The metabolic syndrome represents a cluster of metabolic disorders, which include increased fasting glucose, blood pressure and plasma TAG and decreased HDL-cholesterol concentrations. This syndrome is common among overweight and obese individuals and leads to an increased risk of CVD and type 2 diabetes mellitus( Reference Grundy, Brewer and Cleeman 1 , Reference Beltrán-Sánchez, Harhay and Harhay 2 ). In addition, the metabolic syndrome is associated with increased risk for certain cancers including breast( Reference Bhandari, Kelley and Hartley 3 ), endometrial( Reference Trabert, Wentzensen and Felix 4 ), colorectal( Reference Ishino, Mutoh and Totsuka 5 ) and biliary tract cancers( Reference Wu, He and Yu 6 ), as well as an increased risk of mortality( Reference Lakka, Laaksonen and Lakka 7 , Reference Hu, Qiao and Tuomilehto 8 ). In the USA, approximately 22·9–25·5 % of adults, aged 20 years and older, had the metabolic syndrome from 1999 and 2000 to 2009 and 2010, according to the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2010( Reference Beltrán-Sánchez, Harhay and Harhay 2 ). Thus, given the incidence number and the associated disease risks, the metabolic syndrome is an important public health concern in the USA.
Although the mechanisms behind the metabolic syndrome are not entirely clear, accumulating evidence supports that chronic inflammation and oxidative stress play important roles in its development( Reference Sutherland, McKinley and Eckel 9 , Reference Chung, Kang and Rho 10 ). Further, because an increased BMI can increase inflammation and oxidative stress production, the prevalence of the metabolic syndrome strongly correlates with an increased prevalence of overweight and obese individuals( Reference Mraz and Haluzik 11 – Reference Bondia-Pons, Ryan and Martinez 13 ).
As a natural antioxidant, lycopene can alleviate oxidative stress and decrease tissue inflammation( Reference Ateşşahin, Ceribaşi and Yilmaz 14 – Reference Luvizotto Rde, Nascimento and Imaizumi 17 ). However, previous studies indicate that different BMI statuses can alter the effect of serum lycopene on inflammation and oxidative stress. For example, in a 2013 study by Ghavipour et al. ( Reference Ghavipour, Saedisomeolia and Djalali 18 ), 106 overweight or obese women were recruited and randomly assigned to an intervention group (tomato juice supplementation) or a control group (usual diet with water) for a 20-d intervention. On the basis of the results, serum concentrations of TNF-α and IL-8 were reduced significantly in the intervention group compared with the control group( Reference Ghavipour, Saedisomeolia and Djalali 18 ). However, subgroup analysis of overweight and obese participants clearly showed that the effects of lycopene were only effective for participants who were overweight and not for participants who were obese( Reference Ghavipour, Saedisomeolia and Djalali 18 ). To further examine the effects of lycopene on oxidative stress, a subsequent 2014 study by Ghavipour et al. ( Reference Ghavipour, Sotoudeh and Ghorbani 19 ), examined sixty-four overweight or obese women who were recruited to a randomised-controlled clinical trial as in the previous study. Findings showed that serum superoxide dismutase (SOD), glutathione peroxidase and catalase as well as plasma total antioxidant capacity significantly increased in the treatment group compared with the control group( Reference Ghavipour, Sotoudeh and Ghorbani 19 ). However, in the treatment group, similar results were only found in participants who were overweight and not in those who were obese( Reference Ghavipour, Sotoudeh and Ghorbani 19 ). As such, an alternative explanation for the different effects of lycopene could be due to increased inflammation and oxidative stress production in individuals who are obese compared with those who are overweight( Reference Ghavipour, Saedisomeolia and Djalali 18 , Reference Ghavipour, Sotoudeh and Ghorbani 19 ).
The biological mechanism by which lycopene reduces the risk of the metabolic syndrome mainly depends on alleviating oxidative stress and decreasing inflammation( Reference Sluijs, Beulens and Grobbee 20 – Reference Liu, Shi and Cao 22 ). Therefore, different BMI statuses may alter the positive effects of lycopene in reducing the symptoms of the metabolic syndrome. In turn, information on the effects of lycopene for individuals with different BMI statuses is expected to be important for future dosage recommendations. As such, the objective of this study was to examine the associations between serum lycopene and the prevalence of the metabolic syndrome in individuals with different BMI statuses, for which data were pulled from the NHANES 2001–2006.
