Introduction
The distribution of the Lesser Flamingo Phoeniconaias minor includes Asian and African regions (Del Hoyo et al. Reference Del Hoyo, Elliot and Sargatal1992) with an estimated total non-breeding population of more than 2.6 million individuals (Childress et al. Reference Childress, Nagy and Hughes2008). Lesser Flamingo populations show a globally decreasing trend and are thus considered “Near Threatened” (BirdLife International 2009). The species breeds regularly at only five colonies in India, Namibia, Botswana and Tanzania, while other locations host only occasional or suspected breeding attempts, such as Mauritania (Childress et al. Reference Childress, Nagy and Hughes2008). Despite the large population, the fact that more than 75% of breeding individuals are concentrated at only one site (Lake Natron, Tanzania), with threats to this wetland including salt exploitation and the building of infrastructures (Köenig Reference KÖenig2006), make the species very sensitive to habitat alteration (Childress et al. Reference Childress, Nagy and Hughes2008). Therefore, the Lesser Flamingo is the subject of an International Action Plan under the AEWA-UNEP programme of the Convention on Migratory Species, which sets out the priorities for conservation action in a global context (Childress et al. Reference Childress, Nagy and Hughes2008).
The presence of Lesser Flamingo in the Senegal River delta (West Africa) was first documented in the 19th century, where it was considered a common species (Tremau de Rochebrune Reference Tremeau de Rochebrune1884). In 1965, breeding was confirmed in flooded areas of Aftout es Saheli, in the Toumbos Lagoon, south-west Mauritania, with about 800 individuals observed incubating, but confirmation of fledging was not possible (Naurois Reference Naurois de1965). Between 1986 and 1988, Lesser Flamingo and Greater Flamingo Phoenicopterus roseus settled in a breeding colony at Aftout es Saheli, with a maximum of 9,000 Greater Flamingos and 200 Lesser Flamingos incubating, but the attempt failed (Lamarche Reference Lamarche1988). Breeding of Lesser Flamingo was suspected in 1998 (Hamerlynck and Ould Messaoud Reference Hamerlynck and Ould Messaoud2000) and breeding of Greater Flamingo was observed in 2004 (Hamerlynck Reference Hamerlynck2005) and in 2008 when 800 juveniles fledged (Diawara et al. Reference Diawara, Arnaud, Araujo and Béchet2007, Reference Diawara, Siddaty and Béchet2008). In 2010 a mixed colony of Greater and Lesser Flamingos succeeded, producing 1,500 chicks of both species, of which at least 178 were juvenile Lesser Flamingos (Ould Sidaty and Ould Daf Reference Ould Sidaty and Ould Daf2010). The reasons for the increase in the number of incubating flamingos in 2008–2010 are unknown, but a better knowledge of the area and increased monitoring effort and high levels of annual rainfall could have contributed to this positive trend (Diawara et al. Reference Diawara, Siddaty and Béchet2008, Ould Sidaty and Ould Daf Reference Ould Sidaty and Ould Daf2010).
The breeding success of flamingos at Aftout es Saheli was ultimately very low in all cases, given the pressure of poachers, who traditionally exploit flamingos as a food resource, and abandonment of the colony due to predation by jackals Canis spp. and warthogs Phacochoerus africanus (Ould Sidaty and Ould Daf Reference Ould Sidaty and Ould Daf2010, Parc National du Diawling 2010), as the primary threats. Since the stable reproduction of the Lesser Flamingo in its only Western African locality is of great importance for the conservation of the species (Childress et al. Reference Childress, Nagy and Hughes2008), several management actions were taken in 2011 to reduce the effects of both threatening factors. The results are reported in this paper.
