma 1 ). For measurement of AEE, subjects are confined in a respiration chamber or wear a facemask to assess gaseous exchange while performing daily activities or exercise.
The indicated method to measure AEE without interference by confinement in a respiration chamber or wearing a facemask is the doubly labelled water method. The doubly labelled water method can be applied under free-living conditions, including extreme environments such as high altitude( Reference Westerterp, Kayser and Brouns 2 ) and during competition( Reference Westerterp, Saris and Van Es 3 ), not limiting physical performance. Then, the contribution of AEE to doubly labelled water assessed TDEE is calculated by measuring or estimating the other two components separately( Reference Westerterp, Lovegrove, Hodson and Sharma 1 ). Alternatively, TDEE is adjusted by expressing TDEE as a multiple of REE. The ratio TDEE/REE is the accepted method for expression of the physical activity level (PAL), as adopted for comparison between subjects differing in body size and body composition( 4 , 5 ).
Measuring energy expenditure in relation to energy balance requires assessment of energy expenditure over longer time intervals. Adult human subjects maintain energy balance through the balanced control of energy intake and expenditure. However, on a daily basis, discrepancies between energy intake and energy expenditure can be large. On days with high-energy expenditure, energy intake is usually normal or even below normal. The ‘matching’ increase in energy intake comes a couple of days afterwards. Thus, energy intake correlates stronger with energy expenditure on a weekly than on a daily basis( Reference Edholm, Fletcher and Widdwson 6 ). The doubly labelled water method typically allows the assessment of human energy expenditure over intervals of at least a week( Reference Westerterp 7 ), reflecting energy requirement for the maintenance of energy balance.
The focus of the present review is on application of the doubly labelled water method for the measurement of energy expenditure under daily living conditions, including exercise and extreme environments. Subsequently, studies are reviewed on limits of doubly labelled water-assessed PAL in relation to the maintenance of energy balance and exercise-induced compensatory changes in energy expenditure.
Doubly labelled water assessment of energy expenditure
The doubly labelled water method for the measurement of energy expenditure is based on the difference between the apparent turnover rates of the hydrogen and oxygen of body water as a function of carbon dioxide production( Reference Lifson and McClintock 8 ). In practice, subjects get a measured amount of doubly labelled water (2H2 18O) to increase background enrichment of body water for 18O of 2000 ppm with about 200 ppm and background enrichment of body water for 2H of 150 ppm with 120 ppm. Subsequently, the difference between the apparent turnover rates of the oxygen and hydrogen of body water is assessed from blood, saliva or urine samples, collected at the start and end of the observation interval of 1–3 weeks. Samples are analysed for 18O and 2H with isotope ratio MS( Reference Speakman 9 ). The doubly labelled water method is the indicated method to measure TDEE in any environment, especially with regard to AEE, without interference with the behaviour of the subject. We adopted a standard protocol( Reference Westerterp 7 , Reference Westerterp, Wouters and Van Marken Lichtenbelt 10 ) with adaptations for uncommon situations as described later, including changing baseline tracer levels, high-intensity endurance exercise and extreme diets with a large contribution of sport drinks.
Exercise studies sometimes induce changes in background abundances of 18O and 2H, by changes in diet pattern or environment. To account for differences in background levels, control subjects can be observed, participating in the same activity and not receiving any tracer( Reference Bhutani, Racine and Shriver 11 ). Thus, we observed background isotope levels of 1986·9 (sd 0·9) and 142·4 (sd 0·6) ppm for 18O and 2H, respectively, in subjects at 4 d after reaching the summit of a 6·542 m mountain in Bolivia. The isotope levels showed a further decrease during a 3-week stay on the summit and were well below the initial values of 1999·0 (sd 1·1) and 151·0 (sd 0·8) ppm while at home in Europe( Reference Westerterp, Kayser and Wouters 12 ). Similarly, we observed a mean difference of 15 ppm for 18O and 10 ppm for 2H between background levels in subjects drinking lowland water and on Mt. Everest, where all water for consumption was produced by melting snow( Reference Westerterp, Kayser and Brouns 2 ). The difference in background abundances of 18O and 2H between geographical locations reflects isotope fractionation during evaporation, resulting in higher enrichment of water sources near the equator and lower enrichments near the poles and at high altitude. A recent suggestion to measure simultaneously 17O as a reflection of background fluctuations in 18O and 2H requires further study( Reference Berman, Melanson and Swibas 13 ). Water enriched in 18O, as used to increase background enrichment of body water, is also enriched in 17O. Until then, the optimal study design for the measurement of energy expenditure in subjects changing diet pattern or environment remains the inclusion of control subjects participating in the same activity.
