Introduction
Echinococcosis, listed as one of the twenty neglected tropical diseases by the World Health Organization (WHO) and targeted for control for over several decades, remains an important parasitic disease caused by the tapeworm genus Echinococcus (World Health Organization 2010, 2023). This disease poses a significant threat to both public health and the livestock industry (McManus et al. Reference McManus, Zhang, Li and Bartley2003; Rong et al. Reference Rong, Fan, Zhu and Zheng2021). Globally, it is estimated that over one million people are affected by echinococcosis at any given time (Vuitton & Brunetti Reference Vuitton, Millon, Manciulli, Brunetti and Sing2015; Casulli et al. Reference Casulli, Siles-Lucas, Cretu, Vutova, Akhan, Vural, Cortés Ruiz, Brunetti and Tamarozzi2020; Ahmed et al. Reference Ahmed, Ali, Afzal, Khan, Raza, Shah and Simsek2017). As a zoonotic disease, the annual cost of treatment and losses to the livestock industry is estimated to be $760 million, imposing a substantial economic burden to society (Cadavid Restrepo et al. Reference Cadavid Restrepo, Yang, McManus, Gray, Giraudoux, Barnes, Williams, Soares Magalhães, Hamm and Clements2016; Casulli et al. 2023). In western China, echinococcosis is also highly endemic, with an estimated 50 million people at risk of infection, causing annual losses of at least US$ 0.66 billion (Qian et al. Reference Qian, Abela-Ridder, Wu and Zhou2017). Many regions around the world have implemented control measures to reduce the burden of echinococcosis (Wen et al. Reference Wen, Vuitton, Tuxun, Li, Vuitton, Zhang and McManus2019; Casulli et al. Reference Casulli, Siles-Lucas, Cretu, Vutova, Akhan, Vural, Cortés Ruiz, Brunetti and Tamarozzi2020; Amarir et al. Reference Amarir, Rhalem, Sadak, Raes, Oukessou, Saadi, Bouslikhane, Gauci, Lightowlers, Kirschvink and Marcotty2021). The Chinese government has also consistently implemented measures to alleviate the disease and control its transmission. Monitoring epidemiological trends and evaluating the effectiveness of those preventive and control strategies can facilitate control of the disease spreading locally and globally.
Cystic echinococcosis (CE) and alveolar echinococcosis (AE), caused by species within the E. granulosus sensu lato cluster and E. multilocularis, respectively, represent the primary burden of echinococcosis (McManus et al. Reference McManus, Zhang, Li and Bartley2003; Vuitton & Brunetti Reference Vuitton, Millon, Manciulli, Brunetti and Sing2015). Despite the different hosts involved in the transmission of E. granulosus sensu and E. multilocularis, they share a similar life cycle. Human infection occurs inadvertently through the ingestion of food and water contaminated with parasite eggs or through direct contact with infected definitive hosts (Paternoster et al. Reference Paternoster, Boo, Wang, Minbaeva, Usubalieva, Raimkulov, Zhoroev, Abdykerimov, Kronenberg, Müllhaupt, Furrer, Deplazes and Torgerson2020; Torgerson et al. Reference Torgerson, Robertson, Enemark, Foehr, van der Giessen, Kapel, Klun and Trevisan2020). Once ingested, the oncospheres within the eggs further develop into cysts (CE) or tumor-like lesions (AE), primarily in the liver (Romig et al. Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). The infection can remain asymptomatic for years until it reaches a point that triggers clinical signs or is unexpectedly detected during hospital examinations (Casulli et al. Reference Casulli, Siles-Lucas, Cretu, Vutova, Akhan, Vural, Cortés Ruiz, Brunetti and Tamarozzi2020). Severe clinical syndromes can be life-threatening if mismanaged.