Methods
Data source and study sample
This study used publicly available NHANES data. The NHANES was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC) to assess the health and nutrition condition of adults and children with a multistage, stratified sampling design from all counties in the USA. Before the survey, the institutional review board of CDC approved the survey. The combination of NHANES data from years 2001 to 2006 included 15 431 participants who were at least 20 years old (7341 men and 8090 women) (Fig. 1). A sample of 13 196 participants was used for the metabolic syndrome analyses. Participants with missing information on the metabolic syndrome, BMI and serum concentrations of lycopene (n 2235) were excluded from this study (Fig. 1).

Fig. 1 Diagram of the study design. NHANES, National Health and Nutrition Examination Survey.
Outcome variable
To be diagnosed with the metabolic syndrome, an individual has to meet at least three or more of the following criteria: abdominal obesity (waist circumference≥102 cm for men, or ≥88 cm for women); hypertriacylglycerolaemia (serum TAG≥150 mg/dl or drug treatment for elevated TAG); low HDL-cholesterol (HDL-cholesterol<40 mg/dl for men and <50 mg/dl for women or drug treatment for reduced HDL-cholesterol); hypertension (≥130/85 mmHg or antihypertensive drug treatment in a patient with history of hypertension); and high fasting glucose levels (fasting glucose≥100 mg/dl or drug treatment for elevated glucose)( Reference Beltrán-Sánchez, Harhay and Harhay 2 ).
Exposure variables
For laboratory serum assessment, blood samples were collected by venepuncture in the mobile examination clinics (MEC) according to the standard protocol. Serum was separated by centrifugation after samples were kept at room temperature for 30–60 min. Serum was frozen at −20°C and transported on dry ice to the CDC laboratory. Serum concentrations of trans-lycopene (µmol/l) were measured using HPLC with multi-wavelength photodiode-array absorbance detection( 23 ). The tertile rank method was used to divide 13 196 participants into three groups according to serum concentrations of lycopene (Fig. 1).
Co-variables
As the prevalence of the metabolic syndrome and serum lycopene could be related to the demographic characteristics and some risk factors, statistical analyses need to take into account these common variables. Variables included race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), sex, age group (20–39, 40–59 and ≥60 years), BMI (BMI<24·9 kg/m2, 25 kg/m2≤BMI<29·9 kg/m2 and BMI≥30 kg/m2), smoking status (non-smoker, past smoker and current smoker), alcohol consumption status (non-alcohol consumption, moderate alcohol consumption and heavy alcohol consumption) and physical activity (physical activity was categorised into lack of physical activity, moderate physical activity and heavy physical activity. The categories of physical activity were defined based on the following two questions (1) ‘Over the past 30 d, did you do any vigorous activities for at least 10 min that caused heavy sweating, or large increases in breathing or heart rate?’ and (2) ‘Over the past 30 d, did you do moderate activities for at least 10 min that cause only light sweating or a slight to moderate increase in breathing or heart rate?’. If the answer to the first questions was ‘yes’, participants were classified as ‘heavy physical activity’. If the answer to the second question was ‘yes’ and the answer to the first question was anything other than ‘yes’, participants were classified as ‘moderate physical activity’. If the answers to both questions were ‘no’ or ‘unable to do activity’, participants were classified as ‘lack of physical activity’).
In addition, dietary intakes were also included in the model. For dietary intakes, NHANES collected two 24-h dietary recalls for every participant by using the United States Department of Agriculture’s Automated Multi-Pass Method. The first 24-h recall was conducted at the MEC; the second 24-h recall was conducted by telephone 3–10 d later. The dietary intakes from the 2 d of the 24-h recall were averaged to estimate the following: (1) milk and milk products (g), (2) meat, poultry, fish and mixtures (g), (3) eggs (g), (4) legumes, nuts and seeds (g), (5) grain products (g), (6) fruits (g), (7) vegetables (g), (8) fats, oils and salad dressings (g), (9) sugars and sweets (g), (10) total dietary fibre (g) and (11) total energy intake (kJ/kcal).