Exercise studies sometimes require administration of multiple isotope doses. Examples are studies with a baseline measurement followed by an exercise intervention and studies exceeding the optimal observation interval, where final isotope enrichments get too close to background levels for precise measurement. The optimal observation interval for the measurement of energy expenditure with doubly labelled water is one to three times the biological half-life of the isotopes( Reference Lifson and McClintock 14 ). The biological half-life of 18O and 2H ranges from 3 d in extremely active subjects to about 10 d in very sedentary subjects( Reference Westerterp 7 ). Thus, a typical observation interval is 1 week in endurance athletes, 2 weeks in normally active subjects and 3 weeks in the very sedentary. Multiple dosing rather than increasing the isotope doses can accomplish extension of the observation time beyond the duration mentioned. Larger doses will not significantly increase the observation interval for precise measurements( Reference Schoeller 15 ). For multiple dosing, a second dose can be applied before the tracers from the first dose are completely eliminated from the body( Reference Trabulsi, Troiano and Subar 16 ). One of the first examples of a multiple dose study was the measurement of energy expenditure over 22 d in the Tour de France( Reference Westerterp, Saris and Van Es 3 ). The total dose of 260 g/subject was split up into three portions of 105, 80 and 75 g, given at days 0, 7 and 15 of the 22-d observation interval. Similarly, subjects got three weekly doses to measure energy expenditure the week before departure and throughout a 14-d cycling expedition covering 2706 km from Copenhagen to Nordkapp( Reference Rosenkilde, Morville and Andersen 17 ). In a study in a hypobaric chamber, to simulate the ascent of Mt. Everest, an initial dose was administered on day 1 and a second dose on day 16 of the 31-d observation interval( Reference Westerterp, Meijer and Rubbens 18 ).
The doubly labelled water method allows the assessment of carbon dioxide production as a measure for energy expenditure. Conversion of carbon dioxide production to energy expenditure requires information on substrate utilisation. The energy equivalent of carbon dioxide ranges between 21·1 kJ/l for carbohydrate oxidation and 27·8 kJ/l for fat oxidation( Reference Brouwer 19 ). The energy equivalent is 23·5 kJ/l for subjects consuming a typical Western diet with 55 % carbohydrate, 15 % protein and 30 % fat( Reference Black, Prentice and Coward 20 ). Exercising subjects often consume diets with a large contribution of carbohydrate-rich sport drinks. Sjödin et al. ( Reference Sjödin, Andersson and Högberg 21 ) observed a 16 % contribution of sport drinks to total energy intake, resulting in a relatively higher carbohydrate intake, and a corresponding lower figure for the energy equivalent of carbon dioxide of 23·0 kJ/l. In the Tour de France, the contribution of sport drinks was twice as high, resulting in an energy equivalent of 22·5 kJ/l carbon dioxide. Even then, using the real energy equivalent of 22·5 kJ/l instead of the figure of 23·5 kJ/l for a typical Western diet results in an adjustment <5 % of TDEE. Thus, in practice, a change in substrate utilisation does not have a large effect on energy expenditure as calculated from doubly labelled water-assessed carbon dioxide production.