Methods
Study area
The area where Greater and Lesser Flamingos bred in 2011 and where monitoring and conservation measures were applied was the lagoon of Aftout es Saheli in the Senegal River Transboundary Biosphere Reserve, south-west Mauritania (Figure 1). It consists of a saltmarsh about 100 km2 in area, 45 km north of the Senegal River mouth, separated from the sea by a coastal dune bar. It is part of the vast coastal depression and floodplains of the Senegal delta (Duvail Reference Duvail2001). Aftout es Saheli is connected through various channels to the Aftout dam on the Senegal River that, together with the contribution of groundwater, allows the seasonal inflow of water to the lagoon (Duvail Reference Duvail2001, Hamerlynck and Duvail Reference Hamerlynck and Duvail2003). Four different islands, with a maximum surface area of about 1,600 m2, 1,400 m2, 4,000 m2 and 3,600 m2 respectively, emerge after the flooding of the wetland and subsequent progressive drying. These islands, situated in the north-east of the lagoon, form the flamingo colony, while other islands and trees are breeding sites of cormorants Phalacrocorax lucidus and P. africanus, terns Sterna caspia and S. maxima and Great White Pelicans Pelecanus onocrotalus (Isenmann et al. Reference Isenmann, Benmergui, Browne, Diam Ba, Hamallah, Diawara and Ould Sidaty2010). The study area is located in the Sahel region, a transitional pastoral semi-desert area with an annual rainfall of around 250 mm (Zwarts et al. Reference Zwarts, Biljsma, van der Kamp and Wymenga2009). The habitats surrounding the breeding colony are characterised by sand dunes, seasonal herbaceous vegetation, a wide extent of Salicornia communities and small patches of savanna with Tamarix sp. and Acacia sp. (Hamerlynck and Duvail Reference Hamerlynck and Duvail2003).
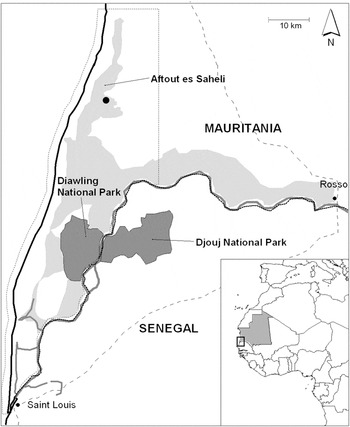
Figure 1. Location of the Greater Flamingo Phoenicopterus roseus and Lesser Flamingo Phoeniconaias minor breeding colony in Aftout es Saheli lagoon (SW Mauritania, black dot), within the Transboundary Biosphere Reserve of the Senegal River in Mauritania (dotted line). The map also shows the Senegal river (dark grey), the flooded areas of the Senegal delta in Mauritania (light grey) and national parks of Diawling and Djouj.
Field work
Monitoring breeding
With the aim of understanding the breeding cycle of flamingos, weekly counts were conducted to record: 1) number of incubating individuals of both species, 2) total number of chicks and identification of the species when possible, 3) number of abandoned eggs, 4) number of individuals (chicks and adults) predated and 5) date of emergence of different nesting islands.
Monitoring and deterring predation
A team of six people, permanently established around the wetland, monitored the breeding colony from 27 January to 31 May. Daily tours around the closest shores to the colony were carried out to detect the presence of people and to identify potential predator species through indirect evidence, camera traps and tracks (Silveira et al. Reference Silveira, Jácomo and Diniz-Filho2003, Long et al. Reference Long, MacKay, Zielinski and Ray2008). Moreover, the following predator deterrent and trapping devices were set up (Neuman et al. Reference Neuman, Page, Stenzel, Warriner and Warriner2004, Muñoz-Igualada et al. Reference Muñoz-Igualada, Shivik, Domínguez, González, Aranda, Fernández-Olalla and Alves2010):
-
–Electric fences. Electrified net 90 cm high, 300 m long, composed of orange braided polyethylene mesh, plastic posts and Solar Electrifier Viper S250 and a recharging battery (Smith et al. Reference Smith, Pullin, Stewart and Sutherland2010a; Figure 2).
-
–Scent stations. Pieces of cotton fabric periodically provided with commercial perfumes.
