INTRODUCTION
Surgical site infections (SSIs) are among the most common hospital-acquired infections in patients undergoing surgery, and can result in extended hospitalization and increased healthcare system costs [Reference Gomez1]. Antimicrobial prophylaxis has been included on infection control options [Reference Mangram2]. During the past three decades, the use of surgical antimicrobial prophylaxis has markedly reduced the incidence of SSIs [Reference Turnbull, Zoutman and Lam3]. The optimal and appropriate use of antimicrobials is an urgent and necessary goal because of the widespread emergence of antibiotic resistance in opportunist pathogens which is promoted by indiscriminate use of broad-spectrum antimicrobial agents by clinicians for prophylaxis and treatment of infections [Reference Dohmen4, Reference Paterson5]. Moreover, antibiotics appear to be used not only in excess but also inappropriately [Reference Akalin6]. Consequently, many studies have underlined the importance of strict adherence to validated guidelines for prescription of antimicrobials and of the development of a monitoring system within each institution of antimicrobial usage [Reference Tourmousoglou7, Reference Talon8]. For specific procedures these local guidelines set out the correct drug, timing, dose and route of administration and their appropriate implementation has been shown to achieve significant improvement in antibiotic use [Reference Mangram2, Reference Dohmen4]. Indeed, studies have shown increases from 50% to 95% in the appropriateness of antibiotic prophylaxis by the strict implementation of an existing protocol [Reference Alerany9–Reference Braxton, Gerstenberger and Cox11].
In Italy, Brusaferro et al. [Reference Brusaferro12] described an improvement in perioperative antibiotic prophylaxis (PAP) compliance following implementation of a specific protocol developed by the hospital health management in collaboration with the hospital infection control committee. The importance of assigning the responsibility of correct choice (and administration) for PAP in each institution was stated in the 2003 Italian national guidelines for PAP implementation [13], and revisited in the 2008 revision of the national recommendations [14]. In particular, according to the findings by Tan et al. [Reference Tan, Naik and Lingard15], which underline the importance of assigning responsibility for achievement of correct PAP timing, the revised version of the Italian national guidelines addresses the role of collaboration between the anaesthesiology ward, surgical ward, operating room personnel, and other professionals involved in infection control, in the actual adoption of local PAP protocols. An Italian health act (Ministry of Health Circular no. 52/85) states that in the hospital Infection Control Committee, among others (hospital health director pharmacologist, hospital pharmacist, nurse manager and specialist nurses), three professional figures are mandatory: infection control physician, microbiologist, and infectious disease specialist.
The aim of this prospective surveillance study was to determine the adherence to the PAP protocol used at a teaching hospital in Central Italy during a 6-year period, to assess the variables associated with inappropriate administration of antibiotics, and to evaluate the intervention by determining the adherence to the PAP protocol by measuring the impact of the rationalization of antibiotic utilization on SSI rates.
METHODS
This study took place at the ‘Ospedali Riuniti’ of Ancona, a 917-bed teaching hospital with 15 surgical wards, situated in Central Italy, from April 2001 to March 2007.
PAP was defined as ‘the use of prophylactic antibiotics in patients undergoing surgical procedures with no evidence of an established infection at the time of surgery’. Surgical procedures were grouped according to the National Nosocomial Infections Surveillance System (NNIS) categories [Reference Horan and Emori16]. Standardized protocols for antibiotic prophylaxis were established for each surgical procedure performed at the hospital, according to international guidelines and local epidemiology of microbial circulation [13, 17]. Each surgical ward chose a member (the referring surgeon) to take part in the review and approve the hospital-based protocols. Microbiologists, infectious disease specialists and pharmacists participated in the intervention. In January–March 2001, several sessions were conducted to explain and share the content of the PAP protocol. A schematic table, showing appropriate PAP in each clinical intervention, was given to participating surgeons to facilitate implementation of the local protocol. In this scheme, each surgical procedure was linked to the adequate drug, the dose required, the timing, and duration of administration. The evaluation of antibiotic prophylaxis was performed by physicians of the Hospital Hygiene Service.
During the intervention period, clinical audits were performed with periodic revision of protocols according to international guidelines [17]; in March 2004, the publication of Italian National Guidelines on PAP [13] constituted the occasion to update the protocols and give specific information to all surgical medical staff.
PAP was considered to be in line with the protocol when all analysed criteria followed hospital-based protocols. If the type of antibiotic prophylaxis administered was not adherent to the protocols, the difference was specified in terms of type of drug, dose, or timing and duration of administration. The compliance of PAP administration was evaluated by calculating the proportion of interventions receiving adequate PAP in each NNIS system category. The following information was retrieved from the clinical charts: type of procedure, type of drug used for prophylaxis, dose, and duration of therapy. Moreover, data on duration of procedure, wound contamination class, patient's American Society of Anesthesiologists (ASA) score (1=healthy; 2=mild systemic disease; 3=severe systemic disease; 4=severe systemic disease that is a constant threat to life; 5=moribund patient) were collected from the local SSI surveillance system [Reference Prospero18]. Patients undergoing two or more procedures requiring more than one incision during the same operation, or those who received antibiotics in the 24-h preoperative period for infection, or other indication, were excluded from the analysis. Feedback to the surgeons consisted of monthly reports on compliance with institutional protocols addressed to the head of each ward. Monthly feedback included description of surveyed procedures (ICD-9 code, ICD-9 specific SSI rate, and ICD-9 specific PAP compliance with institutional protocols), and the general characteristics of the procedures during that month (patient's ASA score, wound contamination class, duration of operation).