Limits of physical activity level and energy balance
The first review of human energy expenditure measured by the doubly labelled method assessed PAL values. The authors suggested a maximum value of 2·0–2·4 for subjects with strenuous work or high leisure activity( Reference Black, Coward and Cole 22 ). Subsequently, FAO/WHO/United Nations University( 5 ) classified the PAL of a subject in three categories: 1·40–1·69 for a sedentary or light active lifestyle, 1·70–1·99 for active or moderately active lifestyles and 2·00–2·40 for a vigorously active lifestyle. Indeed, in the general population, PAL ranges between 1·1 and 2·5 as shown by the frequency distribution of a large sample of measurements performed in Maastricht between the 1980s and the present day (Fig. 1). The sample includes subjects aged at least 18 years, not involved in interventions, and all observed over 2 weeks under free-living conditions.
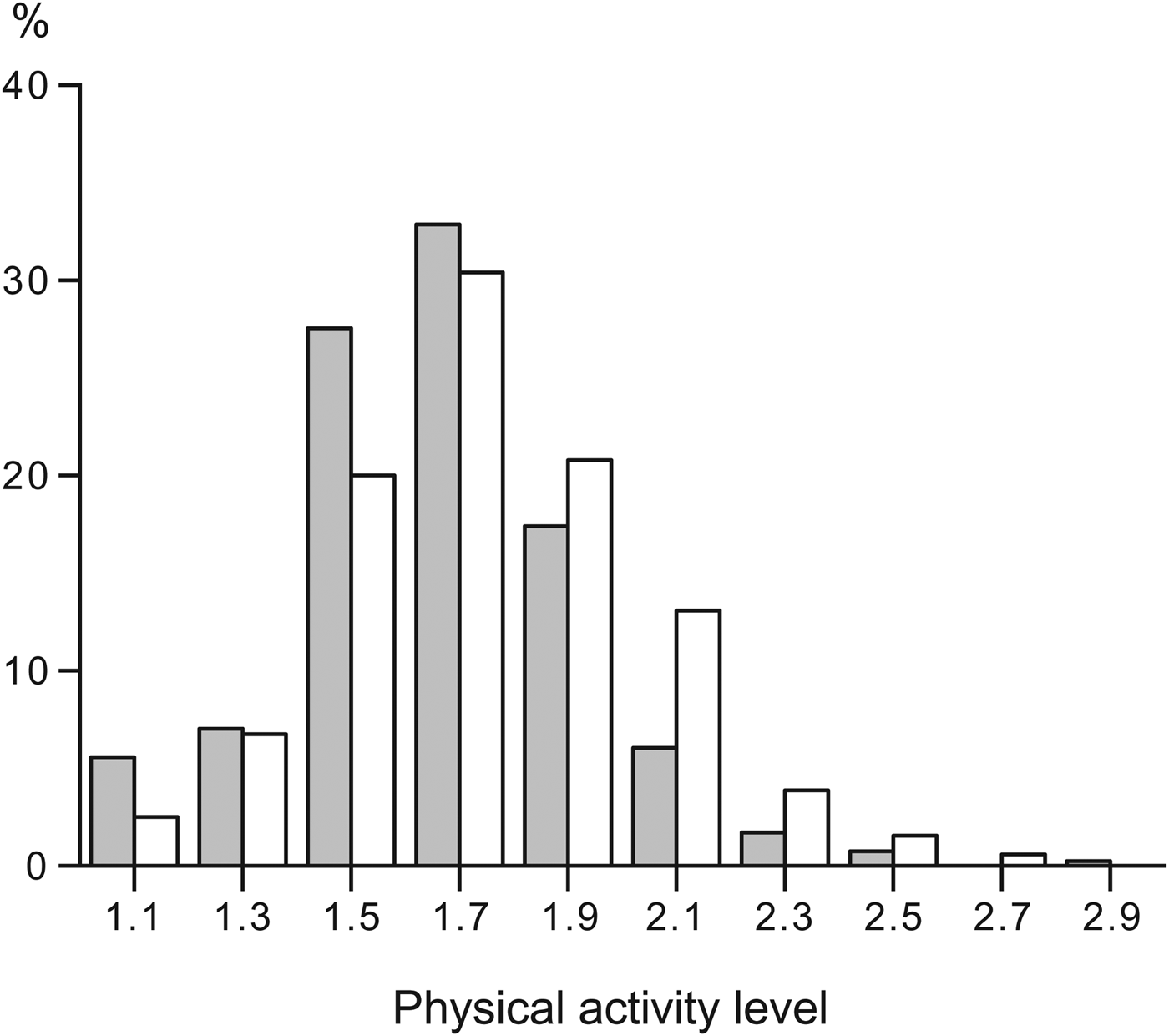
Fig. 1. Frequency distribution of the physical activity level of a large sample of adults, observed over 2 weeks under free-living conditions, living in Maastricht and surroundings. Data from Speakman and Westerterp( Reference Speakman and Westerterp 24 ) and unpublished studies. Grey bars women (n 416) and open bars men (n 550).