-
–Fladry line. A line of about 2,000 m was installed along the shore of the lagoon near the breeding colony; this consisted of a tensioned wire fixed to a post, from which 50 cm plastic strips were suspended every 2–3 m to indicate the presence of a barrier visually and aurally (Shivik et al. Reference Shivik, Treves and Callahan2003; Figure 2).
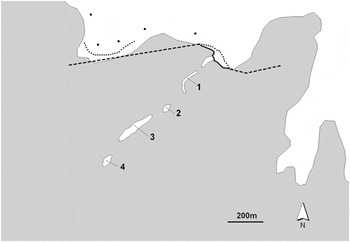
Figure 2. The four islets on which the breeding colony of Lesser Flamingos Phoeniconaias minor and Greater Flamingos Phoenicopterus roseus in Aftout es Saheli, south-west Mauritania, is situated (water in grey and inland in white). Location of the different predator deterrent and trapping devices: electric fence (solid line), fladry line (dashed line), lines of snares (dotted line) and Belisle traps (dots).
The devices used to trap potential mammal predators were selected for their efficacy, selectivity and absence of risk to the welfare of captured animals (Muñoz-Igualada et al. Reference Muñoz-Igualada, Shivik, Domínguez, González, Aranda, Fernández-Olalla and Alves2010):
-
–Line of snares. A barrier consisting of branches of Prosopis sp. was erected to prevent entry by animals. Every 10 m, a 50-cm wide gap was left open to allow the passage of animals. At each gap a top-locked snare was installed (Wisconsin DNR 2004, Muñoz-Igualada et al. Reference Muñoz-Igualada, Shivik, Domínguez, González, Aranda, Fernández-Olalla and Alves2010), to live-trap medium-sized carnivores by their necks. The length of the barrier was expanded sequentially along the shore of the lake in accordance with water level dynamics. The barrier reached 300 m in length (Figure 2).
-
–Walkway snares. After a new access point to the breeding colony was detected, 25 top-locked snares were installed (Wisconsin DNR 2004, Muñoz-Igualada et al. Reference Muñoz-Igualada, Shivik, Domínguez, González, Aranda, Fernández-Olalla and Alves2010) among the vegetation around the pond in order to live-trap medium-sized carnivores by their necks (Figure 2).
-
–Belisle traps. Five Belisle leg-hold traps with scented bait (Les Entreprises Belisle 2011) - were set in the surroundings of the lagoon along target-predator paths (Figure 2).
Trapped animals were released 15 km away from the lagoon. The intention was that trapped predators would experience their attempt to access the breeding colony as traumatic but also remain in their territories, to avoid attracting other individuals of the same species (Pitt et al. Reference Pitt, Box and Frederick. F. Knowlton2003). However, we realised that jackals were not exclusively territorial (McDonald Reference McDonald1979, Loveridge and MacDonald Reference Loveridge and MacDonald2001) and that the number of jackals attracted to the colony increased with the increased breeding activity of the flamingos. For this reason, after 1 April it was decided that trapped individuals would be culled, to reduce predation pressure.
Statistical analyses
To establish the relationship between the number of both species of flamingos hatching and the emergence of the different islands on which the breeding colony was situated as water levels decreased, we performed an ANOVA (Sokal and Rohlf Reference Sokal and Rohlf1995). We considered as a response variable the number of incubating birds on each census date and as a grouping factor the following options: 1) no appearance of a new breeding islet with respect to the preceding census date, 2) appearance of the first breeding islet, 3) appearance of the second islet, 4) appearance of the third islet, and 5) appearance of the fourth islet. Moreover, to assess whether the number of hatching Lesser Flamingos was positively linked to those of Greater Flamingos, we carried out a simple regression analysis (Sokal and Rohlf Reference Sokal and Rohlf1995).