Statistical analysis
The NHANES sample represents the total non-institutionalised civilian population residing in the fifty states and the District of Columbia. A four-stage sample design was used in NHANES. SAS Survey Procedures (i.e. proc surveyfreq and proc surveymeans) were used to take into account survey clusters, strata and weights (SAS version 9.3, SAS Institute). χ 2 Tests were used to examine the associations between the metabolic syndrome and race/ethnicity, sex, age and BMI status. The mean and standard deviation were used for serum concentrations of lycopene. In addition, logistic regression models were performed to evaluate the association between the prevalence of the metabolic syndrome and serum concentrations of lycopene and to calculate the OR and 95 % CI after adjusting for race, sex, age group, alcohol consumption, smoking status and physical activity. A two-sided P-value<0·05 was considered to be statistically significant.
Results
Demographic characteristics, BMI status, intake of dietary components and serum lycopene levels of individuals with the metabolic syndrome and those without the metabolic syndrome
Of the 13 196 participants, 4330 (32·8 %) had a diagnosis of the metabolic syndrome. The prevalence of each the metabolic syndrome condition was as follows: abdominal obesity (OR 50·3 %; 95 % CI 48·7, 51·9), hypertriacylglycerolaemia (OR 36·2 %; 95 % CI 34·9, 37·4), low HDL-cholesterol (OR 37·1 %; 95 % CI 35·8, 38·5), hypertension (OR 39·1 %; 95 % CI 37·7, 40·4) and high fasting glucose levels (OR 21·3 %; 95 % CI 19·8, 22·8). Demographic characteristics, BMI status, intake of dietary components and serum lycopene levels of individuals with the metabolic syndrome and those without the metabolic syndrome are listed in Table 1.
Table 1 Demographic characteristics, BMI status, intake of dietary components and serum lycopene levels of individuals with the metabolic syndrome and those without the metabolic syndromeFootnote * (Numbers and percentages; mean values and standard deviations)

* χ2 Tests were used to examine the associations between the metabolic syndrome and race/ethnicity, sex, age, BMI status, alcohol consumption, smoking status and physical activity. The mean and standard deviation was used for intake of dietary components and serum concentration of lycopene. The data were adjusted for clusters and strata of the complex sample design of the National Health and Nutrition Examination Survey 2001–2006, with incorporation of sample weight.
There was a significant difference in racial/ethnic characteristics between these two groups. For example, the metabolic syndrome group had a higher proportion of white Americans than the non-metabolic syndrome group (75·5 v. 71·1 %). Participants with the metabolic syndrome tended to be older than those without the metabolic syndrome. For example, 36·0 % of those with the metabolic syndrome were in the oldest age group (≥60 years) compared with 15·2 % of those without the metabolic syndrome. There was a substantially higher percent of obese individuals among participants with the metabolic syndrome (58·9 %) than that among participants without the metabolic syndrome (20·2 %). The percent of alcohol consumption (66·2 %) was lower among participants with the metabolic syndrome than that (76·0 %) among participants without the metabolic syndrome. The percent of past smokers (30·7 %) was higher among participants with the metabolic syndrome than that (22·5 %) among participants without the metabolic syndrome. There was a lower percentage of individuals with heavy physical activity among participants with the metabolic syndrome (22·2 %) than that (40·4 %) among participants without the metabolic syndrome.
For intake of dietary components, there were no significant differences between participants with the metabolic syndrome and participants without the metabolic syndrome in relation to dietary milk and milk products, meat, poultry, fish and mixtures, eggs, legumes, nuts and seeds, grain products, vegetables, fats, oils and salad dressings, and sugars and sweets. There were significantly lower means of dietary fibre, fruits and total energy intake among participants with the metabolic syndrome than among participants without the metabolic syndrome. The mean serum concentration of lycopene was significantly lower in participants with the metabolic syndrome (0·38 (sd 0·20) µmol/l) than participants without the metabolic syndrome (0·43 (sd 0·21) µmol/l) (Table 1).