Children spend about 20 % of TDEE in AEE at age 1, when they start to move around independently( Reference Butte, Ekelund and Westerterp 23 ), resulting in an average PAL value of 1·4. The PAL value reaches adult values about age 10. The PAL tends to remain stable in adults until age 50. From then onwards, there is a gradual decline( Reference Speakman and Westerterp 24 ). Combining doubly labelled water-measured PAL data for all ages results in a general model as presented in Fig. 2, showing that PAL reaches a maximum at reproductive age, with slightly higher values for men than for women. This may have an evolutionary significance( Reference Heitmann, Westerterp and Loos 25 ).
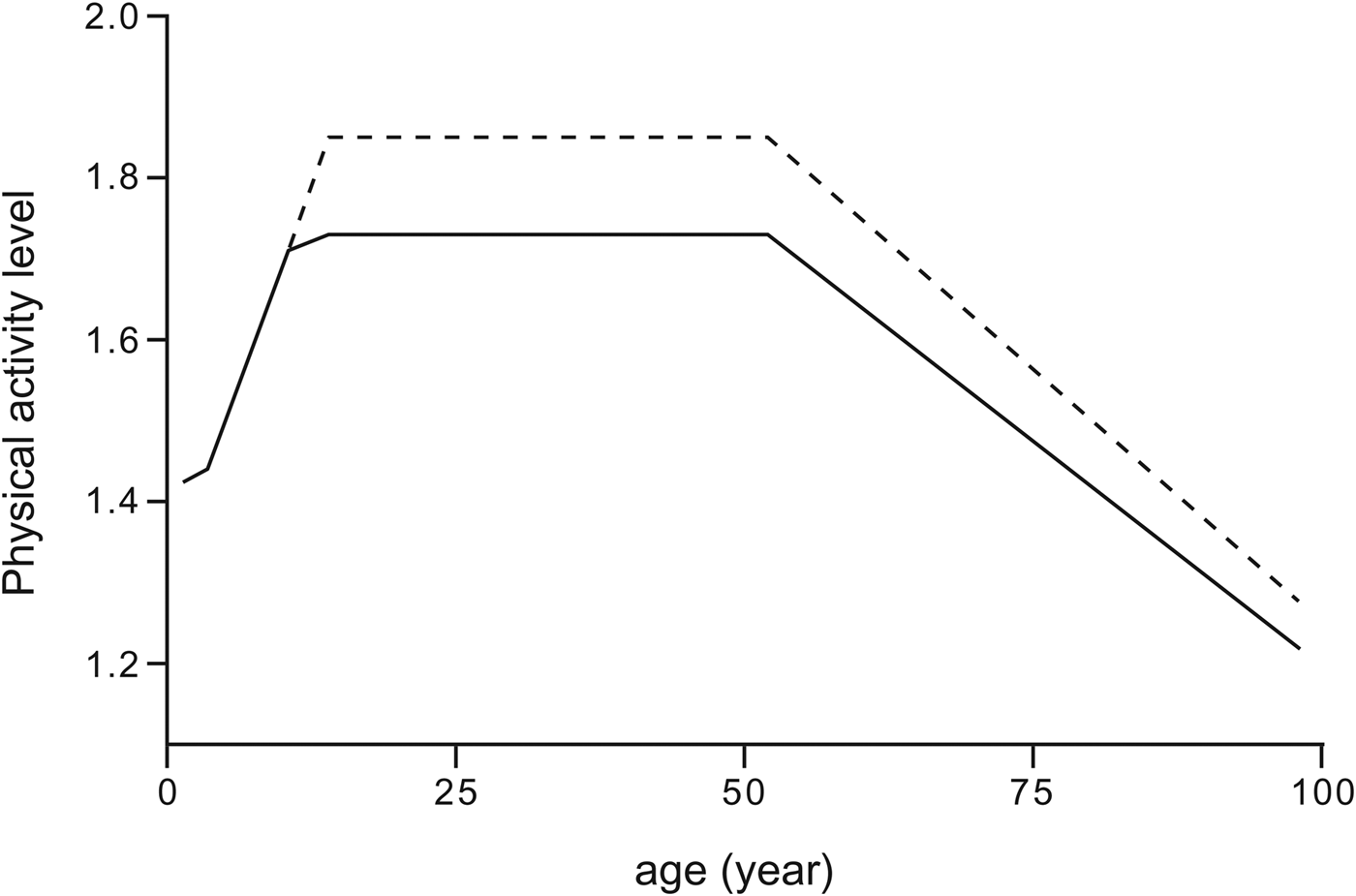
Fig. 2. General model for physical activity level in relation to age for women (continuous line) and men (broken line), derived by combining data from children( 5 ) and adults( Reference Speakman and Westerterp 24 ).
In a search of the literature, ten well-controlled studies were identified that utilised the measurement of PAL to assess the effect of exercise before and during an exercise intervention( Reference Blaak, Westerterp and Bar-Or 26 – Reference Hunter, Wetzstein and Fields 34 ). In these studies, the training mode was aerobic training or resistance training, two to five 40–90 min sessions per week, for 4–40 weeks. In all studies, subjects were sedentary or