Treatment options for echinococcosis include surgery, chemotherapy, and a “watch-and-wait” approach (Wen et al. Reference Wen, Vuitton, Tuxun, Li, Vuitton, Zhang and McManus2019). However, the optimal preventive and curative methods for different types or classifications of echinococcosis remain limited and controversial (Stojković et al. Reference Stojković, Weber and Junghanss2018; Maimaitinijiati et al. Reference Maimaitinijiati, Meng and Chen2022). Emphasis on prevention of infection and disease occurrence could contribute to the reduction of the global echinococcosis burden. The lack of epidemiological data on echinococcosis hinders the implementation of disease control measures. In this work, we examined the echinococcosis infection rate from 2004 to 2022, obtained from the Chinese Center for Disease Control and Prevention (CDC in China), the State Council of the People’s Republic of China, and the China CDC Weekly. Subsequently, we conducted a comprehensive analysis of infection trends over the past several decades. By reviewing the prevention and control policies of the Chinese government at different periods, we summarized China’s practical experience in prevention and control, which may offer insights for future strategies. We hope that our work will support scientific research priorities and facilitate the exploration of echinococcosis prevention and control strategies in other endemic regions of the world.
Materials and Methods
Data sources
The CDC in China records notifiable infectious diseases including laboratory-confirmed and clinically diagnosed cases of echinococcosis. We obtained national data on echinococcosis infection between 2004 and 2020. The data include the annual number of new cases, the incidence rate (IR), and the annual number of new cases by age group (https://www.phsciencedata.cn/Share/en/data.jsp?id=6e5c0cdc-5bb4-49b6-affc-631837d539f9&show=0). To ensure comprehensive data coverage, we also searched The State Council of the People’s Republic of China and China CDC Weekly for echinococcosis infection data between 2020 and 2022 (http://www.nhc.gov.cn/jkj/s3578/202103/f1a448b7df7d4760976fea6d55834966.shtml, http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml, http://www.nhc.gov.cn/wjw/gongb/list.shtml, https://weekly.chinacdc.cn/). Ultimately, we included 19 years of diagnosed echinococcosis cases and IR from 2004 to 2022 as reported in these databases.
Statistical analysis
For the primary analyses, we assessed the IR, defined as the number of newly diagnosed cases of echinococcosis per year per 100,000 population at risk (IR = number of new cases/population at risk * 100000, Centers for Disease Control and Prevention 2020). Population data were obtained from the National Bureau of Statistics of China. We used a joinpoint regression model to examine incidence trends from 2004 to 2020 and to identify changes in trends in echinococcosis incidence. The permutation test was used to select the best-fitting model (Kim et al. Reference Kim, Fay, Feuer and Midthune2000). The maximum number of joinpoints was limited to three based on the number of our data points (National Cancer Institute Surveillance Research Program 2023). Annual percentage changes (APC) and the average annual percent change (AAPC) in incidence were calculated and the parametric method was used to calculate 95% CIs. A P value less than 0.05 was considered statistically significant. The pattern of incidence across geographic regions, defined as provinces, and age group of patients, with no upper age limit, were shown by heatmaps. Joinpoint regression analysis was conducted with the Joinpoint Regression Program (version 4.8.0.1; National Cancer Institute, MD, USA). Schematic diagrams were created using Python (version 3.12.0), BioRender.com, and Adobe Illustrator CC 2019 (Adobe Inc., San Jose, CA, US).
Results and Discussion
Endemic features of Echinococcosis in China
As illustrated in the joinpoint regression model, national echinococcosis IR over the 19-year period in China demonstrated an overall increasing trend, with an AAPC of 9.0593% (95% CI [5.2423, 13.0148]; P < 0.05; Table S5). However, the pattern of trend varied. The infection of echinococcosis showed an increasing trend between 2004 and 2017, reaching a peak incidence of 0.3975 per 100,000 person-years in 2017 (National Population and Health Science Data Sharing Platform 2023). The joinpoint regression analysis indicated an APC of 67.366% (95% CI [40.7818, 98.9703], P < 0.05) from 2004–2007 and an APC of 6.1189% (95% CI [2.8209, 9.5246], P < 0.05) from 2007–2017. However, two notable declines occurred from 2007 to 2010 and again from 2013 to 2014, resulting in a IR decrease from 0.2441 to 0.1884 and 0.2837 to 0.2482 per 100,000 person-years, respectively. Incidence in 2017 was the highest recorded, almost four times higher than that in 2004 (National Population and Health Science Data Sharing Platform 2023). In 2022, the number of new cases of echinococcosis in China decreased by 49.95%, and the IR decreased by 51.09% compared with 2017, with an APC of -10.9174% (95% CI [17.5487, 3.7529], P < 0.05) (Figure 1A, Table S1, and Table S4). Specifically, there were 5485 newly reported cases with an incidence rate of 0.3975 per 100,000 person-years in 2017. By 2022, these numbers were reduced to 2745 reported cases with an incidence rate of 0.1944 per 100,000 person-years (National Population and Health Science Data Sharing Platform 2023).