Significant interaction between the levels of serum lycopene and BMI status on the metabolic syndrome
As mentioned above, the mean of serum lycopene concentration was significantly lower in participants with the metabolic syndrome than in participants without the metabolic syndrome. To further estimate the association between the prevalence of the metabolic syndrome and serum levels of lycopene, three groups of participants were divided by the tertile rank method according to serum concentrations of lycopene. The mean serum concentration of lycopene was 0·206 µmol/l (95 % CI 0·203, 0·209) for the first tertile group, 0·387 µmol/l (95 % CI 0·385, 0·389) for the second tertile group and 0·642 µmol/l (95 % CI 0·635, 0·648) for the third tertile group.
The prevalence of the metabolic syndrome was significantly higher in the first tertile group (OR 38·6 %; 95 % CI 36·9, 40·3) compared with the second tertile group (OR 29·3 %; 95 % CI 27·5, 31·1) and the third tertile group (OR 26·6 %; 95 % CI 24·9, 28·3).
To avoid possible confounding bias between the prevalence of the metabolic syndrome and the serum levels of lycopene, a multivariate logistic analysis was performed to evaluate the associations between the prevalence of the metabolic syndrome and serum levels of lycopene. After adjusting for race, sex, age, BMI status, alcohol consumption, smoking status, physical activity, milk and milk products, meat, poultry, fish and mixtures, eggs, legumes, nuts and seeds, grain products, fruits, vegetables, fats, oils and salad dressings, sugars and sweets, fibre and total energy intake, there was still a significant association between the metabolic syndrome and the levels of serum lycopene (Table 2). Most importantly, there was a significant interaction effect between serum lycopene and BMI status on the metabolic syndrome (P<0·0001).
Table 2 A multivariate logistic model for the associations between the prevalence of the metabolic syndrome and serum levels of lycopeneFootnote * (Odds ratios and 95 % confidence intervals)

* The mean serum concentration of lycopene was 0·206 µmol/l (95 % CI 0·203, 0·209) for the first tertile, 0·387 µmol/l (95 % CI 0·385, 0·389) for the second tertile and 0·642 µmol/l (95 % CI 0·635, 0·648) for the third tertile. The data were adjusted for clusters and strata of the complex sample design of the National Health and Nutrition Examination Survey 2001–2006, with incorporation of sample weight. There were significant interactions between serum levels of lycopene and BMI status (P<0·0001).
Association between the metabolic syndrome and the levels of serum of lycopene stratified by BMI status
There was a significant interaction effect between serum lycopene levels and the BMI status on the metabolic syndrome. Therefore, BMI is an effect modifier of the association between serum lycopene levels and the metabolic syndrome. With effect modification, stratified analysis is an appropriate method to examine the association between exposure and outcome. The associations between serum lycopene levels and the metabolic syndrome stratified by BMI status are shown in Fig. 2.

Fig. 2 The association between the metabolic syndrome and serum concentration of lycopene stratified by BMI status. The mean serum concentration of lycopene was 0·206 µmol/l (95 % CI 0·203, 0·209) for the first tertile, 0·387 µmol/l (95 % CI 0·385, 0·389) for the second tertile and 0·642 µmol/l (95 % CI 0·635, 0·648) for the third tertile. χ2
Tests were used to examine the associations between metabolic syndrome and serum lycopene. a The prevalence of the metabolic syndrome was significantly different (P<0·05) between the first and second tertiles for BMI<24·9 kg/m2. b The prevalence of the metabolic syndrome was significantly different (P<0·05) between the first and the third tertiles for BMI<24·9 kg/m2. c The prevalence of the metabolic syndrome was significantly different (P<0·05) between the first and the second tertiles for BMI: 25–29·9 kg/m2; d The prevalence of the metabolic syndrome was significantly different (P<0·05) between the first and the third tertiles for BMI: 25–29·9 kg/m2. The data were adjusted for clusters and strata of the complex sample design of the National Health and Nutrition Examination Survey 2001–2006, with incorporation of sample weight. ![]() , 24·9 kg/m2;
, 24·9 kg/m2; ![]() , 25–29·9 kg/m2;
, 25–29·9 kg/m2; ![]() , ≥30 kg/m2
, ≥30 kg/m2
For normal-weight participants, the prevalence of the metabolic syndrome was significantly lower in the third tertile group (OR 4·5 %; 95 % CI 3·2, 5·7) compared with the first tertile group (OR 12·9 %; 95 % CI 10·8, 14·9), and the prevalence of the metabolic syndrome was significantly lower in the second tertile group (5·6 %; 95 % CI 4·3, 6·9) compared with the first tertile group (OR 12·9 %; 95 % CI 10·8, 14·9).