In addition, to evaluate the moment at which chick predation rates were higher, we also applied an ANOVA (Sokal and Rohlf Reference Sokal and Rohlf1995), considering as a response variable the number of observed dead chicks and as independent qualitative factor 1) the existence of water at the lagoon or 2) the lack of water around the breeding islets.
Results
Breeding
The first incubating Greater Flamingos were detected on 15 February on the first islet that emerged (Figure 3). Their number increased significantly at the beginning of March, coinciding with the appearance of the third islet (F3,6 = 35.937; P = 0.0031; Figure 3), until they reached the maximum of 10,000 incubating individuals. Lesser Flamingos did not overlap the sequence of incubation with the Greater Flamingo (F1,8 = 0.001; P = 0.9699). Once the fourth islet emerged during late March, the number of hatching Lesser Flamingo reached a maximum (4,800 individuals; F3,6 = 18.246; P = 0.0028; Figure 3).
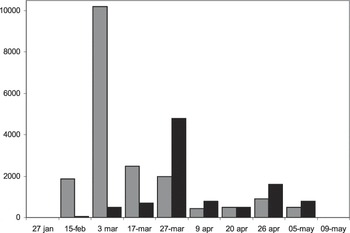
Figure 3. Number of Greater Flamingos Phoenicopterus roseus (grey) and Lesser Flamingos Phoeniconaias minor (black) incubating at Aftout es Saheli in 2011.
The first chicks were observed in early March and their numbers gradually increased to about 11,000 on 26 April (Figure 4). At that time the water level decreased, many breeding areas remained accessible by land and, therefore, the number of predated flamingos increased significantly (F1,11 = 3,787.3; P< 0.0001). Thus, on 5 May more than 4,000 corpses of chicks of both flamingo species were registered. Adding that number to 10,000 live juvenile flamingos yields an approximate total of 14,000 hatched chicks. In late June, after the wetland dried up completely, 4,672 corpses of juveniles were found (482 Lesser Flamingos, 1,720 Greater Flamingos and 2,470 unknown), as well as 147 adult casualties (28 Greater Flamingo, 17 Lesser Flamingo and 102 unknown).
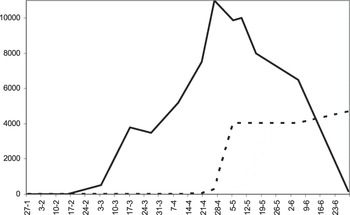
Figure 4. Number of alive chicks of Greater Flamingo Phoenicopterus roseus and Lesser Flamingo Phoeniconaias minor (solid line) and the number of dead/predated chicks (dashed line) at Aftout es Saheli in 2011.
The 2011 season was the most successful in terms of number of birds hatching and fledged juveniles, in relation to all previous breeding attempts (Figure 5).
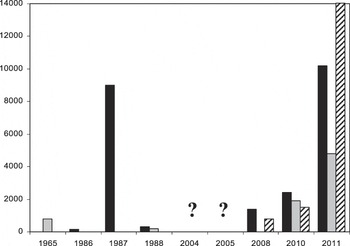
Figure 5. Maximum number of Greater Flamingo Phoenicopterus roseus (black) and Lesser Flamingo Phoeniconaias minor (grey) incubating at Aftout es Saheli and maximum number of chicks recorded of both species (barred). Breeding was confirmed in 2004 and 2005 but no hatching numbers were released. In 2008, only Greater Flamingo juveniles fledged.
Protection against predators and disturbance
Around the colony, golden jackal Canis aureus, side-striped jackal Canis adustus, pale fox Vulpes pallida, striped hyena Hyaena hyaena, honey badger Mellivora capensis, unidentified mongoose species and warthogs were detected. The entry of these species into the colony was prevented by the deterrent and protection systems installed, except on seven occasions, 21 February, 15 March, 19 March, 30 March, 3 April, 4 April and 1 May. All instances, except for 4 April, involved 1–3 jackals. The entry of predators into the colony caused the abandonment of about 1,500 eggs.