In China, the progression of echinococcosis prevention and control can be divided into three distinct phases. In the first two phases, national incidence exhibited an overall upward trend, but there was a notable deceleration of increase in IR between 2007 and 2017 (Figure 1A). The underestimation of IR in the early phase may be attributed to limited screening techniques and methods. Early diagnostic methods for suspected patients mainly relied on ultrasound and serology (Craig et al. Reference Craig, Hegglin, Lightowlers, Torgerson and Wang2017). Unfortunately, the serological tests for echinococcosis were neither standardized nor adapted for epidemiological surveys (Siles-Lucas et al. Reference Siles-Lucas, Casulli, Conraths and Müller2017; Tamarozzi et al. Reference Tamarozzi, Silva, Fittipaldo, Buonfrate, Gottstein and Siles-Lucas2021). Poor public awareness of physical examination also contributed to lower detection rates in the early phase (Qian et al. Reference Qian, Chen, Bergquist, Li, Li, Xiao, Utzinger and Zhou2019). Later, advance in screening techniques and increased financial investment led to a gradual increase in incidence later on (Yu et al. Reference Yu, Xiao, Han, Tian and Zhou2020).
The trend of infection in areas with high incidence (where the total number of cases exceeded 1000) mirrored that of the nation as a whole. In China, the endemic areas of Xinjiang, Sichuan, Qinghai, Gansu, Ningxia, Tibet, and Inner Mongolia accounted for 98.38%, 98.43%, and 97.83% of cases in 2017, 2018, and 2019, respectively. The average decrease in number of cases as a percentage of the endemic population was similar to that of the percentage of the national total population from 2017 to 2019, which was 27.43% (from 5396 to 3916 cases) and 27.02% (from 5485 to 4003), respectively. The average decrease in incidence rate by population in the endemic regions was also similar to that of the national total population from 2017 to 2019, which was 28.38% (from 3.1 to 2.22 per 100,000 person-years) and 27.89% (from 0.3975 to 0.2866 per 100,000 person-years), respectively (Figure 1B, Table S1, and Table S2). The reasons underlying the disparity in echinococcosis infection rates may be attributable to various factors, such as socio-economic conditions, lack of education, limited access to health services, suboptimal hygiene practices, and direct interaction with animal hosts. For example, according to the National Bureau of Statistics (2021), Qinghai has a total grassland area of 36,369,700 hectares, of which 9,215,500 hectares are infested with rodents. This accounts for about 25% of the total grassland area, which is the highest among the seven provinces. The large rodent population facilitates the transmission of E. multilocularis. In the case of Xinjiang, the largest autonomous region in China by area, 2021 data show a population of 25.89 million with 256,700 health workers. This translates to 9.915 healthcare workers per 1,000 people, which is the lowest among the seven provinces (National Bureau of Statistics 2021).
A subgroup analysis of patient demographics revealed that the majority of affected individuals were in the 40–49 year age group (Figure 1C). Echinococcosis is a chronic disease characterized by years of asymptomatic or minimally symptomatic disease, resulting in delayed diagnosis. Consequently, the prevalence of echinococcosis is higher among middle-aged and elderly populations. Given the high incidence of liver cancer in this age group, clinicians should differentiate between the two when diagnosing hepatic space-occupying lesions, especially in endemic areas.