For overweight participants, the prevalence of the metabolic syndrome was significantly lower in the third tertile group (OR 25·0 %; 95 % CI 22·6, 27·3) compared with the first tertile group (OR 39·7 %; 95 % CI 36·6, 42·7), and the prevalence of the metabolic syndrome was significantly lower in the second tertile group (OR 28·5 %; 95 % CI 26·1, 30·9) compared with the first tertile group (39·7 %; 95 % CI 36·6, 42·7).
However, for obese participants, there was no significant difference in the prevalence of the metabolic syndrome among the first tertile group (OR 60·3 %; 95 % CI 57·6, 62·9), the second tertile group (OR 56·1 %; 95 % CI 52·5, 59·6) and the third tertile group (OR 53·8 %; 95 % CI 48·9, 58·7).
To remove the possible confounding bias between the prevalence of the metabolic syndrome and the levels of serum lycopene, multivariate logistic models were performed to evaluate the associations between the prevalence of the metabolic syndrome and serum levels of lycopene for each BMI status group (Table 3). After adjusting for race, sex, age, alcohol consumption, smoking status, physical activity, milk and milk products, meat, poultry, fish and mixtures, eggs, legumes, nuts and seeds, grain products, fruits, vegetables, fats, oils and salad dressings, sugars and sweets, fibre and total energy intake, there were still significant associations between the metabolic syndrome and serum levels of lycopene among participants who were normal weight or overweight.
Table 3 Multivariate logistic models for the associations between the prevalence of the metabolic syndrome and serum levels of lycopene by BMI statusFootnote * (Odds ratios and 95 % confidence intervals)
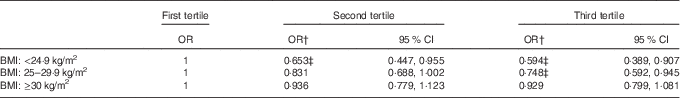
* The mean serum concentration of lycopene was 0·206 µmol/l (95 % CI 0·203, 0·209) for the first tertile, 0·387 µmol/l (95 % CI 0·385, 0·389) for the second tertile and 0·642 µmol/l (95 % CI 0·635, 0·648) for the third tertile.
† Adjusting for race, sex, age, alcohol consumption, smoking status, physical activity, milk and milk products, meat, poultry, fish and mixtures, eggs, legumes, nuts and seeds, grain products, fruits, vegetables, fats, oils and salad dressings, sugars and sweets, fibre and total energy intake. The data were adjusted for clusters and strata of the complex sample design of the National Health and Nutrition Examination Survey 2001–2006, with incorporation of sample weight.
‡ Statistically significant values.
Mean intake of dietary components from food and beverages by BMI status and serum levels of lycopene
Individuals who are overweight and obese tend to have less healthy dietary habits. For example, usually, overweight and obese individuals consume too much energy and consume much less fruits and vegetables( Reference He, Hu and Colditz 24 , Reference Sartorelli, Franco and Cardoso 25 ). To further compare the difference of dietary habits among individuals with different serum levels of lycopene, we also estimated intake of dietary components from foods and beverages by BMI status and serum levels of lycopene (Table 4). Serum levels of lycopene were mainly associated with dietary intake of lycopene for all BMI statuses. For example, the mean intake of dietary lycopene significantly increased from 3·6 mg/d (in first tertile group) to 5·7 mg/d (in second tertile) to 8·5 mg/d (in third tertile) for normal-weight participants. For obese participants, the mean intake of dietary lycopene also significantly increased from 3·7 mg/d (in first tertile group) to 5·8 mg/d (in second tertile) to 8·6 mg/d (in third tertile). In addition to dietary lycopene, participants in the third tertile group had higher intake of dietary fibre, meat, poultry, fish and mixtures, grain products, vegetable and total energy than participants in the first tertile group for all BMI status.