had a light active lifestyle before the intervention started, as shown by a mean PAL value of 1·40–1·69 (Fig. 3). In only five of the ten studies, the exercise intervention induced a significant increase in PAL( Reference Blaak, Westerterp and Bar-Or 26 – Reference Westerterp, Meijer and Janssen 30 ); in four of those studies, subjects reached values of 2·00–2·40 for a vigorously active lifestyle( Reference Blaak, Westerterp and Bar-Or 26 – Reference Van Etten, Westerterp and Verstappen 28 , Reference Westerterp, Meijer and Janssen 30 ). In the five studies not showing an exercise-induced increase in PAL, training was combined with an energy-restricted diet( Reference Kempen, Saris and Westerterp 31 ), or training was preceded by weight loss( Reference Hunter, Fisher and Neumeier 29 ), or subjects were aged above 50 years( Reference Meijer, Goris and Wouters 32 – Reference Hunter, Wetzstein and Fields 34 ). PAL gradually decreases with increasing age( Reference Speakman and Westerterp 24 ). Thus, exercise training can increase PAL in younger subjects with ad libitum access to food. Surprisingly, none of the exercise interventions employed in the ten studies identified( Reference Blaak, Westerterp and Bar-Or 26 – Reference Hunter, Wetzstein and Fields 34 ) increased PAL above the maximum value of 2·0–2·4 for a sustainable lifestyle, i.e. maintenance of energy balance and body weight.