Figure 1. National incidence of echinococcosis and demographic characteristics. (A) Temporal trends in national incidence, 2004–2022. * Indicates that the Annual Percent Change (APC) is significantly different from zero at the alpha = 0.05 level. (B) Geographic distribution of echinococcosis in 2019. The heatmap shows the temporal changes in incidence of the disease at the provincial level from 2004–2019. (C) Age distribution and temporal changes in incidence from 2004 to 2019.
Prevention and control methods of Echinococcosis in China
In 2005, the Chinese government launched a national campaign to prevent and control echinococcosis, focusing on the management of intermediate and definitive hosts, and education to improve public awareness. The detailed and comprehensive measures included management of stray dog populations in endemic areas, deworming of house dogs, safe handling of contaminated or potentially contaminated livestock at slaughtering sites, and personal hygiene and knowledge of the echinococcus infection. In addition, community surveillance and early detection programs, including free screening and treatment, have been implemented (Qian et al. Reference Qian, Chen, Bergquist, Li, Li, Xiao, Utzinger and Zhou2019; Zheng et al. Reference Zheng, Yang, Zhang, Wang, Wu and Yan2020; Rong et al. Reference Rong, Fan, Zhu and Zheng2021). In our analysis, 2004–2016 IR showed significantly decreased APC. A systematic review and meta-analysis of AE prevalence in China showed a resurgence from 2005 to 2007, then a decrease from 2007 to 2010, followed by low but stable prevalence (Wang et al. Reference Wang, Dai, Li, Jia, Guo and Lu2020). This pattern is in line with our analysis of the AE and CE bulk IR during the same period (Figure 1A), indicating the effectiveness of those control measures, particularly the dog deworming that was implemented as of 2005.
Although a separate data set of epidemiology on nationwide CE is unavailable, there is a report of an increase of CE prevalence from 0.09% to 1.67% from 2012 to 2016 and a decrease of CE prevalence to 0.03% from 2016 to 2018 in Sichuan, one of the high endemic regions of echinococcosis in China, where CE accounted for 75.67% of echinococcosis cases based on 2012 surveillance data (Fu et al. Reference Fu, Wang, Han, Guan, Bergquist and Wu2021). According to 2012–2016 national surveillance data, CE accounted for 78.28 % of echinococcosis cases in China (Wu et al. Reference Wu, Wang, Wang, Zhou, Wang, Zheng, Cao, Xiao, Wang, Zhu, Niu, Xue, Zeng, Fang, Han, Yu, Yang, Fu, Bai, Tian, Li, Zhang, Wu, Zhang, Hou, Feng, Ma, Li, Li, Guo, Yang, Wu, Jin, Zhang and Yu2018). The national bulk IR in our analysis also indicated an increase from 2010 to 2017, followed by a decline after year 2017 (Figure 1A). This decline correlated with the campaign of sheep vaccination with EG95 in seven endemic provinces and regions in northwestern China, which was initiated in 2016 (Larrieu et al. Reference Larrieu, Gavidia and Lightowlers2019; Sander et al. Reference Sander, Sánchez López, Mendoza Morales, Ramos Duarte, Corigliano and Clemente2020a, b). Following the implementation of various control measures, the national IR has consistently decreased. The Belt and Road Network for Elimination and Control of Echinococcosis and Cysticercosis was launched in 2017, with the goal of building research and development capacity to achieve the control targets for echinococcosis and Taenia solium cysticercosis by 2030 (Qian & Zhou Reference Qian and Zhou2018). Meanwhile, the new policy implemented in 2019 expanded the management and control area with the deworming of stray and wild canids, improved the deworming strategies that were adaptive to prevalence levels, and enhanced surveillance of infection incidence in wild animals. Improved early screening diagnostics and close follow-up of patient treatment were applied to the management and control program (Can-jun et al. 2020). Hygiene and sanitation facilities have also been improved significantly in most rural areas over the past few decades (Craig et al. Reference Craig, Hegglin, Lightowlers, Torgerson and Wang2017). The pattern of echinococcosis in bulk and that of CE and AE epidemiology correlated with the time points of implementation of dog deworming by drug distribution since 2005 and vaccination of livestock with Eg95 since 2016, respectively, demonstrating the efficiency of the control measures specific to Echinococcus spp.