Eleven potential predators were trapped (Table 1). Of these, three freed themselves from the traps, six were released by the monitoring team in areas far from the breeding colony and two were culled (a golden jackal and a warthog). No poaching attempts or access by people to the environs of the colony were detected.
Table 1. Number of individual predator species trapped around the flamingo breeding colony of Aftout es Saheli in 2011.

Discussion
Breeding
The nesting of flamingos in mixed colonies has interesting ecological aspects that influence development of the reproduction cycle (Kear and Duplaix-Hall Reference Kear and Duplaix-Hall1975, Newton Reference Newton1992). In the study colony, asynchrony of about 15 days in the maximum numbers of incubating individuals of the two flamingo species was observed, probably caused by limited site availability in the breeding colony (McPeek et al. Reference McPeek, Rodenhouse, Holmes and Sherry2001). This limiting factor could determine the maximum number of incubating Lesser Flamingos, as occurs in other flamingo species (Nager et al. Reference Nager, Johnson, Boy, Rendon-Martos, Calderon and Cézilly1996). Specifically, we observed the occupation of the central areas of the colony by Greater Flamingos, possibly indicating an inferior competitive ability of the Lesser Flamingo since they established nests at the periphery of the colony (Newton Reference Newton1992). This could delay laying and hatching dates and make Lesser Flamingo chicks more vulnerable to predation when the lake dried up completely during the first half of May.
Habitat management
The hydrological dynamics of wetlands are a key factor in the reproduction of colonial waterbirds (Cezilly et al. Reference Cezilly, Boy, Green, Hirons and Johnson1997, Bechet et al. Reference Bechet, Germain, Sandoz, Hirons, Green, Walmsley and Johnson2009). In the case of Aftout es Saheli, the most important variables involved are annual rainfall, the management of the Senegal River dams in supplying the delta and associated farmlands (i.e. opening time, water volume transferred) and the evaporation rate (Hamerlynck and Duvail Reference Hamerlynck and Duvail2003, Hamerlynck Reference Hamerlynck2005). Such factors could determine the water level, the flooding period and the approximate dates of total drying out of the lagoon (Hamerlynck and Duvail Reference Hamerlynck and Duvail2003) and also therefore: 1) breeding population size (Cezilly et al. Reference Cezilly, Boy, Green, Hirons and Johnson1997, Bechet and Johnson Reference Bechet and Johnson2008), 2) breeding phenology (Bechet and Johnson Reference Bechet and Johnson2008), and 3) the likelihood of access by predators (Rendón et al. Reference Rendón, Garrido, Ramirez, Rendon-Martos and Amat2001).
Furthermore, the annual variability in factors influencing hydrological dynamics affects the start and end dates of breeding, the nesting of other bird species and the entry of other species into the colonies, such as Great White Pelican (authors’(tm) unpubl. data). Thus, it is necessary to determine an accurate approach to water level management. As an alternative, to ensure the sustainability of flamingo reproduction in the area, managing the size and height of the nesting islands could provide very positive results, as demonstrated in other breeding colonies (Rendón and Johnson Reference Rendon and Johnson1996, Johnson Reference Johnson1997). So, appropriate management of water levels in Aftout es Saheli to ensure the islands remain isolated for an adequate period of time is a crucial issue and should be considered when updating the management policy for the Senegal delta, especially in years with low rainfall. The next official approval of the enlargement of Diawling National Park should integrate a modified management regime for the river dams according to ecological requirements, including appropriate water timing, levels and dynamics that allow flamingos to breed successfully in Aftout es Saheli, in a compatible way with agriculture, fishing and livestock raising by the local people (Reference BaldóBaldó in press).