Fig. 3. Physical activity level before (open bar) and at the end of an exercise training programme (grey bar) for ten studies (A( Reference Blaak, Westerterp and Bar-Or 26 ), B( Reference Bingham, Goldberg and Coward 27 ), C( Reference Van Etten, Westerterp and Verstappen 28 ), D( Reference Hunter, Fisher and Neumeier 29 ), E( Reference Hunter, Fisher and Neumeier 29 ), F( Reference Westerterp, Meijer and Janssen 30 ), G( Reference Kempen, Saris and Westerterp 31 ), H( Reference Meijer, Goris and Wouters 32 ), I( Reference Goran and Poehlman 33 ) and J( Reference Hunter, Wetzstein and Fields 34 )), and presented in sequence of mean age of the participants (years) in brackets. The lower broken line denotes the maximum value of the physical activity level of 1·69 for a light active lifestyle, the upper broken line denotes the minimum value of the physical activity level of 2·0 for a vigorously active lifestyle( 5 ). *P < 0·05; **P < 0·01 for difference with before training programme.
Only one of the ten exercise training studies (study F as presented in Fig. 3) reported an exercise-induced change in energy balance, as indicated by a change in body weight and body composition( Reference Westerterp, Meijer and Janssen 30 ). It included the longest and possibly most demanding exercise training. Sixteen women and sixteen men, aged 21–41 years, BMI 19·4–26·4 kg/m2, not participating in any sport before the start of the experiment, prepared to run a half-marathon competition after 40 weeks. The training consisted of four sessions per week, increasing running time to 30–90 min per training session, finally running 50 km/week. During the study, nine subjects withdrew being unable to keep up with the training programme. They were all in the higher fat mass range with BMI over 24 kg/m2. The remaining subjects showed a mean weight loss of 1·0 (sd 1·8) kg (P < 0·01) over the 40-week period, with more pronounced losses of fat mass (3·7 (sd 2·1) kg, P < 0·001) and gains of fat-free mass (2·6 (sd 1·5) kg, P < 0·001). Assuming an energy equivalent of 38 MJ/kg fat mass and 6·0 MJ/kg fat-free mass( Reference Westerterp, Donkers and Fredrix 35 ), the energy content of the body decreased by 125 MJ/d over 40 weeks, representing an energy mobilisation from body reserves of 0·4 MJ/d. Thus, the main part of the exercise-induced increase in TDEE of 2·3 (sd 1·0) MJ/d must have been covered by an increase in energy intake. Indeed, in the majority of exercise intervention studies, an exercise-induced increase in energy expenditure is compensated by a concomitant increase in energy intake( Reference Thomas, Bouchard and Church 36 ).
There are situations where exercise induces higher PAL, as defined for a vigorously active lifestyle( Reference Piersma 37 ). Cooper et al. ( Reference Cooper, Nguyen and Ruby 38 ) reviewed studies with doubly labelled water-assessed PAL measurements in extremely active subjects, including athletes and military personnel. The highest PAL values, in excess of 5·0, were observed in the world's most demanding cycle race, the Tour de France( Reference Westerterp, Saris and Van Es 3 ), and in subjects attempting to reach the North Pole on foot( Reference Stroud, Coward and Sawyer 39 ). The subjects in the Arctic trek walked from the northernmost point of Siberia, pulling each a 135 kg sledge with food, fuel and equipment, in the direction of the North Pole. After 30 d and 450 km, the sledges were abandoned and supplies were switched to rucksacks. After a further 360 km, the expedition was abandoned on the 48th day. They lost more than 1 kg bodyweight per week. In the Tour de France, subjects managed to maintain energy balance as shown by minimal changes in body weight and body composition. Endurance athletes, like Tour de France participants, consume energy-dense carbohydrate-rich foods and liquid formulas in order to compete at the top level. However, in most studies with PAL values higher than 2·00–2·40, subjects are in negative energy balance such as soldiers during field training( Reference Westerterp 40 ) and exercise interventions in older subjects( Reference Rosenkilde, Morville and Andersen 17 , Reference Ainsli, Campbell and Frayn 41 ).
Exercise-induced compensatory changes in energy expenditure
An exercise-induced increase in energy expenditure can be compensated by a reduction in REE, a reduction in non-exercise physical activity and an increase in exercise efficiency. Examples of each of the three options mentioned are described later.