Available data, particularly from the last six years, suggests that China’s long-term strategy for echinococcosis control has achieved impressive results. Strategies such as intermediate and definitive host controls have been proven to be effective and need to be continued. However, echinococcosis continues to pose a threat to both humans and animals, resulting in substantial economic losses, with 2,745 cases in 2022. Echinococcosis is chronic and its transmission is complex. It is impossible to eliminate the disease in a short term, and prevention and control programs may need to be adjusted over time to achieve greater success.
Future perspective
In the past, considerable efforts were dedicated to the prevention and control of echinococcosis in humans, livestock, and domestic dogs. A 2012–2016 national survey conducted in China revealed a total of 4,161 cases of CE and 1,055 cases of AE among 1,150,723 residents. Among the regions affected by echinococcosis, there were nine endemic provinces (autonomous regions): Inner Mongolia, Sichuan, Tibet, Gansu, Qinghai, Ningxia, Yunnan, Shaanxi, and Xinjiang (Ma et al. Reference Ma, Wang, Hao, Xue, Wang, Han, Wang, Zhao, Ma, Wu, Jiang, Cao, Yang, Feng, Gongsang, Scheffran, Fang, Maude, Zheng, Ding, Wu and Jiang2023). Currently, these nine provinces continue to bear the highest burden of human echinococcosis (Supplementary data). While deworming programs for dogs have been proven effective in village-based communities, the effectiveness in semi-nomadic or pastoral areas was limited (Wen et al. Reference Wen, Vuitton, Tuxun, Li, Vuitton, Zhang and McManus2019). Notably, the life cycle of the parasite can be completed in the wild, as illustrated in Figure 2, allowing for its