The impact of implementation of PAP protocols during the study period was analysed by comparing the proportion of interventions receiving adequate PAP on an annual basis; the statistical significance of differences was measured by the Cochrane–Armitage test for linear trend, and outcome was evaluated by determining SSI rates. A multivariable logistic regression model was designed to assess factors associated with inappropriate PAP (model 1). A second model was used to evaluate parallel changes in SSI rates over the study period after adjusting for common SSI risk factors (model 2). Selection of the variables to be included in the final logistic regression analysis was based on bivariate associations between the selected variables and inappropriate PAP (model 1), and between selected variables and SSI (model 2). The hospital and patient variables with P<0·2 on bivariate analysis were entered into the models; in particular: ASA score (2, 3, 4 or 5 vs. 1, respectively), duration of procedures (originally measured as a continuous variable, was dichotomized according to the NNIS system T time as follows: procedure lasting less than NNIS T system time=0; procedure lasting more than NNIS system T time=1), wound contamination class (2, 3, 4, 5 vs. 1), urgent procedures (defined as operations within 24 h after an unscheduled admission to the hospital; yes=1, no=0), laparoscopic procedure (yes=1, no=0), type of surgical procedure and study year (1 April 2001–31 March 2002=1, 1 April 2002–31 March 2003=2, 1 April 2003–31 March 2004=3, 1 April 2004–31 March 2005=4, 1 April 2005–31 March 2006=5, 1 April 2006–31 March 2007=6) were introduced as independent variables in the model. The level of significance was set at P=0·05. Data were analysed using SOR.R.ISO software (SORveglianza Routinaria delle Infezioni del Sito Operatorio) [Reference D'Errico19] and Stata version 9.0 software [20].
RESULTS
In, total 28 621 patients were surveyed in the study period; baseline characteristics of included procedures are summarized in Table 1. Of the procedures, 41·7% (n=12 243) were general surgery, 26·53% (n=7316) orthopaedic surgery, 14·77% (n=3789) neurosurgery, 7·71% (n=2459) vascular surgery, and 9·28% (n=2814) other surgery. An improvement in total adherence to PAP protocol (P<0·001), between the first and last year of observation was registered for 58·85% of patients; an overall reduction in SSI rates by 1·78% (P<0·001) was identified (Fig. 1). In particular, a relative rise of 25·08% per year (95% CI 10·75–41·25) for adequate PAP was estimated, with a relative fall in the SSI rate of 22·98% per year (95% CI 6·84–36·32).
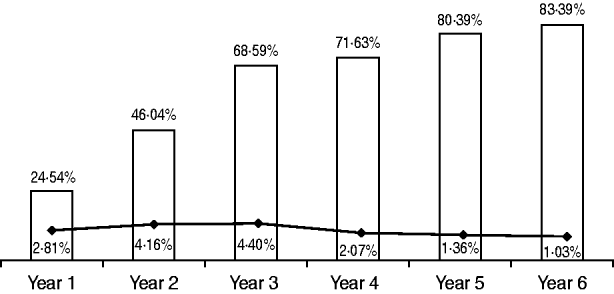
Fig. 1. Distribution of compliance of antibiotic prophylaxis and infection rates, comparison of study years (n=28 621). □, % perioperative antibiotic prophylaxis compliance (P<0·001); –◆–, % surgical site infection (P<0·001).
Table 1. Distribution of baseline characteristics of surveyed procedures (n=28 621) by study year
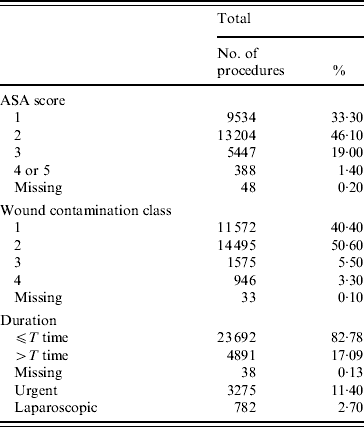
ASA, American Society of Anesthesiologists.
According to the protocols, 74·65% (n=21 366) of patients received an antibiotic during the perioperative period, and 81·95% (n=17 509) of these received it within 1 h prior to surgical incision and in 86·82% (n=18 549) of patients PAP was discontinued within 24 h after surgery.
