To the Editor—The emergence of multidrug-resistant organisms is a threat to healthcare systems worldwide. As the inanimate patient environment is a major reservoir interest in UV-C-disinfection techniques has increased recently.Reference Kanamori, Rutala, Gergen and Weber1 , Reference Rock, Curless and Nowakowski2 Petersson et alReference Petersson, Albrecht, Sedlacek, Gemein, Gebel and Vonberg3 tested a mobile UV-C device and demonstrated that 4 bacterial species could be successfully inactivated on agar plates within a few seconds of irradiation, whereas longer time periods were needed for bacterial spores. Based on their conclusion ultraviolet (UV) light may provide an alternative for the decontamination of medical products, which cannot be treated otherwise. In this study, we examined the effectiveness of 2 UV-C devices on difficult-to-disinfect surfaces in air ambulance helicopters where additional mandatory air worthiness requirements related to material degradation limit the use of some chemical disinfectants.
Material and Methods
In a pretest, 1 test organism (Enterococcus faecium ATCC 6057) was plated in concentrations of 101 to 105 colony-forming units (CFU)/mL on Columbia 5% sheep blood agar plates (Becton Dickinson, Heidelberg, Germany). Serial dilutions were prepared in in sterile 0.9% sodium chloride. Irradiation was performed at a light wand-plate distance of 6 cm with 2 different mobile UV-C devices: (1) the commercially available Verilux CleanWaveSanitizing Wand (Verilux, Waitsfield, Vermont) and (2) a portable light wand prototype (courtesy of Dinies Technologies, Villingendorf, Germany) for 3, 5, and 10 seconds. Plates were incubated for 24 hours at 36°C under aerobic conditions, and the colony-forming units were visually determined. Untreated plates served as a negative control. We observed a reduction factor of >5 log10 units.
Overall, 6 representative difficult-to-disinfect surfaces from high-touch sites extracted from air ambulance helicopters were evaluated (Table 1). Then, 100 µL of the test strains (106 to 107 CFU) were inoculated. After air drying and irradiation, the surface was sampled with flocked swabs (Swab Rinse Kits, Copan, Italy), and serial dilutions were prepared and inoculated on blood agar. Enterococcus faecium ATCC 6057 was tested at irradiation intervals of 3, 5, and 10 seconds on all samples; Acinetobacter baumannii ATCC 19606 was tested only on 2 samples (surface 1, plastic, and surface 6, metal). Additionally, 2 surface samples were tested at a long irradiation of 60 seconds (surfaces 1 and 6). The experiment was repeated 3 times. After each contamination, the surface was disinfected with 70% alcohol. Untreated but contaminated probes served as controls to calculate the reduction factor.
Table 1. Reduction Factors with 2 Different UV-C Devices and 2 Test Organisms on 6 Problematic Test Surfaces of an Air Ambulance Helicopter When Used for 3, 5, 10, or 60 Seconds, Simulating Real-Life Application Conditions
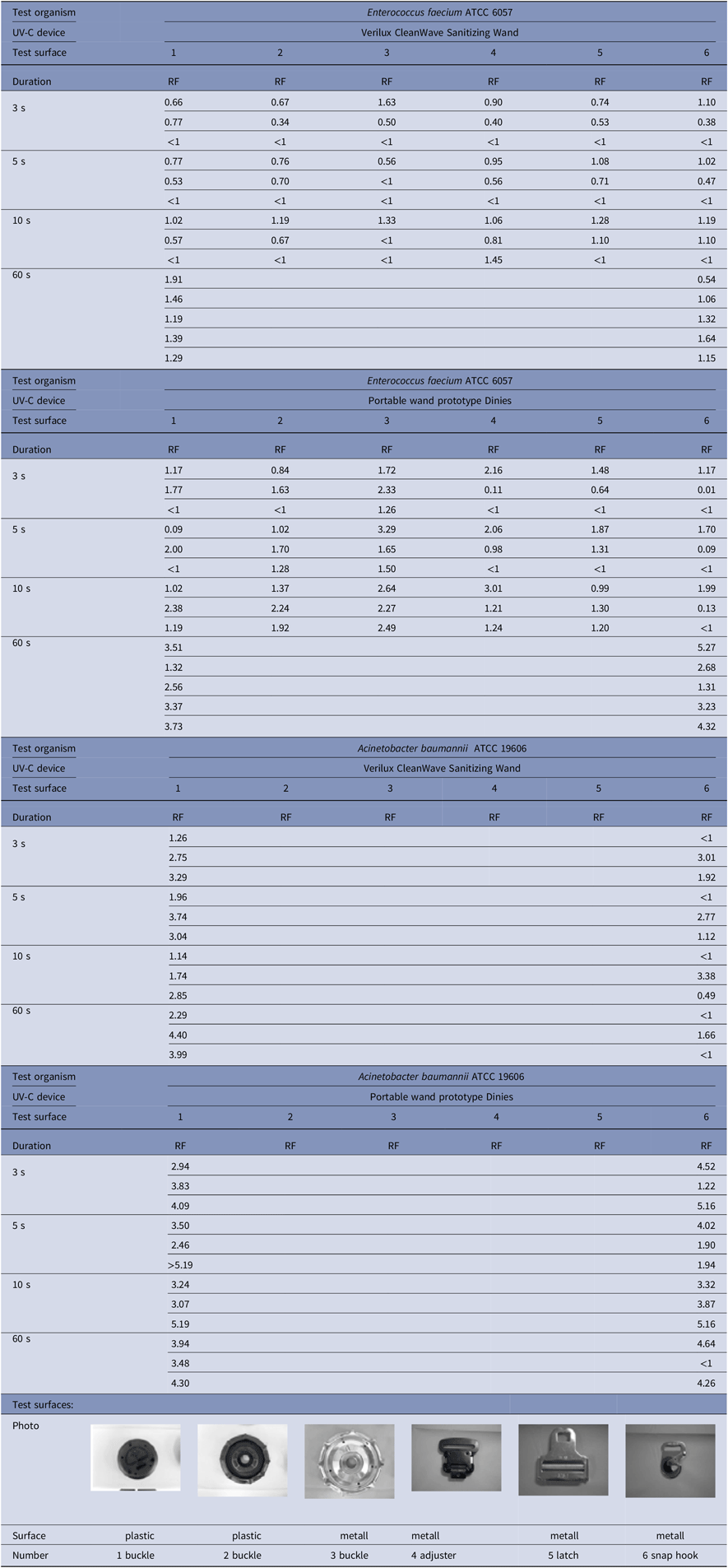
Empty squares: Not analyzed.