survival. However, the control and treatment of echinococcosis infections in wildlife pose significant challenges, leading to potential cross-contamination between wild and domestic animals. Consequently, certain populations inevitably have close contact with infectious eggs in contaminated environments, making it difficult to distance themselves from sources of infection and achieve proper disease eradication. Therefore, we propose that it is essential to enhance disease prevention and control measures aimed at the eradication of echinococcosis in wild animals. We may also adapt control and prevention policies in the light of actual conditions and motivate all stakeholders involved.
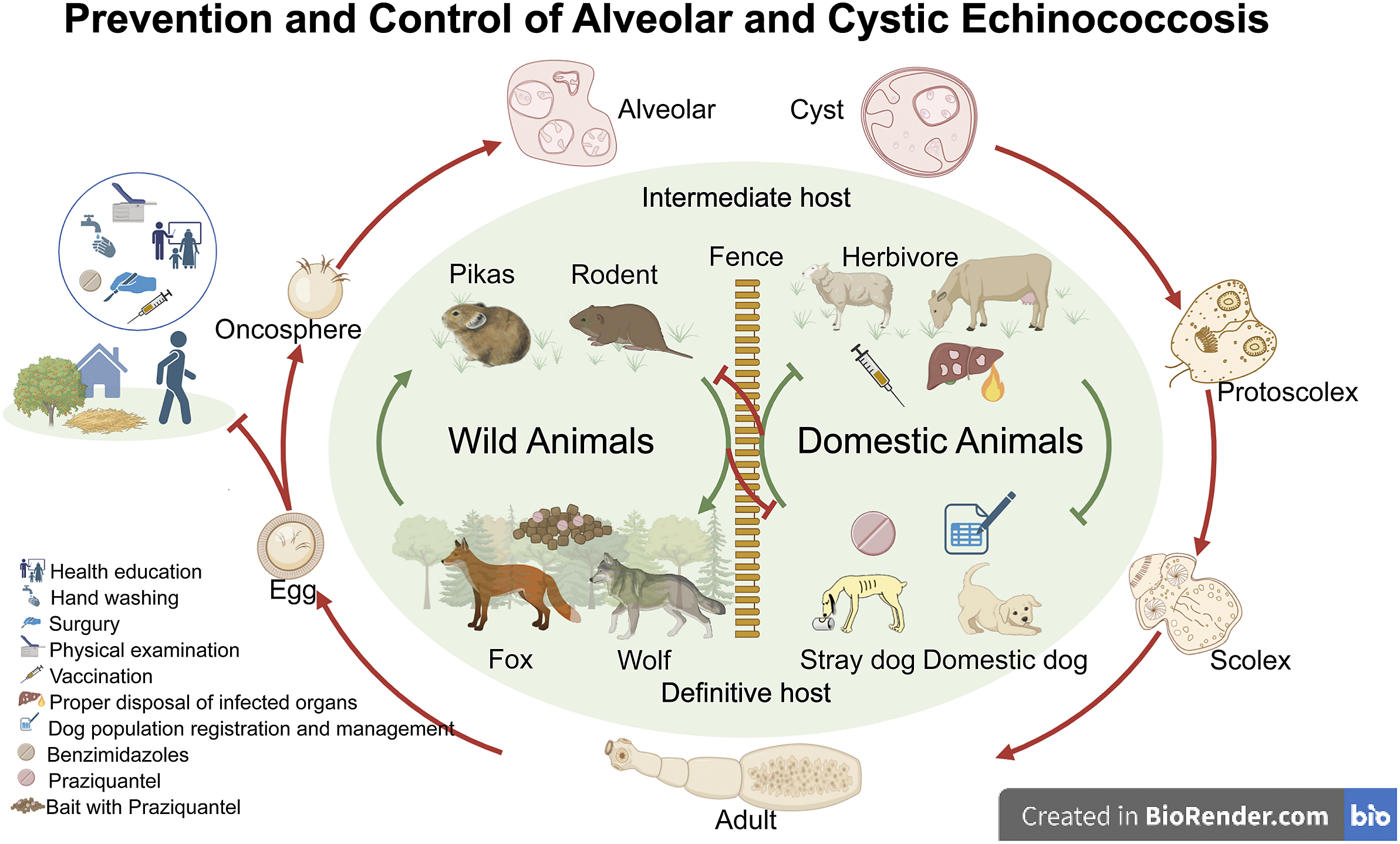
Figure 2. Prevention and control of echinococcosis in China. For outer circulation, we illustrate the morphology of genus Echinococcus and its hosts at different life stages. Echinococcus adult parasites reside in the upper small intestine of terminal hosts, such as dogs, wolves, and red foxes. Gravid proglottids, or eggs, are then excreted in faeces, resulting in contamination of water and grasslands. Intermediate hosts, including pika, vole, sheep, and cattle, become infected when they graze on contaminated grasslands. When infected intermediate hosts are preyed upon by the terminal hosts, the protoscolex in the echinococcus further develop into adults, reproduce, and lay eggs. Humans become infected with these eggs through accidental hand-to-mouth contact with contaminated food or water. The inner circulation depicts the transmission and life cycle of the genus Echinococcus among wild and domestic hosts. Existing control measures and recommended approaches are presented for each part. Created with BioRender.com.