One potential effect of exercise training on energy expenditure is an increase in REE due to increases in fat-free mass( Reference Speakman and Selman 42 ). However, in a study where sedentary subjects were trained to run a half-marathon( Reference Westerterp, Meijer and Janssen 30 ), REE as a function of fat-free mass was lower after 40 weeks training than before training (Fig. 4). REE decreased by 0·3 (sd 0·5) MJ/d, and the decrease was related to a decrease in body mass, possibly as a defence mechanism by the body to maintain body mass( Reference Westerterp, Meijer and Schoffelen 43 ). In a weight loss study maximising fat loss and minimising fat-free mass loss with an intensive 30-week exercise programme, REE decreased by 3·3 (sd 2·0) MJ/d( Reference Johannsen, Knuth and Huizinga 44 ). The decrease in REE was 2 MJ/d greater than accounted for by the change of body weight and composition. Thus, an exercise-induced change in fat-free mass is not necessarily reflected in a proportional change in REE. An exception is elite endurance athletes. Top international-level cross-country skiers showed a 16 % higher REE than sex-matched and fat-free mass-matched sedentary control subjects( Reference Sjödin, Forslund and Westerterp 45 ). Here, a possible explanation is positive energy balance, where a high-energy flux and unchanged eating habits lead to overeating on resting days when REE is measured.

Fig. 4. Resting energy expenditure as a function of fat-free mass in sedentary subjects before (open dots) and after 40 weeks training (closed dots) to run a half-marathon, with the linear regression lines (data from Westerterp et al. ( Reference Westerterp, Meijer and Janssen 30 )).
An exercise intervention may reduce non-exercise physical activity, reducing the effect of exercise on TDEE. Reviews on the effect of exercise training on non-exercise physical activity concluded that, in healthy adults, no statistically or clinically significant change in non-exercise physical activity occurs during exercise training( Reference Washburn, Lambourne and Szabo 46 , Reference Fedewa, Hathaway and Williams 47 ). Indeed, two studies combining doubly labelled water assessment of AEE with accelerometer assessment of activity pattern did not detect a change in non-exercise activity( Reference Van Etten, Westerterp and Verstappen 28 , Reference Meijer, Janssen and Westerterp 48 ). However, in studies not showing an exercise-induced increase in PAL, where training was combined with energy restriction( Reference Kempen, Saris and Westerterp 31 ) or subjects were aged above 50 years( Reference Meijer, Goris and Wouters 32 – Reference Hunter, Wetzstein and Fields 34 ) (Fig. 3), exercise activity probably was compensated by a reduction in non-exercise physical activity( Reference Melanson, Keadle and Donnelly 49 , Reference Melanson 50 ). Kempen et al. ( Reference Kempen, Saris and Westerterp 31 ) observed no difference in PAL between women on an energy-restricted diet with and without exercise. In older subjects, an exercise training programme had no effect on total daily physical activity. Training activity was compensated for by a decrease in non-training physical activity( Reference Meijer, Westerterp and Verstappen 51 ). Apparently, TDEE is constrained by energy restriction and at higher age.
Finally, an exercise-induced increase in energy expenditure can be compensated by an increase in exercise efficiency. Plotting TDEE as a function of accelerometer-assessed physical activity in a comparative analysis between subjects, TDEE increases with physical activity at low-activity levels but plateaus at higher activity levels( Reference Pontzer, Durazo-Arvizu and Dugas 52 ). In a longitudinal study, training sedentary subjects in preparation to run a half-marathon competition after 40 weeks, we observed an initial increase in PAL but no further increase between 8 and 40 weeks training, while training distance was doubled over the same interval (Fig. 5). The initial increase in PAL was nearly twice as high as the predicted cost of the training and could not be attributed to a change in non-training activity as recorded with an accelerometer( Reference Meijer, Janssen and Westerterp 48 ). After the initial increase in PAL, the novice runners increased the efficiency of the exercise as a result of the training. Training increased exercise economy, and thus reduces an exercise-induced increase in energy expenditure, especially in untrained subjects( Reference Van Etten, Westerterp and Verstappen 28 , Reference Valenti, Bonomi and Westerterp 53 ).