On the other hand, construction of infrastructure such as paved roads in areas surrounding the breeding colony or even within the whole Senegal River delta may increase human accessibility to the breeding flamingos and thus increase the number of threats from disturbance or poaching (Martínez-Abrain et al. Reference Martínez-Abrain, Oro, Jiménez, Stewart and Pullin2010, Torres et al. Reference Torres, Palacín, Seoane and Alonso2011). For this reason, a suitable management plan should be developed that considers all the potential threats to this and other protected species (Hamerlynck and Duvail Reference Hamerlynck and Duvail2003, Hamerlynck Reference Hamerlynck2005, Reference HamerlynckBaldó in press).
Predation and poaching
Monitoring of breeding areas most probably contributed to the reduction in poaching pressure, while predation was revealed to be the main threat to both adults and juveniles. Predation was positively related to greater opportunities for access to nesting islands (Rendón et al. Reference Rendón, Garrido, Ramirez, Rendon-Martos and Amat2001) so water level control is one of the best management options for reducing accessibility (Rendon and Johnson Reference Rendon and Johnson1996, Johnson Reference Johnson1997). Water levels could not be controlled in 2011 so predator access to the colony was prevented as described above and, as a consequence, widespread desertion of the colony was avoided (Johnson and Cezilly Reference Johnson and Cezilly2007) such that only a few eggs were abandoned (Smith et al. Reference Smith, Pullin, Stewart and Sutherland2010b).
Implications for conservation
A permanent breeding colony of Lesser Flamingo in West Africa would improve the conservation status of the species by increasing the number of existing colonies, expanding the distribution range, attenuating threats in the few breeding locations and reducing demographic isolation (Childress et al. Reference Childress, Nagy and Hughes2008). Thus, although interchange rates among the four main hotspots for the species (eastern, southern, western Africa and the Indian subcontinent; Childress and Hughes Reference Childress and Hughes2007, Zaccara et al. Reference Zaccara, Crosa, Childress, McCulloch and Harper2008) are not known, accurately the establishment of new breeding colonies would be a step towards more effective wetland conservation policies and improve the connectivity of the species (Bechet et al. Reference Bechet, Germain, Amat, Canas, Rendon-Martos, Garrido, Baccetti, Dall’Antonia, Balkyz, Diawara and Johnson2007, Childress et al. Reference Childress, Hughes, Harper and van den Bossche2007).
The experience gained during 2011 led to preliminary recommendations on optimising the sustainability of Lesser Flamingo reproduction in the area: 1) the hydrological dynamics of the Aftout es Saheli wetland should be investigated in detail and properly managed; 2) constant monitoring efforts would allow a better understanding of flamingo population dynamics at the colony; 3) suitability of the structure of the nesting islands should be assessed to increase the synchrony and availability of space for a greater number of breeding Lesser Flamingo; 4) predation might be managed in an advanced and optimised way using the best known practices; 5) technical training for surveillance, monitoring of the breeding colony and deterrence of predation should be continued; and 6) infrastructure such as paved roads or coastal developments should be avoided in the surroundings of Aftout es Saheli and the whole Senegal River delta.
Acknowledgements
This work was developed within the collaborative framework between the Ministry of Environment and Sustainable Development of Mauritania and the Ministry of Agriculture, Food, and Environment of Spain (MAGRAMA), and implemented by the PARCE-PND-RBT project (Projet d’appui à la restauration et la conservation des ecosystems et de la biodiversité au profit des communautés locales de la Réserve de Biosphere Transfrontière du Bas Delta Mauritanien), the Spanish Agency of International Cooperation (AECID) and Diawling National Park. It was co-funded by MAGRAMA and AECID and was supported by the Senegal River Delta Biosphere Reserve (UNESCO), IUCN and Banc d’Arguin International Foundation (FIBA). Moulaye, Boubacar Ba, Abdallahi Magrega, Albert Roura, Manuel Rendón, Yeli Diawara, Antonio Araujo, Sidi Mohamed, Mohamed Ould Lehlou and Moctar Dada helped in different phases of the work. Olivier Hamerlynck, Phil Atkinson and an anonymous reviewer kindly improved a draft version of this article.