Table 2 summarizes the results of the two logistic regression models performed. Regarding variables associated with inadequate PAP, we found significant risk factors for: patients' ASA score ⩾2 [odds ratios (OR) from 1·28 (95% confidence interval (CI) 1·19–1·37) to 1·87 (95% CI 1·43–2·44)], prolonged duration of surgery (OR 1·68, 95% CI 1·56–1·82) for procedures lasting more than time T, and urgent surgery (OR 2·16, 95% CI 1·96–2·37), with respect to elective surgery. Laparoscopic procedures showed a decreased risk (OR 0·50, 95% CI 0·42–0·61) as did procedures performed in years 2–6 of the project, with odds ratios ranging from 0·29 (95% CI 0·26–0·31) to 0·05 (95% CI 0·04–0·05).
Table 2. Results of logistic regression models for estimates of factors associated with the administration of inadequate perioperative antibiotic prophylaxis (PAP) and with the risk of surgical site infection (SSI) (n=28 621)

OR, Odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists.
For the occurrence of inappropriate PAP the log likelihood ratio was: −13 844·881 (χ2=10 473·81, P<0·0001, pseudo R 2=0·2744).
For the occurrence of SSI the log likelihood ratio was: −2883·33 (χ2=1289·76, pseudo R 2=0·1828, P<0·0001).
† Reference category.
* P<0·05.
The decrease in the number of SSIs during the study years was confirmed by multivariable analysis, after adjusting for common SSI risk factors (patient's ASA score, wound contamination class, duration of operation, non-laparoscopic surgery, urgent surgery, PAP) and surgical categories (Table 2).
DISCUSSION
The global reduction of 58·85% in inadequate PAP observed between the first and the last year of this study represents the efficacy of continuous surveillance of antimicrobial utilization. The reduction registered in SSI rates is reassuring and possibly reflects efficient prescription of the correct drug at the optimal dose and duration. These findings are supported by the meta-analysis by Bowater et al. [Reference Bowater, Stirling and Lilford21] which highlights the importance of antibiotic prophylaxis as an effective intervention for preventing wound infection over a broad range of different surgical procedures as measured by relative reductions in the risk of wound infection.
The analysis of risk factors associated with inadequate PAP administration raises the possible influence on decision making of duration of surgery (i.e. interventions lasting more than time T vs. procedures lasting less than time T; OR 1·68), together with patients' characteristics before the intervention (odds ratios of inadequate PAP increasing from 1·28, in ASA 2 patients to 1·87 in ASA 4 patients). It is well known that prolonged duration of surgical procedures is associated with an increased risk of infection [Reference Mangram2]; modern surgical techniques allow surgeons to reduce operation times and subsequently the risk of infection. Minimally invasive surgery, such as laparoscopic surgery, is associated with improved immune function compared to open surgery; therefore the improved immune function results in significantly decreased infectious complications [Reference Richards22, Reference Yoshida23]. Recently Varela et al. [Reference Varela, Wilson and Nguyen24] found that laparoscopic surgical techniques significantly decreased the incidence of SSI.
To minimize the influence that variables such as ASA score, wound contamination class, and duration and type of surgery might have on PAP compliance and SSI rates, two logistic regression models were used. Nevertheless, it is possible that some other factors not taken into account by the model, such as those related to the patients' specific clinical condition, could have affected our outcomes. Improvements in the use of an appropriate antimicrobial regimen, and shorter duration of administration have defined more clearly the impact of this approach in reducing the number of post-operative wound infections [Reference Nichols25]. Although the effectiveness of guidelines and protocols in promoting professional quality is debatable, it is clear that monitoring and intervention can be effective in increasing adherence to a protocol. This has been shown in studies in which the appropriateness of antibiotic prophylaxis was increased from around 50% to 95–100% by the stricter implementation of an existing protocol [Reference Alerany9–Reference Brusaferro12, Reference Adams26, Reference Mol27]. In agreement with other authors, our experience has shown that the introduction of a hospital-based protocol, based on international and national guidelines, could have an important impact on PAP use especially when actively disseminated, when its ‘ownership’ is increased by fine-tuning recommendations with targeted physicians, and when easy access to feedback information is provided [Reference Adams26]. Moreover, testing the feasibility and acceptance of clinical guidelines among the target group is important for their effective implementation [Reference Van Kasteren28, Reference Van Kasteren29]. In fact, in our experience, besides infection control professionals, surgeons, anaesthesiologists and pharmacists, the protocols have been discussed also with clinical microbiologists and infectious disease specialists in order to encompass all possible stakeholders in the process of SSI prevention, control and treatment.
A limitation of the study is that other possibly confounding covariates, particularly those related to patients' clinical situation, were not included in the logistic regression models, and may have influenced the results obtained. Moreover, the timing of antibiotic prophylaxis administration was based on a widely accepted protocol rather than direct observation. Nevertheless, given the local effectiveness of this methodology, we consider that the implementation of similar experiences in other settings could be of interest, in order to improve the general applicability of the intervention.
DECLARATION OF INTEREST
None.