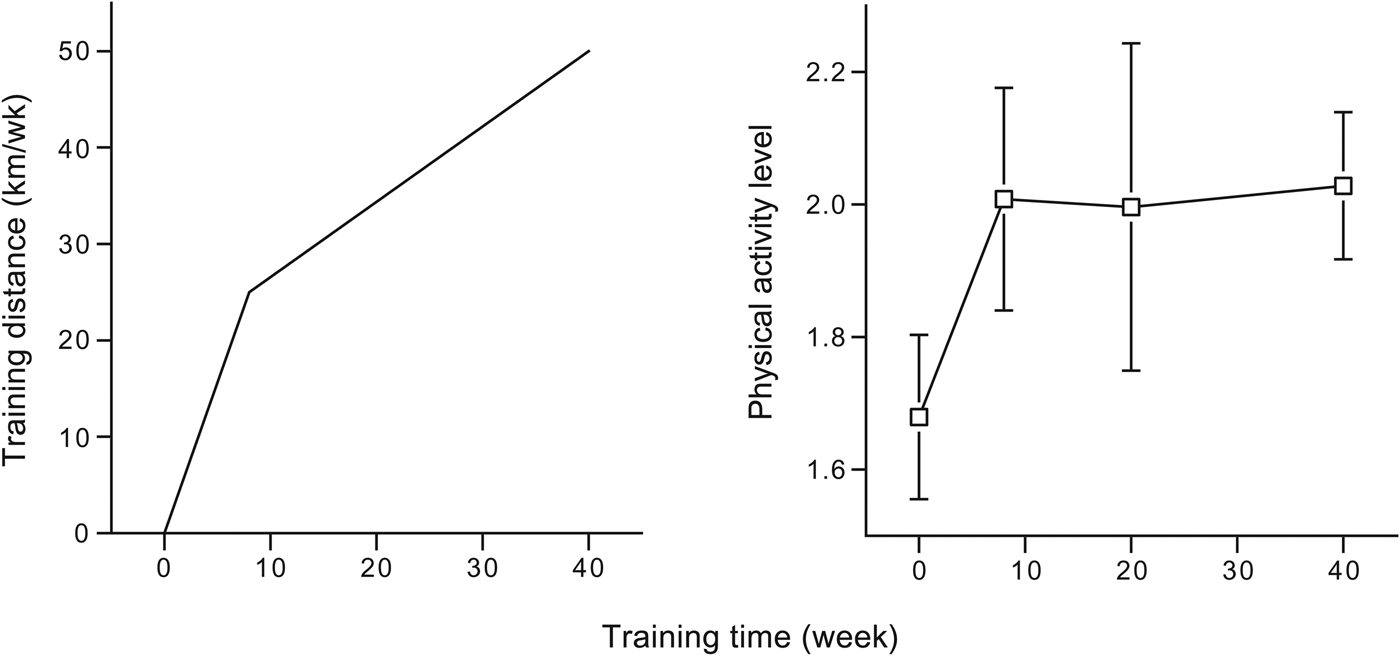
Fig. 5. Training distance and physical activity level in subjects before and during a 40-week preparation to run a half-marathon (data from Westerterp et al. ( Reference Westerterp, Meijer and Janssen 30 )).
Conclusions
The doubly labelled water method allows the assessment of TDEE without interference with activity behaviour of subjects. The observation interval is at least 1 week, covering the weekly cycle of day-to-day variation in activity pattern. TDEE reaches a maximum value of 2·00–2·40 times REE in subjects with strenuous work or high leisure activity. Higher values are observed in professional endurance athletes consuming additional energy-dense foods to maintain energy balance. An exercise-induced increase in activity energy expenditure can be compensated by a reduction of non-training activity energy expenditure and REE and an increase in exercise efficiency.
Financial Support
None.
Conflicts of Interest
None.
Authorship
The author was solely responsible for all aspects of preparation of this paper.