Wildlife management
Canids, especially stray dogs, have been identified as the main definitive host of both E. granulosus and E. multilocularis, with the highest risk of transmitting CE and AE to humans in China due to their popularity, ability to roam freely in pastoral areas, habit of preying on slaughtered livestock, and close relationship with human activities (Wang et al. Reference Wang, Gongsang, Pang, Qin, Wang, Li, Frutos and Gavotte2022). The prevalence of CE in dogs varies from 0.45 to 52.78%, which is the highest among the animals surveyed (van Kesteren et al. Reference van Kesteren, Qi, Tao, Feng, Mastin, Craig, Vuitton, Duan, Chu, Zhu and Wen2015; Fu et al. Reference Fu, Wang, Han, Guan, Bergquist and Wu2021). Dogs can also carry E. multilocularis into a transmission ecosystem when they prey on small mammals (Craig Reference Craig2006; Wang et al. Reference Wang, Liu, Zuo, Mu, Weng, Sun, Wang, Boufana, Craig, Giraudoux, Raoul and Wang2018). Since the implementation of deworming of dogs and other canines as an approach for wildlife management from 2019, the national incidence rate decreased from 0.2866 to 0.1944 per 100,000 person-years in 2022, a decrease of 32.18% (Table S2). In Europe and Japan, where foxes were the predominant source of E. multilocularis for environmental contamination, with prevalence ranging from 17.7% to over 70%, deworming of foxes in the wild with praziquantel-containing baits reduced the burden of echinococcosis (Ito et al. Reference Ito, Romig and Takahashi2003; Romig et al. Reference Romig, Thoma and Weible2006; Hegglin & Deplazes Reference Hegglin and Deplazes2013). Deworming not only eliminates the environmental source of the parasitic pathogen, it also blocks the transmission of the pathogen from wildlife hosts to humans. We anticipate that continued and expanded implementation of wildlife deworming will further reduce the prevalence of echinococcosis in humans. In addition, completion of the parasite life cycle requires spatial overlap between intermediate hosts and definitive host faeces (Atkinson et al. Reference Atkinson, Gray, Clements, Barnes, McManus and Yang2013). The establishment of separate livestock activity areas in rangeland regions may help minimize spatial overlap between wildlife and livestock, potentially reducing contamination of grassland, thereby reducing the incidence of echinococcosis in livestock.
Case-based policy making
The dynamics of tapeworm transmission are complex (Figure 2). Interventions adapted to local conditions can be implemented based on several factors, including sex, occupation, education level, geography, animal density, numbers of animals slaughtered, altitude, pasture and forest area, temperature, and gross domestic product (GDP). These factors were found to be associated with the prevalence of echinococcosis (Ma et al. Reference Ma, Wang, Hao, Xue, Wang, Han, Wang, Zhao, Ma, Wu, Jiang, Cao, Yang, Feng, Gongsang, Scheffran, Fang, Maude, Zheng, Ding, Wu and Jiang2023). In regions with high incidence rates, it is essential to promote disease awareness among the population, especially through health education of preschool children and promotion of scientific knowledge among the elderly. In addition, it is crucial to strengthen disease screening among high-risk groups, such as increasing the frequency of screening and expanding the coverage of screening initiatives. The establishment of isolation zones should be considered in areas with extensive grasslands and forests, especially where wild animals frequently interact with domestic animals and humans. In addition, specific prevention and control measures can be formulated for targeted populations, including shepherds and farmers working in endemic areas.
Vaccine development
Vaccination holds promise as a long-term preventive measure. Vaccination of sheep with the Eg95 vaccine has been promoted as an intervention to reduce the disease burden in China (Larrieu et al. Reference Larrieu, Mujica, Gauci, Vizcaychipi, Seleiman, Herrero, Labanchi, Araya, Sepúlveda, Grizmado, Calabro, Talmon, Poggio, Crowley, Cespedes, Santillán, García Cachau, Lamberti, Gino, Donadeu and Lightowlers2015; Gauci et al. Reference Gauci, Jenkins and Lightowlers2022). Our data show that since the implementation of vaccination in 2016, the national incidence has declined annually (Figure 1). The Eg95 vaccine was developed to prevent E. granulosus infection (Lightowlers et al. Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Heath1996). However, we did not have segregated data available on changes in the incidence of AE and CE. Due to the lack of specific data, it is uncertain whether the implementation of vaccination alone has led to a significant reduction in the incidence of CE although studies have reported the effectiveness of vaccination, in general.