Table 4 Mean intake of dietary components from food and beverages by BMI status and serum levels of lycopeneFootnote * (Numbers, mean values and 95 % confidence intervals)

* The mean serum concentration of lycopene was 0·206 µmol/l (95 % CI 0·203, 0·209) for the first tertile, 0·387 µmol/l (95 % CI 0·385, 0·389) for the second tertile and 0·642 µmol/l (95 % CI 0·635, 0·648) for the third tertile. The data were adjusted for clusters and strata of the complex sample design of the National Health and Nutrition Examination Survey 2001–2006, with incorporation of sample weight.
Discussion
To our knowledge, this is the first study to examine the role of BMI on the association between the prevalence of the metabolic syndrome and the levels of serum lycopene. Consistent with the findings of previous studies( Reference Sluijs, Beulens and Grobbee 20 – Reference Liu, Shi and Cao 22 ), higher serum concentrations of lycopene are associated with the reduced prevalence of the metabolic syndrome. However, the associations are only significant for participants who are normal weight and overweight, but not significant for participants who are obese. Our results are similar to the findings of a previous study, which showed that the effect of lycopene was not significant for obese participants( Reference Ghavipour, Saedisomeolia and Djalali 18 , Reference Ghavipour, Sotoudeh and Ghorbani 19 ). These studies explored the effect of lycopene on inflammation and anti-oxidative biomarkers between overweight and obese individuals( Reference Ghavipour, Saedisomeolia and Djalali 18 , Reference Ghavipour, Sotoudeh and Ghorbani 19 ). Our present study provides further evidence that BMI status has an important influence on the association between serum lycopene and health outcome – the metabolic syndrome.
In addition to demographic characteristics, some other factors such as alcohol consumption( Reference Xiao, Huang and Xu 26 ), smoking status( Reference Jia 27 ), physical activity( Reference Moore, Davis and Baxter 28 ) and dietary components( Reference Pot, Hardy and Stephen 29 – Reference Esmaillzadeh, Kimiagar and Mehrabi 31 ) have significant associations with the metabolic syndrome. Therefore, to remove the possible confounding bias between the prevalence of the metabolic syndrome and the levels of serum lycopene, in the present study, we evaluated the association between the prevalence of the metabolic syndrome and serum levels of lycopene after adjusting for race, sex, age, alcohol consumption, smoking status, physical activity, milk and milk products, meat, poultry, fish and mixtures, eggs, legumes, nuts and seeds, grain products, fruits, vegetables, fats, oils and salad dressings, sugars and sweets, fibre and total energy intake. Consistent with the above results, the association between the prevalence of the metabolic syndrome and serum levels of lycopene was still significant after adjusting for demographic characteristics and these related risk factors.