Note. ATCC, American Type Culture Collection; RF, reduction factor.
Results
Table 1 shows the detailed reduction factors that were heterogeneous for the different surfaces and the 2 chosen species. Reduction factors of ≥3 log10 units were achieved for E. faecium with the Dinies prototype after 60 seconds irradiation and for A. baumannii with both light wands after a much shorter irradiation time. Despite a highly standardized irradiation procedure, there was large variation between individual tests and devices.
Discussion
In an air ambulance helicopter, surface materials are heterogeneous (metal, plastic, and others) and by design often difficult to clean. Due to air worthiness regulations and material degradation, not all chemical disinfectants can be used. A mobile, nonchemical device would therefore be a valuable alternative for a targeted surface disinfection. Cadnum et alReference Cadnum, Jencson and Gestrich4 compared multiple UV decontamination devices in a radiology suite. Moreover, 4 standard, vertical-tower, low-pressure, mercury devices achieved reductions of VRE or MRSA ≥2 log10 units and of C. difficle at ~1 log10 unit, whereas a pulsed-xenon device resulted in less reduction of the pathogens. Compared with the vertical tower low-pressure mercury devices, equal or greater reductions of the pathogens were achieved by 3 nonstandard low-pressure mercury devices that included either adjustable bulbs that could be oriented directly over the exam table or 3 vertical towers operated simultaneously.Reference Cadnum, Jencson and Gestrich4 Our results in achievable reduction factors in a test environment simulating real-life conditions of manual application are comparable to those of Cadnum et al. However, 60 seconds were needed with our surfaces compared to the fast reduction achieved on agar plates by Petersson et alReference Petersson, Albrecht, Sedlacek, Gemein, Gebel and Vonberg3 and in our pretest. The large variability of log10 unit reduction between tests and devices might be due to unavoidable differences in angulation of the device toward the surface, changing reflection artefacts, and/or the different surfaces, which are to be expected in a real-life environment. Even in a hospital setting, the efficiency of UV-C devices remains controversial. Ontario Health concludes in a health technology assessment report: “We are unable to make a firm conclusion about the effectiveness of this technology on HAIs given the very low to low quality of evidence.”5
This study has several limitations. We analyzed only 2 bacterial species and only a few representative surfaces. Based on our experiments and the reviewed literature UV-C disinfection with mobile light wand devices should only be used as an add-on technique after thorough cleaning and requires a prolonged application time for some bacterial species. No experiment showed a reduction >5 log10 units defining disinfection. However, after cleaning, a low-level reduction may be acceptable because fewer CFU can be expected on the surface than in our experiment. The light wand device can also be used as an extra disinfection after terminal cleaning and disinfection for complex surfaces (eg, buttons of the endotracheal suction system), as shown by Wendel et al.Reference Wendel, Malecki, Otchwemah, Tellez-Castillo, Sakka and Mattner6 Additional material degradation testing is needed before air worthiness approval in an air ambulance. Occupational safety regulations regarding UV-C use need to be observed with manual application procedures.
Acknowledgments
We thank Mr Cajus Dinies (Dinies Technologies GmbH, Gewerbestr. 5, D-78667 Villingendorf) for letting us use his prototype device. We also thank Ingo Winterfeld (Institute of Hygiene, Cologne) for technical assistance.
Financial support
This research was funded by institutional funds only.
Conflicts of interest
S.S.S. is shareholder of Deutsches Beratungszentrum für Hygiene (BZH GmbH) and receives royalties from Springer, Thieme, Deutsche Krankenhausverlagsgesellschaft and Consilium Infectiorum. All other authors report no conflicts of interest relevant to this article.