In addition to sheep, experiments of immunizing yaks have been conducted, and the vaccine has been shown to be effective in preventing CE infection (Ai-guo et al. Reference Ai-guo, Ming-zhong, Dong-bo, Li, Wei, Zhi-ping, Xi, Guang-qiong, Chao-hui, Guo-teng, Lin, Hao, Duo, Bo and Zheng2017; Yang et al. Reference Yang, Zhao, Li, Gu, Zhang, Du, Cai, Li, Zhang, Zhao and Hu2020). The vaccine is expected to be widely used in livestock. A vaccine candidate targeting the definitive host has also been developed. This vaccine was expected to prevent dog infection and CE transmission. However, field test results were challenged with poor efficacy and unreproducible outcomes (Torgerson et al. 2009). Although vaccination stands as an ideal strategy for disease prevention and control, substantial progress is requisite in the research and development of vaccines.
Early diagnosis and enhanced surveillance
Data from the past 19 years have shown that early detection and community surveillance programs are effective in reducing the incidence of echinococcosis infections. Subgroup analysis shows that the majority of affected individuals are in the 40–49 age group, according to subgroup analysis. Further improvements in screening techniques and enhanced surveillance may facilitate the elimination of echinococcosis.
Serological tests and portable ultrasonography can be convenient and rapid methods and can assist patients in early diagnosis and obtaining timely treatment (Craig et al. Reference Craig, Mastin, van Kesteren and Boufana2015). But field-friendly and validated serum examinations with high sensitivity and specificity are not available for surveillance application (Carmena et al. Reference Carmena, Benito and Eraso2007; Qian et al. Reference Qian, Abela-Ridder, Wu and Zhou2017). For the livestock industry, early diagnosis of echinococcus infection in animals can cut economic loss. The main and most reliable surveillance method for livestock farmers is necropsy. Several studies suggest that ultrasound and serology surveillance could also be beneficial (Alvi et al. Reference Alvi, Ali, Khan, Saqib, Qamar, Li, Fu, Yan and Jia2023). However, there is no worldwide consensus on an affordable, accessible, and highly effective non-invasive method (Siles-Lucas et al. Reference Siles-Lucas, Casulli, Conraths and Müller2017; Alvi et al. Reference Alvi, Ali, Khan, Saqib, Qamar, Li, Fu, Yan and Jia2023). Taking into account the relatively limited economic level of high-risk areas and the difficulty of prevention, it will be necessary to increase investment in echinococcosis research and develop more cost-effective and convenient detection methods for echinococcosis diagnosis in both humans and animals (Stojković et al. Reference Stojković, Weber and Junghanss2018; Gauci et al. Reference Gauci, Jenkins and Lightowlers2022).
In this study, we obtained a comprehensive dataset of echinococcosis infections from the China CDC, covering over nearly 19 years. Unfortunately, we were unable to obtain infection data for AE and CE separately, which hindered further analysis to evaluate the efficacy of Eg95 vaccination, although CE and AE have different pathological characteristics, clinical presentations, and host preferences. Given these fundamental differences, we recommended the implementation of an enhanced surveillance system that would record and report AE and CE cases separately. Such a refined approach to data collection would greatly facilitate a more detailed understanding of the epidemiology and clinical dynamics of these two variants of echinococcosis.
Conclusion
Given the chronic onset of these diseases, it is challenging to demonstrate effective prevention and control over a short period of time. The lack of filed investigations in this area limits in-depth understanding of true prevalence and evaluation of the effectiveness of policy implementation. Efforts to raise awareness, improve surveillance systems, and strengthen collaborative initiatives will be critical to reducing the burden of echinococcosis and improving global health outcomes.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X24000014.
Financial support
This study was funded by the Clinical Research Incubation Program, West China Hospital, Sichuan University (2019HXFH074); the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21046); the 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH001); the Natural Science Foundation of Sichuan Province (2022NSFSC0806); the National Natural Science Foundation of China for Young Scientists Fund (82203650, 82203782); the Sichuan Science and Technology Program (2021YJ0132, 2021YFS0100); the Fellowship of China Postdoctoral Science Foundation (2021M692277); the Sichuan University-Zigong School-Local Cooperation Project (2021CDZG-23); the Science and Technology Project of the Health Planning Committee of Sichuan (21PJ046); the Sichuan University-Sui Lin School-Local Cooperation Project (2022CDSN-18); the Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH127).
Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.