Andersen et al.( Reference Andersen, Jacobs and Gross 32 ) reported an inverse correlation between BMI and serum carotenoids including carotenes, cryptoxanthin and lutein, except for lycopene. The authors found that the relationship between BMI and serum lycopene was weak. In our study using a larger sample size, the protective effect of lycopene was associated with normal BMI<24·9 kg/m2 and BMI between 25 and 29·9 kg/m2 but not with BMI>30 kg/m2. It is also likely that individuals with normal BMI consume a healthier diet than obese individuals, and, as such, these factors may include consuming less energy content and more intake of fruits and vegetables. Therefore, it is also possible that other dietary factors can contribute to the association between lycopene and the metabolic syndrome. With increased BMI, participants are prone to have more inflammation and oxidative stress( Reference Ghavipour, Saedisomeolia and Djalali 18 , Reference Ghavipour, Sotoudeh and Ghorbani 19 ). In addition, the antioxidant enzymes such as decreased SOD, glutathione peroxidase and oxygenase-2( Reference Bełtowski, Wójcicka and Górny 33 – Reference Lee, Kim and Choi 35 ) as well as the total plasma antioxidant capacity are suppressed in those with overweight and obesity condition. The biological mechanism by which lycopene reduces the risk of the metabolic syndrome mainly depends on alleviating oxidative stress and decreasing inflammation( Reference Sluijs, Beulens and Grobbee 20 – Reference Liu, Shi and Cao 22 ). Therefore, with the equal level of serum lycopene, the effects of serum lycopene on the prevalence of the metabolic syndrome are only significant for participants who are normal weight or overweight, but not significant for participants who are obese. In addition, after absorption from the intestine, 60–72 % of lycopene is distributed in adipose tissue( Reference Moran, Erdman and Clinton 36 ). Lycopene concentrations at different adipose tissue sites (abdomen, buttocks and thighs) have positive correlations with serum lycopene concentrations( Reference Chung, Ferreira and Epstein 37 ). As lycopene is lipid soluble, it is possible that lycopene may be sequestered in the adipose tissue with higher BMI (>30 kg/m2) leading to a decline in its antioxidant capacity in obese individuals.
However, accumulating evidence also supports that lycopene inhibits inflammation and oxidative stress in a dose-dependent manner( Reference Park, Hwang and Moon 38 , Reference Kang, Park and Seo 39 ). Therefore, we propose that highly efficient serum concentrations of lycopene may be needed to produce significant effects on participants who are obese. However, due to the observational study, the highest serum concentration of lycopene was only 0·642 µmol/l in the third tertile group, which might not be sufficient to elucidate protective effects in obese participants. In agreement, our results support the notion that measuring serum concentrations of lycopene may not be sufficient to elucidate an effect of lycopene in those with high BMI, because the prevalence of the metabolic syndrome is not significantly reduced in the third tertile group when compared with the second tertile group for normal-weight and overweight participants.
Therefore, new analysis methods for serum lycopene assessment (e.g. relative serum lycopene to the amount of inflammation and oxidative stress in the body) or further clinical trials are needed to confirm the hypothesis. Measuring serum lycopene concentration relative to the amount of inflammation and oxidative stress will take into account two important factors related to the effects of lycopene, serum concentration of lycopene and the amount of inflammation and oxidative stress in the body. In addition, serum concentration of lycopene can be high enough in clinical trials when subjects take more fruits and vegetables enriched in lycopene or more lycopene supplement, because our results show that serum levels of lycopene were mainly associated with dietary intake of lycopene for all BMI status.
There are several limitations to this study. First, the cross-sectional study cannot build a causal association between serum lycopene and the metabolic syndrome. Second, the cross-sectional study can lead to a prevalence–incidence bias. Third, the effect of serum lycopene may be underestimated in the present study. Fourth, although race, sex, age, alcohol consumption, smoking status, physical activity, milk and milk products, meat, poultry, fish and mixtures, eggs, legumes, nuts and seeds, grain products, fruits, vegetables, fats, oils and salad dressings, sugars and sweets, fibre and total energy intake were taken into account between the prevalence of the metabolic syndrome and serum lycopene, there is a possible residual confounding by other unmeasured covariates.
In summary, these findings from a nationally representative sample of US adults indicate that BMI status has an important influence on the association between serum lycopene levels and the metabolic syndrome. Current serum concentration of lycopene does not have a significant effect on the prevalence of the metabolic syndrome among obese individuals. New analysis methods for the determination of serum lycopene levels (e.g. serum lycopene concentration relative to the amount of inflammation and oxidative stress) or further clinical trials are needed to test the effects of serum lycopene among obese individuals.
Acknowledgements
All the authors contributed to the study design. In addition, G.-M. H. performed statistical analyses and wrote the manuscript. J. L. M., G. A. S., K. M. M. I. and S. W. revised the manuscript. All the authors read and approved the final manuscript.
None of the authors reported a conflict of interest related to the study.









